Abstract
Background
Omission of family and caregiver health spillovers from the economic evaluation of healthcare interventions remains common practice. When reported, a high degree of methodological inconsistency in incorporating spillovers has been observed.
Aim
To promote emerging good practice, this paper from the Spillovers in Health Economic Evaluation and Research (SHEER) task force aims to provide guidance on the incorporation of family and caregiver health spillovers in cost-effectiveness and cost-utility analysis. SHEER also seeks to inform the basis for a spillover research agenda and future practice.
Methods
A modified nominal group technique was used to reach consensus on a set of recommendations, representative of the views of participating subject-matter experts. Through the structured discussions of the group, as well as on the basis of evidence identified during a review process, recommendations were proposed and voted upon, with voting being held over two rounds.
Results
This report describes 11 consensus recommendations for emerging good practice. SHEER advocates for the incorporation of health spillovers into analyses conducted from a healthcare/health payer perspective, and more generally inclusive perspectives such as a societal perspective. Where possible, spillovers related to displaced/foregone activities should be considered, as should the distributional consequences of inclusion. Time horizons ought to be sufficient to capture all relevant impacts. Currently, the collection of primary spillover data is preferred and clear justification should be provided when using secondary data. Transparency and consistency when reporting on the incorporation of health spillovers are crucial. In addition, given that the evidence base relating to health spillovers remains limited and requires much development, 12 avenues for future research are proposed.
Conclusions
Consideration of health spillovers in economic evaluations has been called for by researchers and policymakers alike. Accordingly, it is hoped that the consensus recommendations of SHEER will motivate more widespread incorporation of health spillovers into analyses. The developing nature of spillover research necessitates that this guidance be viewed as an initial roadmap, rather than a strict checklist. Moreover, there is a need for balance between consistency in approach, where valuable in a decision making context, and variation in application, to reflect differing decision maker perspectives and to support innovation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-023-01321-3.
Key Points for Decision Makers
| Evidence suggests that omission of family and caregiver health spillovers from the economic evaluation of healthcare interventions remains common practice. When accounted for, a high degree of methodological inconsistency has been observed. |
| The Spillovers in Health Economic Evaluation and Research (SHEER) task force has developed consensus guidance on the incorporation of family and caregiver health spillovers in cost-effectiveness and cost-utility analysis. |
| It is hoped that this report, detailing 11 consensus recommendations for emerging good practice and 12 avenues for future research, will motivate more widespread incorporation of health spillovers and expedite development of the spillover knowledge base. |
Introduction
It has been more than 50 years since the earliest consideration in the health economics literature of the wider consequences of an individual’s health for the health and wider welfare of others [1, 2], and almost 20 years since Basu and Meltzer [3] denoted these impacts as “spillover effects” in the specific context of a cost-effectiveness analysis (CEA) [4]. Subsequently, greater awareness of the importance of family health spillovers has developed [5, 6]. Building on a number of key papers [7–11], the evidence base on the topic continues to grow. This literature describes an extensive range of spillover effects, including impacts on physical health [12] and psychological well-being [13–16]. These impacts have been shown to occur among persons “caring for” and “caring about” the affected individual [14, 17–19], originating from their emotional response, adaptation of their attitudes and behaviours, and changes in the level of informal care provided by caregivers (i.e., those “caring for” the patient) [20].
As a result, healthcare interventions that improve the lives of patients may offer wider benefits to a network of family members and “significant others” [13, 18, 21]. When accounted for in health economic evaluations, the implications of including such benefits for the incremental cost-effectiveness ratio have often been shown to impact funding recommendations [20, 22, 23]. As such, there have been many calls for the inclusion of the wider health effects of interventions in analyses, which may alternatively be thought of as ameliorated or prevented impacts of illness [24–27]. Others, however, have urged caution in incorporating spillovers owing to the potential for unintended distributional impacts of spillover incorporation, which may challenge the equity of resource allocation decisions [28, 29]. Moreover, quantification of health spillovers is non-trivial, and as such, concerns as to the appropriateness of inclusion also relate to the extent to which they can be accurately identified, via cross-sectional study designs for example [28]. Nevertheless, guidelines on the conduct of economic evaluations published by the National Institute for Health and Care Excellence (NICE) [30], the Second Panel on Cost-Effectiveness in Health and Medicine [31] and others [32, 33] also now advocate for family health spillover incorporation into the reference case. Although these guidelines recommend inclusion, little methodologic direction is provided.
Despite these calls for inclusion, evidence suggests that accounting for the spillover effects of health conditions and/or healthcare interventions is far from common practice. For example, a recent review of NICE evaluations revealed that only 3% of technology appraisals included caregiver health-related quality of life (HRQoL) in cost-utility analyses (CUAs) [34]. Similarly, Lamsal [35] found that of 139 CUAs of maternal-perinatal treatments or programmes, 38 (27%) included health outcomes of the mother and child in analyses, and of 747 paediatric CUAs, 20 (3%) accounted for family health spillover effects. This tendency toward omission was also observed by Scope et al. [20], who reported just 40 of all identified CUAs of patient interventions incorporating family member quality-adjusted life-years (QALYs). A high level of methodological variation in those analyses incorporating family QALYs was noted, with a “conspicuous need for standardisation of methodologies” being emphasised [20]. This view has been echoed by Leech et al. [36], in arguing for the establishment of “consensus guidelines” to promote best practice in accounting for health spillovers, and Dawoud et al. [37], in calling for “consensus of academic opinion on appropriate methods” when including spillovers in economic models.
Accordingly, the Spillovers in Health Economic Evaluation and Research (SHEER) task force was convened. Through this paper, SHEER aims to provide consensus guidance to the health economics and outcomes research community working in academia, regulatory agencies, health technology assessment bodies, the life sciences industry, and elsewhere on the incorporation of family and caregiver health spillovers into CEA and CUA (hereafter, ‘CEA’ refers to both CEA and CUA). The task force also aims to identify and explicitly describe issues requiring additional evidence in order to inform a spillover research agenda and future practice. With little published guidance on the incorporation of heath spillovers having been developed, these recommendations are framed as initial guidelines for emerging good practice [38]. The SHEER task force continues to acknowledge that much additional research is required to develop good or best practice guidance. Furthermore, it is hoped that these initial recommendations will act as a catalyst to wider public discussion of the issues presented and encourage innovation in the field, advancing the science underlying the measurement and incorporation of spillover effects, allowing stakeholders to arrive at a set of widely agreed-upon practices [39]. The remainder of this report is structured as follows: Sect. 2 details the methods used in developing this guidance; Sect. 3 presents the recommendations for emerging good practice and future research; Sect. 4 discusses the overarching themes emerging through task force interactions and the potential implications of this work; and Sect. 5 provides conclusions.
Methods
To reach consensus on a set of recommendations on the incorporation of family and caregiver health spillovers into CEA, SHEER implemented a modified nominal group technique. This structured small-group discussion approach, devised by Delbecq et al. [40, 41], seeks to systematically measure and develop general agreement or convergence of opinion on a given topic [41–44]. It is one of the three most frequently used consensus methods in healthcare research, namely, the nominal group technique, the Delphi method and the RAND/UCLA appropriateness method [45, 46]. The nominal group technique allows for a large number of ideas to be generated independently and subsequently offers a platform for constructive discourse and debate [44, 47]. Furthermore, the method seeks to empower all participants to share their opinions, preventing dominant voices from unduly influencing proceedings [44]. The RAND/UCLA method, conversely, is not intended for idea generation and is chiefly employed in assessing the appropriateness of medical or surgical procedures [48, 49]. The Delphi method limits the opportunity for discussion among participants [49], and while it has previously been favoured in allowing for the inclusion of geographically dispersed respondents, improvements in video conferencing software limit the continued saliency of this advantage. Consensus studies will often refine components to achieve their specific aims [45, 47], and thus, a number of phases of the nominal group technique were adapted to our specific context, as described in Sect. 2.2.
Composition of the SHEER Task Force
Task force members were invited to participate on the basis of subject-matter expertise [50]. This was broadly defined as an identifiable track record of:
Peer-reviewed publication or presentation of research on the subject of family and caregiver health spillovers and/or applied economic evaluations in areas such as social care, dementia, and paediatrics (i.e., those areas where family and caregiver health spillovers are likely to be present) [22, 51, 52]; and/or,
Involvement in the development or application of guidelines for applied economic evaluations in the public or private sector.
Diversity of perspective has been described as critical in developing well-balanced guidance with broad applicability [38]. Insights were, therefore, sought from a range of work environments and jurisdictions. The purposively sampled group comprises 17 members drawn from academia (i.e., universities and research institutes/organisations), regulatory agencies and health technology assessment bodies (e.g., NICE and the Health Intervention and Technology Assessment Program [HITAP]), and the life sciences industry, working (or having worked) in Europe, North America, Australasia, and Africa. The full membership of SHEER is detailed in Appendix A, Fig. A1 of the Electronic Supplementary Material (ESM).
Task Force Process and Interactions
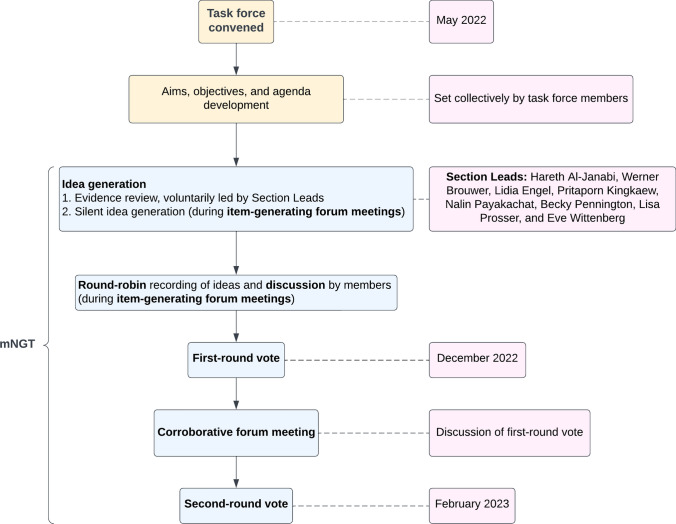
The task force was convened in May 2022 and member inputs were initially sought in developing the aims and objectives of SHEER, as well as in formulating an agenda to address these aims. Multiple face-to-face interactions (in the form of video conferences) were supplemented by an online discussion board, allowing members to share their thoughts and ideas remote to the meetings. The programme of group interactions culminated in three forum-style meetings (two item-generating meetings and one corroborative meeting, relating to separate phases of the modified nominal group technique, see below).
The nominal group technique typically involves four phases: silent idea generation, where participants silently reflect or record their individual ideas; round-robin recording of these ideas (in this instance, primarily recommendations proposed by members), with each participant sharing one idea from their list until all ideas have been shared; discussion of the ideas by the group; and voting to assign priority to generated ideas [41, 42]. The elements of the process adapted to our context were three-fold. First, the silent idea phase was expanded to incorporate an informal evidence review (specific to each item of the agenda), voluntarily led by select members, designated as ‘Section Leads’, working in conjunction with the SHEER rapporteur (member appointed to report on task force proceedings). This evidence review was shared with other members of the task force prior to the forum-style meetings, and not only allowed for the generation of ideas ahead of these meetings, but also ensured all members were made aware of developments within the literature distinct to their own specific area of research/expertise. Second, the round-robin recording of ideas and discussion phases were held consecutively for each of the items of the agenda to allow for the discussion of applicable groups of proposed recommendations immediately following their original suggestion. Finally, voting was held over two rounds, with the number of rounds having been determined a priori.
Through the structured discussions at the forum meetings, as well as on the basis of evidence identified during the review process, emerging good practice recommendations were proposed and put to a consensus vote. Where the necessity for further investigation was identified, recommendations for a future research agenda were also specified, and priority rated by members. 14 emerging good practice and 11 future research recommendations were voted upon in the first round. The results of the first vote (held after the item-generating forum meetings) were presented and discussed at the final (corroborative) forum meeting. This presentation/discussion entailed: a presentation of round one descriptive statistics; determination of where consensus had been reached at round one; and a summary of the comments and feedback of members provided during the vote. The second vote was held immediately following this final meeting. The round one distribution of votes and median scores specific to each recommendation, as well as member comments from the first vote and respondents’ personal votes, were provided to each member individually at the time of voting. Although the nominal group technique did not allow for anonymity during task force discussions, anonymity was maintained during voting, where members were reminded that their views did not need to conform to the opinion of other members. All 17 members completed both rounds of voting. The vote was conducted using Qualtrics, an online survey platform. Result collation and analysis were performed using Microsoft Excel (2020). This task force has sought, where possible, to conform to recommendations for demonstrating methodological rigor in consensus methods from Humphrey-Murto et al. [49]. The steps in this modified nominal group technique are summarised in Fig. 1.
Fig. 1.
Summary of the process and interactions of the Spillovers in Health Economic Evaluation and Research (SHEER) task force. mNGT modified nominal group technique
Definition and Assessment of Consensus
Definitions of consensus were developed by task force co-chairs and subsequently agreed upon by the wider membership. While consensus definitions vary across studies depending on the aims/objectives of the research [41], our definitions of agreement are consistent with those most commonly employed in the literature [49].
Members were initially asked to endorse a ‘reference perspective’ and a number of working definitions to be included in the report. In this context, consensus was defined as having been reached if 70% of respondents expressed their endorsement (yes/no response) for the reference perspective and each definition. With regard to the proposed recommendations for emerging good practice, members were asked to score each in terms of appropriateness (i.e., suitability as a guide to the conduct of CEA) on an 8-point scale, with 0 = inappropriate and 7 = most appropriate. Consensus on a recommendation as appropriate for inclusion in this guidance was defined as having been reached if 70% of member ratings fell within an ‘appropriate’ bracket, which was defined as a rating of 5 or above. Agreement on omission of recommendations was defined as 70% of ratings falling within an ‘inappropriate’ bracket of 2 or below. Where consensus was not achieved on emerging good practice, recommendations are still mentioned in the text of this report as indicative of issues that remain, as yet, disputed within the field. Last, respondents were asked to assign priority to areas of future research, proposed during any and all preceding task force interactions (0 = lowest priority, 7 = highest priority). These proposed avenues for future research were then ranked based on the proportion of ratings falling within a ‘high priority’ bracket (defined as ratings of 5 or above).
In keeping with our definition of consensus, these guidelines represent a convergence rather than unanimity of opinion. A synopsis of member discussions is contained in Appendix C of the ESM.
Report Compilation and Internal Review
The resulting consensus recommendation set and a supporting text (based on summaries of the evidence review process and dialogue of group) comprise this report of the SHEER task force. Following incorporation of comments from members, the document then underwent ‘internal review’ by a further group of subject-matter experts and other stakeholders. Specifically, as the SHEER task force is acutely aware of the importance of giving voice to the views of patients and families in health and social care research, patient and caregiver representatives were invited to share their perspectives on the recommendations as part of this review. Contributors were identified and recruited through consultation with charitable organisations, caregiver advocacy groups, the Irish Health Service Executive and the PPI Ignite Network (a national network developed to promote and advance the involvement of patients, caregivers, and the public in health and social care research in Ireland) [53]. An online meeting was held with the patient and caregiver contributors to allow for presentation and discussion of the various aspects of the consensus guidelines. Feedback and comments received during the internal review phase of the process were incorporated into the text of the report prior to submission for publication.
Results
Table 1 presents the agenda of the task force, developed through preliminary meetings and initial e-mail correspondence of its 17 members. The agenda encapsulates the key issues and common methodological challenges likely to be encountered by analysts seeking to incorporate health spillovers into CEA.
Table 1.
Agenda of the SHEER task force
| No. | Agenda item |
|---|---|
| 1 | Definition of family and caregiver health spillovers |
| 2 | When health spillovers should be incorporated into CEA |
| 3 | Outcome measurement |
| 4 | Sources of data: use of primary vs. secondary data |
| 5 | Aggregation methods |
| 6 | Outcome value weighting |
| 7 | Outcome double counting |
| 8 | Consistency in inclusion and reporting of spillovers |
CEA cost-effectiveness analysis and cost-utility analysis, SHEER Spillovers in Health Economic Evaluation and Research
Reference Perspective
A ‘reference perspective’ is necessary in order to inform the basis for the definition of health spillovers to be employed by the group, and to outline the context in which it is envisioned that these recommendations will primarily be applied. With a view to producing a practicable recommendation set, aligned with the primary aim of the task force, it is proposed that this guidance focus on incorporation of family and caregiver health spillovers (as defined subsequently) into a CEA conducted from a healthcare/health payer perspective. Such a perspective includes only those costs that fall under the healthcare budget and only health effects as outcomes/benefits [6]. Our interpretation of the healthcare/health payer perspective is analogous to that observed most commonly in a European context, where a single healthcare provider is more likely to predominate in a given jurisdiction. It is important to note, however, that in some contexts (e.g., the USA), where there are multiple healthcare providers, health costs and outcomes will most likely fall under the remit of multiple providers.
Definition of ‘Family’, ‘Caregiver’ and ‘Family and Caregiver Health Spillovers’
Working definitions of the terms ‘family’, ‘caregiver’ and ‘family and caregiver health spillovers’ were considered essential given the multiplicity of meanings that may be attached to each. All definitions contained within this paper are colour coded in green.
The definition of ‘family’ below is deliberately far-reaching and, moreover, specific to health economic evaluations, acknowledging that network composition and size will differ within and across health, cultural and other contexts. Yet, some measure of consistency is required in order to make valid comparisons between evaluations, as the wider the circle, the more ‘spillover effects’ are likely to be captured:
Linked with these definitions, and focusing on the impact of an individual’s illness on others’ HRQoL, the following working definition of ‘family and caregiver health spillovers’ has been endorsed by the task force:
This definition, purposely restricted to encompass only health spillovers onto family and caregiver HRQoL, does not negate the potential for impacts among persons outside this group, but rather focuses, for practical purposes, on those for whom the effects are anticipated to be largest. Deciding who is included or not inevitably requires some discretion on the part of the analyst, and likely depends, at least in part, on the health condition and age of the patient. Where there is ambiguity, sensitivity analyses with a wider/narrower set of included individuals may be undertaken (see recommendation 7 in relation to emerging good practice). As an economic evaluation compares different interventions, it also important to note that the effect of the intervention on the caregiver/family member may arise through a change in the patient’s health status and/or via another pathway [54]. For example, an ‘organisational intervention’ to discharge patients from hospital earlier is likely to affect family caregivers by shifting the location of care to the home and increasing the amount of informal care provided, rather than by changing the patient’s health status.
Emerging Good Practice and Related Future Research Recommendations
The succeeding sections detail 11 consensus recommendations for emerging good practice and 12 in relation to future research. The emerging good practice recommendations are summarised in Table 2, with their full text being presented thereafter. Recommendations for emerging good practice are colour coded in blue. As mentioned, a supporting text, expanding upon the discussions of the task force and the evidence review, accompanies the recommendation set. Where relevant, applicable recommendations for future research are highlighted in red. Table 3 outlines these and other avenues for further study.
Table 2.
Summarised consensus recommendations for emerging good practice
| No. | Summary of recommendation |
|---|---|
| 1 | While this task force seeks primarily to inform the conduct of CEA from a healthcare/health payer perspective, family and caregiver health spillovers should also be included in economic evaluations from a societal or public sector perspective |
| 2 | If opportunity costs within the healthcare sector are explicitly considered, health spillovers related to displaced/foregone activities should, ideally, also be considered |
| 3 | The distributional consequences of inclusion (or omission) of health spillovers in CEA should be considered |
| 4 | Health spillovers should be measured over a time horizon sufficient to capture all relevant impacts of the health condition(s)/intervention(s), in as balanced and accurate a manner as possible |
| 5 |
(a) Where specified, adhere to the jurisdiction’s health technology assessment guidelines on outcome measurement among family and caregivers unless it is empirically demonstrated that another method of measurement is more appropriate (b) Consider the use of the same measure for spillover and patient outcomes if the aim is to aggregate both in the analysis. Be mindful of how spillover outcomes are to be compared to outcomes related to displaced/foregone activities (c) Where outcome measures differ, consider employing a cost-consequence analysis |
| 6 | Collect primary data where possible, using secondary data sources when primary data cannot be collected, justifying the use of that secondary data |
| 7 |
Step 1 Spillovers should be estimated (a) among specific caregivers and other family members (e.g., parents, siblings, grandparents) OR (b) for the ‘closest’ network member and second ‘closest’ network member Step 2 QALY gains/losses should be reported separately for patients and caregivers/family members Step 3 Spillovers should be aggregated with patient effects via additive summation Step 4 Sensitivity analysis should supplement the reference case |
| 8 | Equal weighting should be applied to all impacts of the health condition and/or intervention (be they patient or spillover health impacts), unless empirical evidence suggests that the decision maker and/or society value family/caregiver outcomes differently than those of the patient in the context under investigation |
| 9 | Consider and discuss the extent of the risk presented by double counting, given the measurement technique employed (direct or indirect), flagging this as a potential source of uncertainty or bias |
| 10 | An explicit statement of assumptions about spillovers should be made. CEA should be presented in a way that enables methods of incorporation, data sources and results to be clearly discernible |
| 11 | Characterise and present the uncertainty surrounding spillover estimates, together with an assessment of implications of that uncertainty |
CEA cost-effectiveness and cost-utility analysis, QALY quality-adjusted life-year
Table 3.
Recommendations for future research
| Future research recommendation | Proportion rating as ‘high priority’a (%) | Priority rank |
|---|---|---|
|
Distributional consequences Exploration of ways of establishing, presenting and considering the distributional consequences of family and caregiver health spillovers |
94 | 1 |
|
Longitudinal study designs Adoption of longitudinal study designs to assess outcome measure sensitivity to change and allow for more in-depth investigation of the directionality and reciprocity of spillover effects |
76 | 2 |
|
Joint utility elicitation methods Further examination of joint patient-caregiver utility elicitation methods, and estimation of QALY gains/losses at the family or household level |
76 | 2 |
|
Context sensitivity of spillover magnitude/scale, social value and double counting Investigation of how: (i) the magnitude/scale of health spillover; (ii) the social value of these spillover health impacts; (iii) the functional form of the distance-decay function (used to project total spillover); and (iv) the risk and/or extent of double counting, vary on the basis of health condition/intervention, sociodemographic, cultural and other contexts, as well as in relation to the utility elicitation technique |
75b | 4 |
|
Modelling caregiver and family member health spillover as a function of available data Further examination of the potential for projecting/modelling spillover as a function of data such as patient health status, measures of sociodemographic status or caregiver burden |
71 | 5 |
|
Inclusion of family and caregivers in decision models In the context of decision models, examination of the implications of inclusion of caregivers and family members as separate individuals (with their own utilities) vs as disutilities/utility increments applied to patient outcomes (to account for the effect of the patient intervention on caregivers and/or others in the family network) |
69b | 6 |
|
Adjusting for double counting Develop methodological approaches to adjust for double counting when applying spillover estimates from the literature to analyses, or when quantified in the context of primary data collection |
53 | 7 |
|
Displacement Calculation of the average family and caregiver health spillovers in relation to displaced/foregone activity (to, e.g., inform thresholds empirically set on the basis of opportunity cost) |
53 | 7 |
|
Modelling total network effects as a function of partial network outcome data Further investigation of how total network effects might be estimated from partial network outcome data (i.e., when net spillover effects are to be estimated but only data on the patient and closest network member can be collected) |
44b | 9 |
|
Outcome measure appraisal Development of novel generic preference-based measures aimed at capturing spillovers (beyond those in current use or development), underpinned by psychometric assessment of the construct validity and responsiveness of the measure among family and/or caregiver populations. |
35 | 10 |
|
Social value weight estimation Investigation of methods for elicitation (e.g., person trade-off, discrete choice experiment) of spillover equity weights, giving particular focus to how estimated weights may vary by method used |
29 | 11 |
|
Reporting bias Investigate the extent of reporting bias either related to social desirability or when using the same preference-based HRQoL measure for caregiver/family member HRQoL and patient HRQoL (including self-report and proxy report) |
24 | 12 |
DCE discrete choice experiment, HRQoL health-related quality of life, PTO person trade-off, QALY quality-adjusted life-year
aRanked based on the proportion of member votes falling within a ‘high priority’ bracket (i.e., ratings 5, 6, 7) on an 8-point scale (0 = lowest priority, 7 = highest priority)
b16 of 17 members voted on this recommendation
The full results of the second-round vote are included in Appendix B of the ESM. Consensus was not reached as to the appropriateness of two proposed, emerging good practice recommendations; none of the proposed recommendations was deemed inappropriate.
When to Incorporate Health Spillovers into a CEA
There are two main schools of thought in this context, which relate to commonly espoused viewpoints in economic evaluations, often phrased as ‘perspective’ [55]. Under the healthcare/health payer perspective, the goal of the decision maker (to be informed by the economic evaluation) is typically assumed to be to maximise health from a fixed budget [56]. The decision maker, thus, aims to produce as much health (i.e., QALYs) as possible for the population from that fixed budget. If this is the decision rule, two things become important: (i) the measurement and inclusion of all relevant health changes in order to ensure health maximisation [8] and (ii) the capture of health effects related to displaced/foregone activity as well as those resulting from the new intervention [7]. Using a societal perspective, in contrast, the goal of the informed decision maker is assumed to be the maximisation of social welfare from a more flexible budget [57]. Here, all costs and benefits are relevant, regardless of where or on whom they fall [24].
Acknowledging that a payer perspective is the least inclusive of those most frequently employed [58], albeit adopted by NICE [30], the task force advocates for the consideration of health spillovers in all economic evaluations conducted from this and those more inclusive perspectives, such as the societal perspective, which is the default in the USA and the Netherlands, for example. In other words, family and caregiver health spillovers should be included in economic evaluations from all of the more frequently employed perspectives (i.e., healthcare/health payer, societal or public sector perspective). Consensus among members has been reached as to the need for an express statement to that effect, embodied in the recommendation below:
Opportunity Costs
If one assumes interventions are funded from a fixed budget, the opportunity costs of healthcare spending become relevant, including the health spillovers related to healthcare interventions displaced or foregone when a new intervention is adopted. However, the wider impacts of that care that is displaced/foregone will likely not have been considered when establishing benchmarks/thresholds of cost effectiveness. This may lead to a greater observed quantity of health displacement when funding new interventions where these effects have been considered [59]. To combat this and establish the value of the net gain in health, one should seek to account for all relevant gains of a healthcare intervention, including health spillovers, minus the lost health (also including health spillovers) due to care displaced or foregone [6, 57].
Given the focus of this report on health spillovers, the task force has refrained from further discussion of the ‘broader costs’ mentioned in the recommendation. It should, however, be recognised that caregivers and the wider family will also incur time and other costs, both within and outside healthcare sector.
In the absence of any guidance on disinvestment, healthcare interventions that are displaced or foregone are typically unknown, and as such, little is known about lost spillover effects related to this activity [6]. Much research is therefore needed to develop methods to quantify the average displaced/foregone spillover and this motivates the following future research recommendation:
Distributional Consequences of Inclusion
The distributional consequences of spillover inclusion (as well as omission) provoke much debate in the literature [5, 21, 29]. While systematic exploration of the trade-off between the maximisation of aggregate health and preservation of health equity [28] is not practicable at the level of an individual economic evaluation, the decision maker should at least be forewarned of this challenging issue to allow for informed decisions [6].
The implication of this recommendation is that consideration of spillovers could introduce bias in resource allocation (as accumulation of QALY gains among more isolated patient groups may be reduced and interventions targeting these groups would appear less cost effective). The limited evidence base in this regard compels further investigation of the extent and acceptability of these potential equity impacts, via, for example, the application of analytic approaches [60–62], as proposed by Dixon and Round [28].
Spillover Time Horizon
In addition to how wide a net to cast (see recommendation 7, below), there is also a question of how long, in terms of duration, to measure effects for (i.e., how to define the analytic time horizon). Typically, health costs and benefits cease at the time beyond which no incremental benefits of an intervention can be attributed to a cure or successful treatment, or at the end of life—all related to the patient. When considering health spillovers, however, family members may survive the patient and bereavement may be an important consideration in their HRQoL. Moreover, long-term health consequences of caregiving may surpass the time horizon of a patient’s illness, even when the patient survives. With respect to the time horizons applied to health spillovers, one must principally consider whether to match the patient’s timeline, both in treatment benefits/costs and survival, or to impose different timelines for family members/caregivers, so as to acknowledge that effects may not entirely coincide. Balance and consistency in accounting for these impacts are also crucial, across trial arms for example [27]. Last, it must be noted that a lack of established methods for including the impacts of bereavement, and the unpredictably of those effects (especially as the analytic horizon extends), make the consideration of bereavement particularly challenging [59]. Clearly, much research is needed in this regard.
Outcome Measurement
Both direct [10, 63] and indirect measurement methods [64–69] have been used in estimating spillover health (dis)utility. There is, however, no clear guidance on which methods or instruments are optimal. Relatedly, while expressing the need for the robust capture of caregiver health spillovers, a recent review by NICE described a dearth of good-quality evidence to inform the relative impact of new interventions on caregiver HRQoL, compared with current practice [37]. A lack of empirical evidence, therefore, makes the task of method/instrument selection challenging.
The group also discussed the potential for employing estimates of the relative value of care-related quality-of-life and HRQoL outcomes (e.g., Carer Experience Scale [70] or the CarerQol instrument [71] relative to EQ-5D outcomes). These empirical weightings (or “exchange rates”) could be used to convert caregiver outcomes captured in the form of care-related quality of life to HRQoL outcomes. This would allow for the aggregation of caregiver outcomes with patient outcomes measured via HRQoL [72, 73]. The threshold of consensus was, however, not reached for this method to be included as a recommendation. With regard to avenues for future research in measuring caregiver and family outcomes, the development of novel preference-based measures aimed at capturing health spillovers was proposed (which inherently necessitates determination of the measures’ underlying conceptual domains and attribute/item selection prior to preference-based valuation [69, 74]).
The task force has refrained from explicit recommendations on whether to include family and caregiver spillover outcomes as utilities (in which case the caregiver/family member is treated as a separate entity in the analysis) or disutilities/utility increments (where the extent to which caregiver/family member impacts are relieved or exacerbated as a result of patient health changes, implying, for example, that caregiver/family member disutility would improve when the patient dies) in decision models [27]. Implicitly, recommendation 4, in advocating for spillover measurement over a time horizon sufficient to capture all relevant impacts (including bereavement), suggests consideration of family members and caregivers as separate entities is favoured. Appropriate means of addressing this issue will require further exploration.
Sources of Data: Use of Primary Versus Secondary Data
Given our context, the SHEER membership have endorsed the following definitions of ‘primary’ and ‘secondary’ data for the purposes of this recommendation.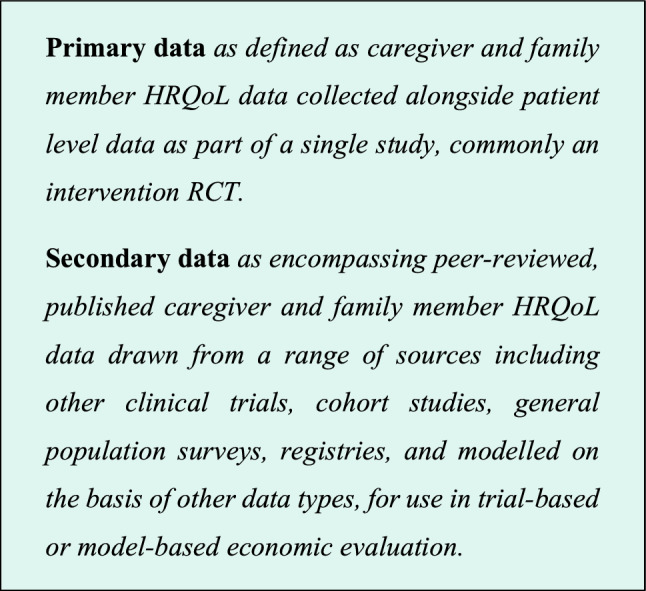
As one might expect, the evidence review identified trial-based evaluations as tending to include primary data more frequently, whereas models used secondary data. Described barriers to the inclusion of primary data related to missing data on family/caregiver outcomes [20], insufficient time horizons in trials to detect change in utilities resulting from spillovers [19, 75], and differing outcome measurement methods being employed among patients and family/caregivers [76]. Challenges to the use of secondary data pertained to the context specificity of spillovers [13], and differing interpretation of estimates from the same source [20, 27]. Taking this exacting data environment into consideration, the task force recognises that secondary data sources will often need to be employed, especially where impacts are to be modelled over the lifetime of the specified family/caregiver cohorts [77]. Nevertheless, the collection of primary data is currently favoured.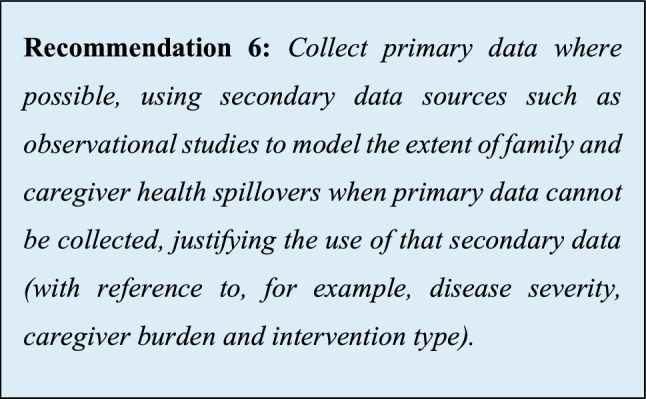
Aggregation Methods
In view of the utilitarian foundations of health economic evaluations, the objective, and conventional practice, is the identification and additive summing of all relevant effects (regardless of the source). Effects may be quite broad [11, 21] and, accordant with our working definition of family and caregiving health spillovers, there is a need to pragmatically capture these within an economic evaluation, given resources and other constraints. The task force has developed a stepwise aggregation procedure, as delineated below:
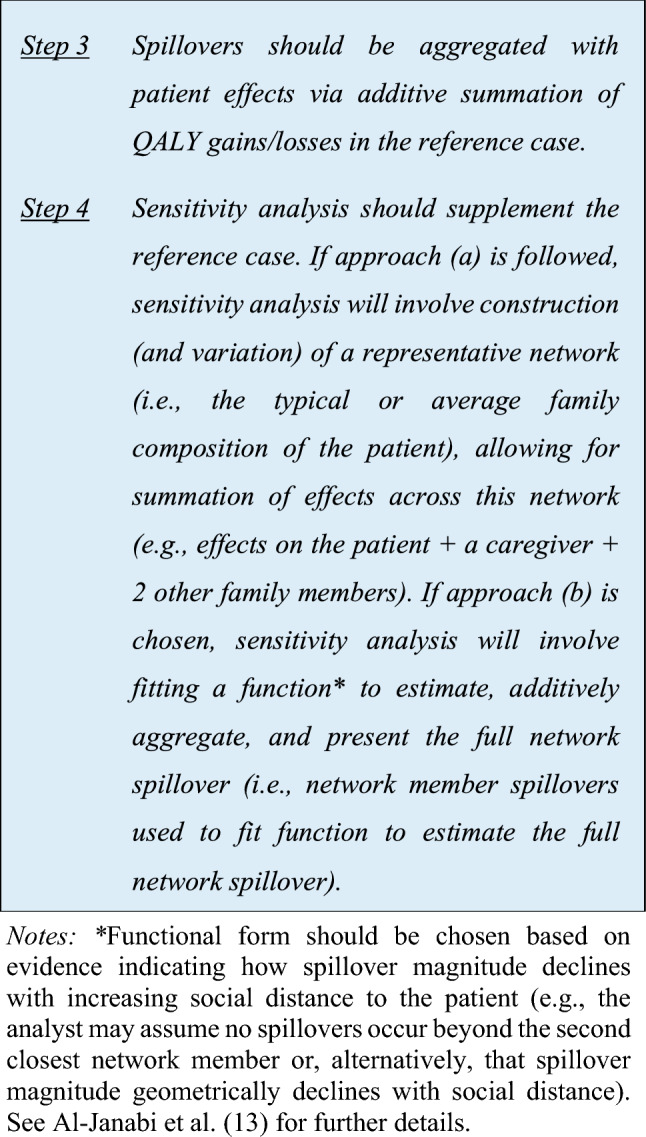
Although we recommend additive summation of spillover health effects with patient health effects, this summation may obscure the cost per QALY for patients, hampering comparisons of efficiency with evaluations where spillovers are omitted. A ‘multiplier approach’ (where spillover health effects are stated in a ratio to patient health effects, with this ratio being applied to the incremental cost-effectiveness ratio) has been proposed to counter this issue (see Al-Janabi et al. [7] for further details on this approach). Consensus was, however, not reached on recommending that spillovers and patient effects be presented in the form of a multiplier.
Outcome Value Weighting
Criteria for resource allocation are usually context specific, depending on social values and judgement. In particular, a small but growing literature shows that the social value of QALYs may vary across settings and individuals [72]. In the context of health spillovers, debate has developed as to the appropriateness of differentially weighting family/caregiver and patient outcomes. Some argue for a lower weighting to be applied to family/caregiver outcomes in order to focus on patients [76] or combat perceived inequity [28], others for higher weighting of caregiver outcomes, owing to a duty of care to family caregivers, given their role in supporting society [78]. Considering the limited peer-reviewed empirical basis for differential weighting of family/caregiver outcomes [72], and the abovementioned utilitarian foundations of health economic evaluations, a general agreement among members has emerged for the application of equal weighting in the reference case.
It is important to note that this recommendation pertains to equity weighting (reflecting social value and societal preference) and not to weights applied to caregiver outcomes in instances where different metrics are used among patients and caregivers (i.e., the earlier mentioned “exchange rate” between care-related quality of life and HRQoL [72, 73]). Future research is needed to investigate methods for the elicitation of spillover equity weights.
Outcome Double Counting
When deriving utilities using indirect techniques (i.e., generic preference-based HRQoL instruments), health spillovers may inadvertently be included, either at the measurement stage or the valuation stage, posing a risk of double counting. In relation to measurement, patients may, for example, take into account the impact of their condition on family members when reporting their own HRQoL [79], such that spillover effects are reflected in utilities. Similarly, the double counting of spillover effects may occur if the caregiver/family member fails to compartmentalise changes in their own health resulting from the health status of the patient [80]. There is also evidence that when valuing health states, respondents consider being a burden to others [81, 82]. Problems with double counting can also occur when the effects of informal caregiving are reflected in the monetary value of informal care time [4, 83]. The nature and extent of double counting requires further exploration and, given the state of current practice, the analyst should seek to minimise, rather than eliminate, the risk of double counting.

Consistency in Inclusion and Reporting of Spillovers
There is varied practice in including spillovers in economic evaluations [20, 22, 52, 84, 85]. This poses a challenge for decision makers in understanding the relative merits of investing in different interventions and improving allocative efficiency across the health system. The desire for greater consistency in this area underpins the rationale for convening this task force.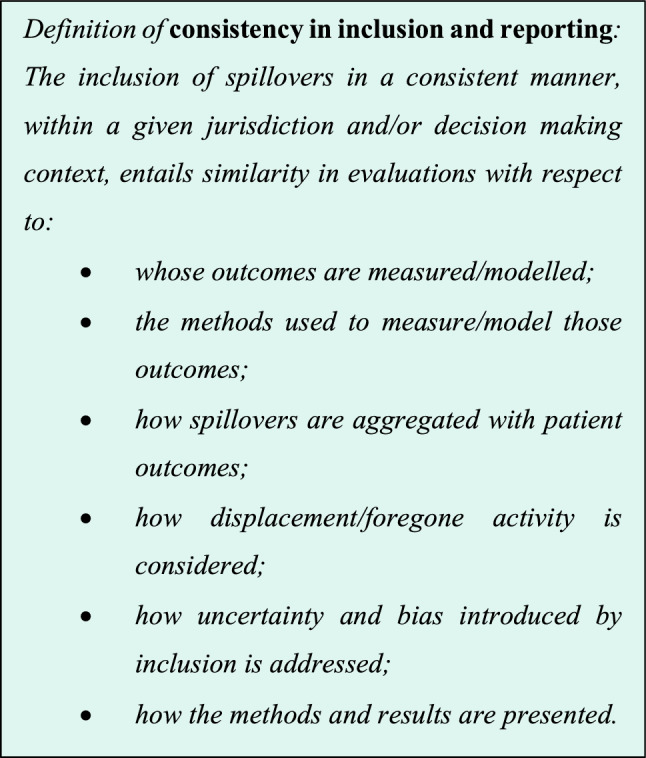
A consistent approach to reporting of health spillover inclusion is pivotal to elucidate the assumptions adopted in accounting for spillovers and to enable the replication of methods and/or results.
However, having globally standardised methods (as opposed to reporting practices) may not necessarily be a good thing. Different health systems may (and do) take contrasting views on their objectives, and thus, the evidence needed to support resource allocation. Striving for globally uniform methods of incorporation is, therefore, unrealistic and moreover, undesired because such practices would stifle the sort of research innovation which is particularly important in an emerging topic such as this. That is to say, there is a need for balance between consistency, where valuable in a decision making context, and variation to reflect differing decision maker perspectives and support innovation.
Characterising and Presenting Uncertainty When Including Spillovers
Last, with regard to the recommendations for emerging good practice, the aforementioned call for consistency extends to how the uncertainty [87] introduced by spillover inclusion is characterised and presented.
It is important to note that, preferably, these probability distributions will be applied at the level of the constituent variables of the economic model, for example, caregiver utility estimate. In such instances, uncertainty surrounding spillover estimates will be reflected within probabilistic sensitivity analyses.
Recommendations for Future Research
The still nascent character of the spillover evidence base necessitates that the task force seeks to inform a spillover research agenda. Thus far in the report, a number of future research recommendations relating to various aspects of emerging good practice have been foregrounded. Throughout our interactions, members have, however, drawn attention to numerous other potential avenues for further investigation. As such, in Table 3, those previously stated recommendations for future research are compiled alongside the other areas proposed as necessitating investigation, and a priority ranking based on member preferences is assigned to this complete listing.
Patient and Caregiver Perspectives on the Recommendations
In the main, the contributors present at the patient and caregiver meeting favoured the inclusion of health spillovers in economic evaluations, on the grounds that incorporation provides a more concrete basis for consideration of their lived experience in decision making. Accordingly, the recommendations of SHEER were welcomed by the group. In particular, the meeting attendees felt that recommendation 4 (related to the time horizon over which health spillovers should be measured) was of particular importance, as they felt time frames should be sufficient to recognise that mental health (and other) impacts may occur remote to the experience itself. Furthermore, the additive summation of health spillovers with patient effects (recommendation 7) was suggested as being accordant with what one contributor viewed as “an accumulation of symptoms” across the family network. A summary of the proceedings of this meeting is included in Appendix D of the ESM.
Discussion
While researchers and policymakers alike have called for the consideration of health spillovers in economic evaluations, the vast majority of analyses continue to omit these wider manifestations of illness and treatment [13], [20]. Although nuance and complexity are intrinsic to the phenomenon, it is hoped that the dialogue and resulting recommendations of the SHEER task force will not only aid in providing more analysts with the means and motivation to incorporate family and caregiver health spillovers, but also in defining a future research agenda to better understand their complex nature.
As is more widely the case in methods research, an overarching theme of ‘principle versus practicability’ pervaded almost all issues discussed by the group. Ideal practices will often not be made feasible on the basis of data constraints and other barriers to inclusion. Only through clear explication of the nature of these barriers (in analyses where health spillovers are omitted or included suboptimally) will greater attention be drawn to such obstacles, and potential avenues of circumvention identified. For this purpose, addition of an item to the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) checklist [88] could provide a formal approach to encouraging disclosure of the challenges surrounding incorporation, in addition to supporting transparency and consistency in methods of inclusion more generally.
Although beyond the terms of reference of the task force, SHEER also peripherally discussed the potential for establishing a registry/tariff of utilities and disutilities associated with family and caregiver health spillovers. This would not only facilitate spillover incorporation where data cannot be collected prospectively, but also encourage further estimation efforts in the context of diverse intervention types, illnesses, family compositions, sociocultural milieus and welfare state models, among a multitude of other settings. Such efforts will hopefully leave the field more fully equipped to establish an ‘average spillover’ resulting from a given activity [59]. This, in turn, might offer one potential route by which the impacts of displacement and the distributional consequences of spillover inclusion might be mitigated.
The main limitation of this guidance is that it represents the consensus view of a purposively sampled group of subject-matter experts. The task force includes members from, or with experience of working in, North America, Europe, Australasia, and Africa. Notably missing are individuals from Latin America and more work is needed to understand the practices and implications of spillover incorporation within the jurisdictions of this and other regions. Furthermore, although care was taken to invite the participation of subject-matter experts with varying opinions, acceptance of invitations may have resulted in a select group that shared many views on the topic at hand—the importance of inclusion of family and caregiver health spillovers. As such, it bears repeating that these emerging good practice recommendations seek to stimulate wider public discussion of the methodological challenges and ethical issues raised by spillover incorporation into applied economic evaluations. For instance, inclusion of spillovers places an additional burden on analysts undertaking evaluations that are already complex and resource intensive. Conceptual models of the likely lifetime costs and outcomes of interventions may therefore prove necessary in order to prioritise which specific impacts to focus on. This would also draw the attention of funders and clinical collaborators to the distinctive data requirements of economic evaluations.
With a view to producing a practical guidance set, discussions centred around spillovers onto family member and caregiver HRQoL. The potential for impacts to manifest in individuals outside this group, and for these impacts to extend to wider welfare, must, however, be recognised. Furthermore, restriction of the perspective to that of the healthcare system/health payer limits full examination of the interaction of more broadly incurred costs, and the characteristics and extent of spillovers. For example, limited consideration has been given to spillover effects associated with the sharing of genetic information, also termed “cascade effects” [89], which may inform disease risks not only for the patient but for other members of the family. The task force also forwent differentiation between ‘first-order’ and ‘second-order’ spillovers. By way of illustration, in a three-person family (two partners and a child), if one partner provides care to the other partner (who has a health condition), the first-order spillover experienced by the partner providing care can, in turn, spill over on to their child’s outcomes (health or otherwise). Whether that child’s ‘first-order’ and ‘second-order’ sources of spillover ought to be disentangled is a normative question worthy of attention in itself, but beyond the scope of this report. Last, in terms of limitations, some may view restricting our consultation with patients and caregivers to the latter stages of the overall process as limiting the potential for their inputs to shape discussions and ensure issues addressed were relevant to them [90].
Conclusions
The prevalence of family and caregiver health spillovers is likely to rise as levels of dependency and comorbidity increase, family compositions change, health institutions shift care to the community and households, and new healthcare technologies prolong life. Consequently, the importance of the consistent consideration of spillovers in health economic evaluations will continue to grow. The SHEER task force consensus recommendations are intended to inform these incorporation efforts in taking account of health spillovers in a fair and judicious manner. However, the emergent nature of spillover research impels that this guidance be viewed as an initial roadmap, as opposed to a strict checklist. In this regard, it is hoped that by articulating recommendations and setting out a research agenda, this task force might also both instigate and expedite development of the spillover knowledge base, improving our understanding of this complex phenomenon.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are deeply appreciative of valuable comments on earlier drafts of this report provided by internal reviewers Anirban Basu and Paula Lorgelly. Likewise, the contributions of the following patient and caregiver representatives are very much appreciated: Elaine Houlihan, Lette Moloney, Michelle O’Neill and Claudia Rathje. We also thank Dawn Lee and audience members for their discussion of a preliminary report of this task force at the Health Economists’ Study Group Winter 2023 Meeting in Manchester, as well as seminar participants at the Discipline of Economics, University of Galway, at the Center for Health Decision Science, Harvard T.H. Chan School of Public Health, and at the Center for the Evaluation of Value and Risk in Health at the Institute for Clinical Research and Health Policy Studies, Tufts Medical Center. Session participants at the 7th EuroQol Academy 2023 and attendees at the 18th Biennial European Conference of the Society for Medical Decision Making, and three anonymous reviewers are thanked for their feedback. We are grateful to Joanna Coast for her input. We also thank Family Carers Ireland, the Health Service Executive Integrated Care Programme for Prevention and Management of Chronic Disease, Croí – The West of Ireland Cardiac and Stroke Foundation, and Martha Killilea of the PPI Ignite Network @ University of Galway for their assistance, advice and support. Wendy J. Ungar (https://orcid.org/0000-0002-0762-0101) is supported by the Canada Research Chair in Economic Evaluation and Technology Assessment in Child Health.
Declarations
Funding
This research was funded by a University of Galway Hardiman Scholarship and the Irish Research Council under grant number GOIPG/2021/107. The funders had no role in the study design, the collection, analysis or interpretation of data, in the writing of this report or in the decision to submit it for publication. This report represents the consensus opinion of the task force membership and does not necessarily reflect the views of the institutions to which they are affiliated.
Conflicts of interest/competing interests
Edward Henry reports receiving an Irish Research Council grant (GOIPG/2021/107) and a University of Galway Hardiman Scholarship that has funded this and other aspects of his PhD programme of study, and receiving funding from the EuroQol Group to cover travel and accommodation to attend a EuroQol Academy Meeting and speak about this project. Hareth Al-Janabi reports consulting fees in the last 36 months relating to advice on the methods for economic evaluations and carer quality-of-life measurement from Curta, Ferring, PHMR and Roche. Werner Brouwer reports previous funding from the European Union, ZonMW and pharmaceutical companies to the Erasmus University Rotterdam in relation to spillover work. Andrew Lloyd reports receiving support from the EuroQol Group for travel costs to discuss aspects of this work at a meeting in Milan, Italy (2023). Nalin Payakachat is a full-time employee at Eli Lilly and Company. However, this work is her individual contribution. Eli Lily and Company has no financial interest in subject matter discussed in this manuscript. Becky Pennington reports receiving funding from the National Institute for Health Research (300160) and consulting fees from Roche/Genentech, Takeda and the Office for Health Economics for work related to carers’ health-related quality of life, and received funding to cover travel expenses and accommodation from the EuroQol Group at a plenary meeting. Becky Pennington is a member of National Institute for Health and Care Excellence (NICE) Technology Appraisal Committee A. This work does not represent the views of any of these funders or organisations. Koonal Shah is employed by NICE. The views expressed in this article do not necessarily reflect the views of NICE. John Cullinan, Lidia Engel, Susan Griffin, Claire Hulme, Pritaporn Kingkaew, Luz María Peña-Longobardo, Lisa A. Prosser, Wendy J. Ungar, Thomas Wilkinson and Eve Wittenberg have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Availability of data and material
All data pertaining to this study are available from the corresponding authors upon reasonable request.
Code availability
Not applicable.
Authors’ contributions
EH, HA, JC and EW contributed to the conception and design of the work. All authors engaged in the preparation and conduct of the modified nominal group technique. EH performed the data analysis and drafted the initial report. All authors reviewed the manuscript critically for important intellectual content and approved the final version.
Consent for Publication
All authors give consent for publication.
Contributor Information
Edward Henry, Email: e.henry4@universityofgalway.ie.
Werner Brouwer, Email: brouwer@eshpm.eur.nl.
References
- 1.Culyer AJ. The nature of the commodity 'health care' and its efficient allocation. Oxf Econ Pap. 1971;23(2):189–211. [Google Scholar]
- 2.Lees D. Efficiency in government spending social services. Health Publ Financ. 1967;22(1–2):176–189. [Google Scholar]
- 3.Basu A, Meltzer D. Implications of spillover effects within the family for medical cost-effectiveness analysis. J Health Econ. 2005;24(4):751–773. doi: 10.1016/j.jhealeco.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Grosse SD, Pike J, Soelaeman R, Tilford JM. Quantifying family spillover effects in economic evaluations: measurement and valuation of informal care time. Pharmacoeconomics. 2019;37(4):461–473. doi: 10.1007/s40273-019-00782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wittenberg E, James LP, Prosser LA. Spillover effects on caregivers’ and family members’ utility: a systematic review of the literature. Pharmacoeconomics. 2019;37(4):475–499. doi: 10.1007/s40273-019-00768-7. [DOI] [PubMed] [Google Scholar]
- 6.Brouwer WB. The inclusion of spillover effects in economic evaluations: not an optional extra. Pharmacoeconomics. 2019;37(4):451–456. doi: 10.1007/s40273-018-0730-6. [DOI] [PubMed] [Google Scholar]
- 7.Al-Janabi H, Van Exel J, Brouwer W, Coast J. A framework for including family health spillovers in economic evaluation. Med Decis Making. 2016;36(2):176–186. doi: 10.1177/0272989X15605094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoefman RJ, van Exel J, Brouwer W. How to include informal care in economic evaluations. Pharmacoeconomics. 2013;31(12):1105–1119. doi: 10.1007/s40273-013-0104-z. [DOI] [PubMed] [Google Scholar]
- 9.Wittenberg E, Ritter GA, Prosser LA. Evidence of spillover of illness among household members: EQ-5D scores from a US sample. Med Decis Making. 2013;33(2):235–243. doi: 10.1177/0272989X12464434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prosser LA, Lamarand K, Gebremariam A, Wittenberg E. Measuring family HRQoL spillover effects using direct health utility assessment. Med Decis Making. 2015;35(1):81–93. doi: 10.1177/0272989X14541328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavelle TA, Wittenberg E, Lamarand K, Prosser LA. Variation in the spillover effects of illness on parents, spouses, and children of the chronically ill. Appl Health Econ Health Pol. 2014;12(2):117–124. doi: 10.1007/s40258-014-0079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinquart M, Sörensen S. Correlates of physical health of informal caregivers: a meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2007;62(2):P126–P137. doi: 10.1093/geronb/62.2.p126. [DOI] [PubMed] [Google Scholar]
- 13.Al-Janabi H, Van Exel J, Brouwer W, Trotter C, Glennie L, Hannigan L, et al. Measuring health spillovers for economic evaluation: a case study in meningitis. Health Econ. 2016;25(12):1529–1544. doi: 10.1002/hec.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry E, Cullinan J. Mental health spillovers from serious family illness: doubly robust estimation using EQ-5D-5L population normative data. Soc Sci Med. 2021;279:113996. doi: 10.1016/j.socscimed.2021.113996. [DOI] [PubMed] [Google Scholar]
- 15.Cooper C, Balamurali T, Livingston G. A systematic review of the prevalence and covariates of anxiety in caregivers of people with dementia. Int Psychogeriatr. 2007;19(2):175–195. doi: 10.1017/S1041610206004297. [DOI] [PubMed] [Google Scholar]
- 16.Hearson B, McClement S. Sleep disturbance in family caregivers of patients with advanced cancer. Int J Palliat Nurs. 2007;13(10):495–501. doi: 10.12968/ijpn.2007.13.10.27493. [DOI] [PubMed] [Google Scholar]
- 17.Bobinac A, van Exel NJA, Rutten FF, Brouwer WB. Health effects in significant others: separating family and care-giving effects. Med Decis Making. 2011;31(2):292–298. doi: 10.1177/0272989X10374212. [DOI] [PubMed] [Google Scholar]
- 18.Bobinac A, van Exel NJA, Rutten FF, Brouwer WB. Caring for and caring about: disentangling the caregiver effect and the family effect. J Health Econ. 2010;29(4):549–556. doi: 10.1016/j.jhealeco.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Bhadhuri A, Jowett S, Jolly K, Al-Janabi H. A comparison of the validity and responsiveness of the EQ-5D-5L and SF-6D for measuring health spillovers: a study of the family impact of meningitis. Med Decis Making. 2017;37(8):882–893. doi: 10.1177/0272989X17706355. [DOI] [PubMed] [Google Scholar]
- 20.Scope A, Bhadhuri A, Pennington B. Systematic review of cost-utility analyses that have included carer and family member health-related quality of life. Value Health. 2022;25(9):1644–1653. doi: 10.1016/j.jval.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Canaway A, Al-Janabi H, Kinghorn P, Bailey C, Coast J. Close-person spill-overs in end-of-life care: using hierarchical mapping to identify whose outcomes to include in economic evaluations. Pharmacoeconomics. 2019;37(4):573–583. doi: 10.1007/s40273-019-00786-5. [DOI] [PubMed] [Google Scholar]
- 22.Lavelle TA, D’Cruz BN, Mohit B, Ungar WJ, Prosser LA, Tsiplova K, et al. Family spillover effects in pediatric cost-utility analyses. Appl Health Econ Health Pol. 2019;17(2):163–174. doi: 10.1007/s40258-018-0436-0. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Al-Janabi H, Mallett A, Quinlan C, Scheffer IE, Howell KB, et al. Parental health spillover effects of paediatric rare genetic conditions. Qual Life Res. 2020;29(9):2445–2454. doi: 10.1007/s11136-020-02497-3. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein MC, Russell LB, Gold MR, Siegel JE. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 25.Al-Janabi H, Flynn TN, Coast J. QALYs and carers. Pharmacoeconomics. 2011;29(12):1015–1023. doi: 10.2165/11593940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Brouwer WB, van Exel NJA, Koopmanschap MA, Rutten FF. The valuation of informal care in economic appraisal: a consideration of individual choice and societal costs of time. Int J Technol Assess Health Care. 1999;15(1):147–160. doi: 10.1017/s0266462399152346. [DOI] [PubMed] [Google Scholar]
- 27.Pennington B, Eaton J, Hatswell AJ, Taylor H. Carers’ health-related quality of life in global health technology assessment: guidance, case studies and recommendations. Pharmacoeconomics. 2022;40(9):837–850. doi: 10.1007/s40273-022-01164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dixon P, Round J. Caring for carers: positive and normative challenges for future research on carer Spillover effects in economic evaluation. Value Health. 2019;22(5):549–554. doi: 10.1016/j.jval.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCabe C. Expanding the scope of costs and benefits for economic evaluations in health: some words of caution. Pharmacoeconomics. 2019;37(4):457–460. doi: 10.1007/s40273-018-0729-z. [DOI] [PubMed] [Google Scholar]
- 30.NICE. NICE health technology evaluations: the manual. 2022. https://www.nice.org.uk/process/pmg36/resources/nice-health-technology-evaluations-the-manual-pdf-72286779244741. Accessed 16 Feb 2022.
- 31.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-effectiveness in health and medicine. 2. New York: Oxford University Press; 2016. [Google Scholar]
- 32.HIQA. Guidelines for the economic evaluation of health technologies in Ireland. Dublin: HIQA; 2020. Available from: https://www.hiqa.ie/sites/default/files/2020-09/HTA-Economic-Guidelines-2020.pdf. Accessed 16 Oct 2020.
- 33.Zorginstituut Nederland. Guideline for conducting economic evaluations in healthcare. 2016. Available from: https://english.zorginstituutnederland.nl/publications/reports/2016/06/16/guideline-for-economic-evaluations-in-healthcare. Accessed 13 Oct 2020.
- 34.Pennington B. Inclusion of carer health-related quality of life in National Institute for Health and Care Excellence appraisals. Value Health. 2020;23(10):1349–1357. doi: 10.1016/j.jval.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Lamsal R. Measuring and incorporating family spillover cost and health consequences in economic evaluation of child health interventions [PhD dissertation]. University of Toronto, 2022.
- 36.Leech AA, Lin P-J, D’Cruz B, Parsons SK, Lavelle TA. Family spillover effects: are economic evaluations misrepresenting the value of healthcare interventions to society? Appl Health Econ Health Policy. 2022;21(1):5–10. doi: 10.1007/s40258-022-00755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dawoud D, Lamb A, Moore A, Bregman C, Rupniewska E, Paling T, et al. Capturing what matters: updating NICE methods guidance on measuring and valuing health. Qual Life Res. 2022;31(7):2167–2173. doi: 10.1007/s11136-022-03101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malone DC, Ramsey SD, Patrick DL, Johnson FR, Mullins CD, Roberts MS, et al. Criteria and process for initiating and developing an ISPOR good practices task force report. Value Health. 2020;23(4):409–415. doi: 10.1016/j.jval.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Berger ML, Sox H, Willke RJ, Brixner DL, Eichler HG, Goettsch W, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Value Health. 2017;20(8):1003–1008. doi: 10.1016/j.jval.2017.08.3019. [DOI] [PubMed] [Google Scholar]
- 40.Delbecq AL, Van de Ven AH, Gustafson DH. Group techniques for program planning: a guide to nominal group and Delphi processes. Glenview: Scott, Foresman and Company; 1975.
- 41.McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm. 2016;38(3):655–662. doi: 10.1007/s11096-016-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manera KE, Hanson CS, Gutman T, Tong A. Consensus methods: nominal group technique. In: Liamputtong P, editor. Handbook of research methods in health social sciences. Singapore: Springer; 2019. pp. 737–750. [Google Scholar]
- 43.Manera KE, Johnson DW, Craig JC, Shen JI, Ruiz L, Wang AY-M, et al. Patient and caregiver priorities for outcomes in peritoneal dialysis: multinational nominal group technique study. Clin J Am Soc Nephrol. 2019;14(1):74–83. [DOI] [PMC free article] [PubMed]
- 44.Centers for Disease Control and Prevention Gaining consensus among stakeholders through the nominal group technique. Eval Briefs. 2018;7:1–3. [Google Scholar]
- 45.Raine R, Nic a’Bháird C, Xanthopoulou P, Wallace I, Ardron D, Harris M, et al. Use of a formal consensus development technique to produce recommendations for improving the effectiveness of adult mental health multidisciplinary team meetings. BMC Psychiatry. 2015;15(1):1–12. [DOI] [PMC free article] [PubMed]
- 46.Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy M, Black N, Lamping D, McKee C, Sanderson C, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998;2(3):i–88. [PubMed] [Google Scholar]
- 48.Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR, Lazaro P, et al. RAND/UCLA appropriateness method user's manual. Santa Monica: RAND Corporation; 2000. [Google Scholar]
- 49.Humphrey-Murto S, Varpio L, Gonsalves C, Wood TJ. Using consensus group methods such as Delphi and Nominal Group in medical education research. Med Teach. 2017;39(1):14–19. doi: 10.1080/0142159X.2017.1245856. [DOI] [PubMed] [Google Scholar]
- 50.Hussain JA, White IR, Johnson MJ, Byrne A, Preston NJ, Haines A, et al. Development of guidelines to reduce, handle and report missing data in palliative care trials: a multi-stakeholder modified nominal group technique. Palliat Med. 2022;36(1):59–70. doi: 10.1177/02692163211065597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Janabi H, Efstathiou N, McLoughlin C, Calvert M, Oyebode J. The scope of carer effects and their inclusion in decision-making: a UK-based Delphi study. BMC Health Serv Res. 2021;21(1):1–10. doi: 10.1186/s12913-021-06742-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin P-J, D’Cruz B, Leech AA, Neumann PJ, Aigbogun MS, Oberdhan D, et al. Family and caregiver spillover effects in cost-utility analyses of Alzheimer’s disease interventions. Pharmacoeconomics. 2019;37(4):597–608. doi: 10.1007/s40273-019-00788-3. [DOI] [PubMed] [Google Scholar]
- 53.Horgan F, Lennon O, Hickey A, Sorensen J, Kroll T, McCartan D, et al. A protocol to evaluate the impact of embedding public and patient involvement in a structured PhD program for stroke care. Front Rehabil Sci. 2022;3:877598. doi: 10.3389/fresc.2022.877598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Janabi H, McLoughlin C, Oyebode J, Efstathiou N, Calvert M. Six mechanisms behind carer wellbeing effects: a qualitative study of healthcare delivery. Soc Sci Med. 2019;235:112382. doi: 10.1016/j.socscimed.2019.112382. [DOI] [PubMed] [Google Scholar]
- 55.YHEC. Perspective. York: York Health Economics Consortium; 2016. https://yhec.co.uk/glossary/perspective/. Accessed 2 May 2023.
- 56.Claxton K, Palmer S, Sculpher M, Walker S. Appropriate perspectives for health care decisions (CHE research paper no. 54). York: Centre for Health Economics; 2010.
- 57.Brouwer W, van Baal P, van Exel J, Versteegh M. When is it too expensive? Cost-effectiveness thresholds and health care decision-making. Eur J Health Econ. 2019;20(2):175–180. doi: 10.1007/s10198-018-1000-4. [DOI] [PubMed] [Google Scholar]
- 58.Garrison LP, Jr, Pauly MV, Willke RJ, Neumann PJ. An overview of value, perspective, and decision context: a health economics approach: an ISPOR Special Task Force report [2] Value Health. 2018;21(2):124–130. doi: 10.1016/j.jval.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 59.NICE. Health related quality of life task and finish group report. 2020. https://www.nice.org.uk/Media/Default/About/what-we-do/our-programmes/nice-guidance/chte-methods-consultation/Health-related-quality-of-life-task-and-finish-group-report.docx. Accessed 14 Dec 2021.
- 60.Asaria M, Griffin S, Cookson R. Distributional cost-effectiveness analysis: a tutorial. Med Decis Making. 2016;36(1):8–19. doi: 10.1177/0272989X15583266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cookson RA, Mirelman A, Asaria M, Dawkins B, Griffin S. Fairer decisions, better health for all: health equity and cost-effectiveness analysis (CHE research paper no. 135). York: Centre for Health Economics; 2016.
- 62.Avanceña AL, Prosser LA. Examining equity effects of health interventions in cost-effectiveness analysis: a systematic review. Value Health. 2021;24(1):136–143. doi: 10.1016/j.jval.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 63.Basu A, Dale W, Elstein A, Meltzer D. A time tradeoff method for eliciting partner’s quality of life due to patient’s health states in prostate cancer. Med Decis Making. 2010;30(3):355–365. doi: 10.1177/0272989X09349959. [DOI] [PubMed] [Google Scholar]
- 64.Bell CM, Araki SS, Neumann PJ. The association between caregiver burden and caregiver health-related quality of life in Alzheimer disease. Alzheimer Dis Assoc Disord. 2001;15(3):129–136. doi: 10.1097/00002093-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Tilford JM, Grosse SD, Robbins JM, Pyne JM, Cleves MA, Hobbs CA. Health state preference scores of children with spina bifida and their caregivers. Qual Life Res. 2005;14(4):1087–1098. doi: 10.1007/s11136-004-3305-2. [DOI] [PubMed] [Google Scholar]
- 66.Payakachat NJA, Tilford JM, Brouwer WB, van Exel N, Grosse SD. Measuring health and well-being effects in family caregivers of children with craniofacial malformations. Qual Life Res. 2011;20(9):1487–1495. doi: 10.1007/s11136-011-9870-2. [DOI] [PubMed] [Google Scholar]
- 67.Tilford JM, Payakachat N, Kuhlthau KA, Pyne JM, Kovacs E, Bellando J, et al. Treatment for sleep problems in children with autism and caregiver spillover effects. J Autism Dev Disord. 2015;45(11):3613–3623. doi: 10.1007/s10803-015-2507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoefman R, Payakachat N, van Exel J, Kuhlthau K, Kovacs E, Pyne J, et al. Caring for a child with autism spectrum disorder and parents’ quality of life: application of the CarerQol. J Autism Dev Disord. 2014;44(8):1933–1945. doi: 10.1007/s10803-014-2066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Janabi H, Flynn TN, Coast J. Estimation of a preference-based carer experience scale. Med Decis Making. 2011;31(3):458–468. doi: 10.1177/0272989X10381280. [DOI] [PubMed] [Google Scholar]
- 70.Al-Janabi H, Coast J, Flynn TN. What do people value when they provide unpaid care for an older person? A meta-ethnography with interview follow-up. Soc Sci Med. 2008;67(1):111–121. doi: 10.1016/j.socscimed.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 71.Brouwer W, Van Exel NJA, Van Gorp B, Redekop W. The CarerQol instrument: a new instrument to measure care-related quality of life of informal caregivers for use in economic evaluations. Qual Life Res. 2006;15:1005–1021. doi: 10.1007/s11136-005-5994-6. [DOI] [PubMed] [Google Scholar]
- 72.Al-Janabi H, Wittenberg E, Donaldson C, Brouwer W. The relative value of carer and patient quality of life: a person trade-off (PTO) study. Soc Sci Med. 2022;292:114556. doi: 10.1016/j.socscimed.2021.114556. [DOI] [PubMed] [Google Scholar]
- 73.Dhanji N, Brouwer W, Donaldson C, Wittenberg E, Al-Janabi H. Estimating an exchange-rate between care-related and health-related quality of life outcomes for economic evaluation: an application of the wellbeing valuation method. Health Econ. 2021;30(11):2847–2857. doi: 10.1002/hec.4411. [DOI] [PubMed] [Google Scholar]
- 74.Brazier J, Peasgood T, Mukuria C, Marten O, Kreimeier S, Luo N, et al. The EQ-HWB: overview of the development of a measure of health and wellbeing and key results. Value Health. 2022;25(4):482–491. doi: 10.1016/j.jval.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 75.McLoughlin C, Goranitis I, Al-Janabi H. Validity and responsiveness of preference-based quality-of-life measures in informal carers: a comparison of 5 measures across 4 conditions. Value Health. 2020;23(6):782–790. doi: 10.1016/j.jval.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tubeuf S, Saloniki E-C, Cottrell D. Parental health spillover in cost-effectiveness analysis: evidence from self-harming adolescents in England. Pharmacoeconomics. 2019;37(4):513–530. doi: 10.1007/s40273-018-0722-6. [DOI] [PubMed] [Google Scholar]
- 77.O’Mahony JF, Newall AT, van Rosmalen J. Dealing with time in health economic evaluation: methodological issues and recommendations for practice. Pharmacoeconomics. 2015;33:1255–1268. doi: 10.1007/s40273-015-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arksey H, Morée M. Supporting working carers: do policies in England and The Netherlands reflect ‘doulia rights’? Health Soc Care Community. 2008;16(6):649–657. doi: 10.1111/j.1365-2524.2008.00791.x. [DOI] [PubMed] [Google Scholar]
- 79.McPherson CJ, Wilson KG, Murray MA. Feeling like a burden to others: a systematic review focusing on the end of life. Palliat Med. 2007;21(2):115–128. doi: 10.1177/0269216307076345. [DOI] [PubMed] [Google Scholar]
- 80.Wittenberg E, Prosser LA. Disutility of illness for caregivers and families: a systematic review of the literature. Pharmacoeconomics. 2013;31(6):489–500. doi: 10.1007/s40273-013-0040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krol M, Attema AE, van Exel J, Brouwer W. Altruistic preferences in time tradeoff: consideration of effects on others in health state valuations. Med Decis Making. 2016;36(2):187–198. doi: 10.1177/0272989X15615870. [DOI] [PubMed] [Google Scholar]
- 82.Karimi M, Brazier J, Paisley S. How do individuals value health states? A qualitative investigation. Soc Sci Med. 2017;172:80–88. doi: 10.1016/j.socscimed.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 83.Koopmanschap MA, van Exel NJA, van den Berg B, Brouwer WB. An overview of methods and applications to value informal care in economic evaluations of healthcare. Pharmacoeconomics. 2008;26(4):269–280. doi: 10.2165/00019053-200826040-00001. [DOI] [PubMed] [Google Scholar]
- 84.Goodrich K, Kaambwa B, Al-Janabi H. The inclusion of informal care in applied economic evaluation: a review. Value Health. 2012;15(6):975–981. doi: 10.1016/j.jval.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 85.Krol M, Papenburg J, van Exel J. Does including informal care in economic evaluations matter? A systematic review of inclusion and impact of informal care in cost-effectiveness studies. Pharmacoeconomics. 2015;33:123–135. doi: 10.1007/s40273-014-0218-y. [DOI] [PubMed] [Google Scholar]
- 86.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 87.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 4. Oxford: Oxford University Press; 2015. [Google Scholar]
- 88.Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Pharmacoeconomics. 2022;40(6):601–609. doi: 10.1007/s40273-021-01112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cernat A, Hayeems RZ, Prosser LA, Ungar WJ. Incorporating cascade effects of genetic testing in economic evaluation: a scoping review of methodological challenges. Children. 2021;8(5):346. doi: 10.3390/children8050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358:j3453. doi: 10.1136/bmj.j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.