Abstract
To analyze the roles of interleukin-12 (IL-12) and the IL-12-dependent Th1 response in resistance to Cryptococcus neoformans, we have established a chronic infection model in wild-type mice and in mice with targeted disruptions of the genes for the IL-12p35 and IL-12p40 subunits (IL-12p35−/− and IL-12p40−/− mice, respectively) as well as in mice with a targeted disruption of the IL-4 gene. Long-term application of exogenous IL-12 prevented death of infected wild-type mice for the entire period of the experiment (up to 180 days) but did not resolve the infection. Infected IL-12p35−/− and IL-12p40−/− mice died significantly earlier than infected wild-type mice, whereas infection of IL-4-deficient mice led to prolonged survival. Interestingly, infected IL-12p40−/− mice died earlier and developed higher organ burdens than IL-12p35−/− mice, which, for the first time in an infection model, suggests a protective role of the IL-12p40 subunit independent of the IL-12 heterodimer. The fungal organ burdens of IL-4-deficient mice and IL-12-treated wild-type mice were significantly reduced compared to those of untreated wild-type mice and IL-12-deficient mice. Histopathological analysis revealed reduction of the number of granulomatous lesions following treatment with IL-12. Susceptibility of both IL-12p35−/− and IL-12p40−/− mice was associated with marginal production of gamma interferon and elevated levels of IL-4 from CD4+ T cells, which indicates Th2 polarization in the absence of IL-12, whereas wild-type mice developed a Th1 response. Taken together, our data emphasize the essential role of IL-12 for protective Th1 responses against C. neoformans.
Interleukin 12 (IL-12) is a heterodimeric cytokine with a relative molecular mass of 75 kDa (IL-12p75) (26). The p35 subunit (IL-12p35) is constitutively expressed but not secreted in somatic tissues (6, 7, 46). The p40 subunit (IL-12p40) is expressed in a highly regulated manner in cells of the immune system, especially macrophages and dendritic cells (5, 36). Expression of IL-12p40 seems to be necessary for the secretion of IL-12p35 which occurs only in the IL-12 heterodimer. Both subunits are linked by a single disulfide bond. Besides the occurrence in the IL-12 heterodimer, p40 is secreted as a monomer and homodimer as well (7, 43). Both are secreted in excess to the IL-12 heterodimer, and both are reported to act as antagonists of IL-12 at its receptor (9, 32).
Cryptococcus neoformans is a heterobasidiomycetic fungus with both filamentous growth (syn. Filobasidiella neoformans) and yeast-like growth stages (C. neoformans). The latter occur as opportunistic pathogens especially in immunocompromised patients like transplant recipients or human immunodeficiency virus-infected individuals with advanced AIDS symptoms. With an incidence of 6 to 8%, it is the major cause of meningitis in AIDS patients (49).
Usually, infective spores enter the host through the respiratory tract. Birds, especially pigeons and canaries, are carriers of these fungi (8, 41, 42). They spread large quantities of blastoconidia in their droppings. The spore’s diameter is reduced by drying to a size that enables its entrance into the finest bronchioli and alveoli. Immunocompetent hosts are able to restrict the primary infection in the lungs, but in immunosuppressed hosts, this ability is severely hampered. Fungal cells spread all over the organism and finally into the brain as well.
Phagocytic cells are most important in defeating invading cryptococci after opsonization primarily by complement, as the response takes place without prior contact to the pathogen. However, protective activity by an additional specific humoral response was recently shown as well (47). A T-cell response, as well as the presence of natural killer cells, is essential, since both T-cell-deficient nude mice (nu/nu) and NK cell-deficient beige (bg/bg) mice are more susceptible to cryptococcal infections than the respective wild-type mice (11, 13). Nitric oxide synthase activity has been shown to be involved in sequestration of fungi (10, 28, 29). It is known that CD4+ cells are crucial in the defense of cryptococcal infections, but CD8+ cells are also necessary for sequestration of the fungi (16, 22). However, the roles of Th1 and Th2 cells in immunity against C. neoformans are not completely understood. In a pulmonary model of cryptococcosis, the importance of Th1 cell differentiation and of IL-12 and gamma interferon (IFN-γ) for protection was shown (17, 18), whereas IL-5-dependent eosinophil recruitment was found to be nonprotective (20). Mice deficient for IFN-γ were reported to be more susceptible to cryptococcal infections (47), while treatment with IL-12 reduced fungal load and prolonged survival of wild-type mice infected with C. neoformans, especially in combination with conventional antifungal therapy (3, 24, 25). The goal of this study was to further define the role of IL-12 and the importance of Th1 cell differentiation in resistance to C. neoformans in IL-12-deficient mice.
MATERIALS AND METHODS
Mouse infection model.
All experiments were performed with (C57BL/6 × 129/Sv/Ev)F2 mice (8 to 10 weeks old). Mice were bred at BRL, Füllinsdorf, Switzerland. IL-12-deficient mice were produced as reported previously (30, 33). The (C57BL/6 × 129/Sv/Ev)F2 mice with a targeted disruption of the IL-4 gene (IL-4−/−) were kindly provided by Horst Blüthmann, F. Hoffmann-La Roche AG, Basel, Switzerland (27).
In order to study long-term T-cell responses to the pathogen C. neoformans, we established a chronic infection model in mice by intravenous (i.v.) infection with 104 CFU. While only minor differences between mouse strains with acute infections were observed, the differences in mice with chronic infections were more pronounced. Thus, acute infection of both BALB/c and 129/Sv/Ev mice with inocula of 107 and 106 CFU led to death within 1 and 2 weeks, respectively. In contrast, using an inoculum of 104 CFU, 129/Sv/Ev, C57BL/6, and F2 hybrids of wild-type mice of the two strains developed chronic infections and had a median survival time of about 60 days compared to 40 days in BALB/c mice. Thus, BALB/c mice are relatively susceptible and 129/Sv/Ev or C57BL/6 mice or F2 hybrids of the two strains are relatively resistant. There was no significant variation in survival periods between the relatively resistant strains (data not shown).
C. neoformans.
Strain 1841 encapsulated cryptococci (serotype D) were kept as frozen stock in skim milk and proliferated in Sabouraud dextrose medium (2% glucose, 1% peptone) in cultures grown overnight. Cells were counted in a hematocytometer, and inocula were resuspended at 5 × 104/ml in sterile phosphate-buffered saline. Mice were chronically infected by i.v. injection of 200 μl containing 104 CFU per mouse.
Organ CFU determination.
For CFU determinations, organs were taken sterilely from asphyxiated mice and homogenized in 2 ml of sterile phosphate-buffered saline. Serial dilutions of the homogenates were plated on Sabouraud dextrose agar plates, and colonies were counted after an incubation period of 48 h at 30°C.
Histopathological analysis.
For histopathological analysis, organs were fixed in 10% buffered formalin and embedded in paraffin. Sections were stained with hematoxylin and eosine. They were used to estimate the extent of granulomatous lesions and cyst-like (cyst) formation in the various organs. For quantification of liver lesions, 20 randomized areas of 0.08 mm2 in the liver sections were chosen in which granulomatous lesions were counted.
Lymphocyte cultures.
Spleens taken from asphyxiated mice were ground through a sieve with 100-μm mesh, depleted of erythrocytes, and washed with Hanks balanced salt solution (HBSS). After the cells were counted in a hematocytometer, cells were resuspended at 2.5 × 106/ml in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 7.5% fetal calf serum (FCS), l-glutamine, 2-mercaptoethanol, and antibiotics (complete IMDM), and plated at 0.5 ml/well in 48-well plates. For polyclonal activation, wells were coated with anti-CD3 antibody (5 μg/ml). For specific activation, heat-killed cryptococci (3 × 106 to 3 × 107/ml) were added. In order to prevent autoconsumption of IL-4, neutralizing rat anti-murine IL-4 receptor antibody (Genzyme, Cambridge, Mass.) was added to the cultures (5 μg/ml). Incubation was performed for 48 h at 37°C in a humidified atmosphere of 5% CO2.
For depletion of CD4+ and CD8+ T cells from total spleen cells, splenocytes were incubated at room temperature for 30 min in HBSS containing 1% FCS in the presence of either biotinylated anti-L3T4 monoclonal antibody (MAb) GK1.5 (Pharmingen) or biotinylated anti-Ly-2 MAb 53-6.7 (Pharmingen) (each at 5 μg/107 cells/ml), respectively. Cells were washed three times in HBSS with 1% FCS. Subsequently, magnetic microbeads coupled with streptavidin (Dynal) were added and rotated for 45 min at 4°C. Microbeads with adherent cells were removed with a magnet, the remaining cells were washed twice in HBSS with 1% FCS, and the remainder of the splenocytes were plated at 2.5 × 106 cells/ml in complete IMDM for 48 h with and without heat-killed cryptococci. Subsequent fluorescence-activated cell sorter analysis (Becton Dickinson & Co.) of remaining cells revealed in all cases complete (100%) depletion of either CD4+ or CD8+ T cells from total spleen cells.
Cytokine analysis.
Cytokine concentrations were determined by sandwich enzyme-linked immunosorbent assay systems with unlabeled capture antibodies and labeled detection antibodies. To determine the concentration of IL-4, MAb 11B11 (rat immunoglobulin G1 [IgG1]) was used as the capture antibody (37) and biotin-labeled BVD6-24G2 (rat IgG1; Pharmingen) was used as the detection antibody followed by incubation with peroxidase-labeled streptavidin. To determine the concentration of IFN-γ, MAb AN18 (rat IgG1) was coated as the capture antibody (40) and peroxidase-labeled XMG1.2 (rat IgG1) was used in the detection step (2). The levels of sensitivity of IL-4 and IFN-γ detection were 0.15 and 0.04 ng/ml, respectively.
Statistical analysis.
Evaluation of statistical differences between data obtained from wild-type and mutant mice was performed by the two-tailed Student’s t test (lung load and number of granulomas as shown in Fig. 2 and 4, respectively) or the Mann-Whitney rank sum test (survival time as shown in Fig. 1 and Table 1).
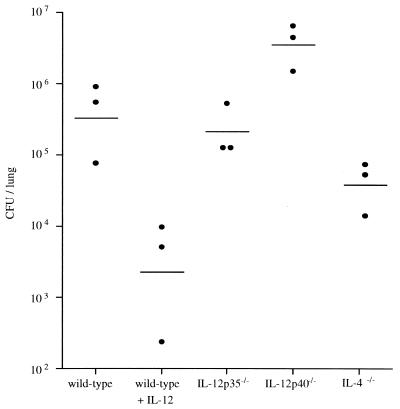
FIG. 2.
Fungal burden in lungs of mice 21 days after infection with C. neoformans. Mice were infected by i.v. injection with 104 CFU of C. neoformans. For the group of IL-12-treated wild-type mice, daily treatment with IL-12 (0.25 μg/mouse intraperitoneally) was started 1 day before infection and continued for the first week. Treatment was continued twice weekly with 0.1 μg/mouse for the next 2 weeks. Twenty-one days after infection, the mice were killed by CO2 asphyxiation and organs were homogenized. Serial dilutions of the homogenates were plated on Sabouraud agar, and colonies were counted after 48 h of incubation at 30°C. Each point is the value for one mouse, and the median values are indicated by the short horizontal lines. Values shown in the figure are adjusted to the whole organ. This figure shows the results of one representative experiment of 12 similar experiments.
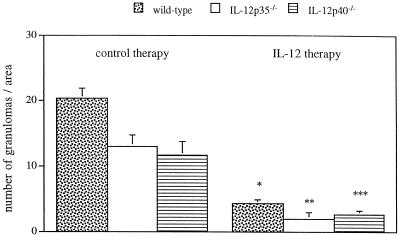
FIG. 4.
Number of granulomatous lesions in livers from IL-12-treated or untreated mice 21 days after infection with C. neoformans. Mice were infected with 104 CFU of C. neoformans and treated with IL-12 for 3 weeks as described in the legend to Fig. 2. The number of granulomatous lesions in the liver from infected mice was determined by counting the mean number of granulomatous lesions in 20 randomized areas of 0.08 mm2. The mean value and standard deviation for three animals per group is shown. The differences between values with and without IL-12 treatment were found to be statistically significant by the two-tailed Student’s t test (P = 0.001 [∗]; P = 0.002 [∗∗]; P = 0.014 [∗∗∗].
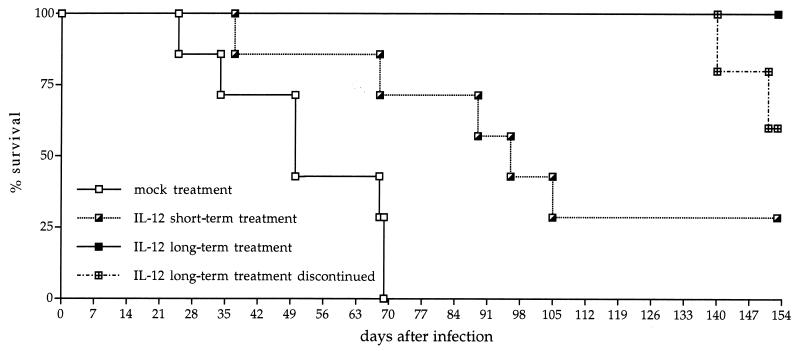
FIG. 1.
Effect of IL-12 treatment on survival of mice infected with C. neoformans. Mice were infected by i.v. injection with 104 CFU of C. neoformans and treated intraperitoneally daily during the first week of infection with either buffer or IL-12 (0.25 μg/mouse) beginning 1 day before infection. Treatment was continued twice weekly with 0.1 μg/mouse for the long-term treatment group. Seven to ten mice per group were monitored. At day 134 after infection, the long-term treatment group was divided. For five mice, treatment with IL-12 was continued, while for the other five mice, treatment was terminated. The mice receiving continuous treatment with IL-12 survived until day 180, the end of the experiment (not shown). The figure represents the results of one typical experiment of six similar experiments.
TABLE 1.
Survival period of wild-type, IL-12p35−/−, IL-12p40−/−, and IL-4−/− mice infected with C. neoformansa
| Mice | No. of expts | No. of mice | Survival periodb | P valuec |
|---|---|---|---|---|
| Wild-type | 5 | 37 | 56 | |
| IL-12p35−/− | 5 | 38 | 32d | <0.0001 |
| IL-12p40−/− | 5 | 38 | 26d | <0.0001 |
| IL-4−/− | 1 | 7 | >100e | NAf |
| 1 | 7 | >77e | NA |
Mice were infected by i.v. injection of 104 CFU of C. neoformans.
Median survival period (days).
Statistical significance of the difference in the survival periods of mutant versus wild-type mice by the Mann-Whitney rank sum test.
Significantly different survival periods between IL-12p35−/− and IL-12p40−/− mice (P < 0.0001) by the Mann-Whitney rank sum test.
Period of experiment (days).
NA, not applicable.
RESULTS
Long-term application of IL-12 prevents death of infected wild-type mice during the entire period of the infection but does not resolve the infection.
To analyze the effect of IL-12 treatment on survival of infected wild-type mice, a 1-week treatment (short-term treatment) or a repetitive treatment over the entire period of the experiment (twice a week; long-term treatment) was done. Short-term IL-12 treatment nearly doubled the mean survival time compared to that of untreated controls (Fig. 1). While untreated wild-type mice survived for a median period of 52 days, short-term-treated mice lived for 100 days after infection (P = 0.013). Protection of infected mice from death was most striking after long-term treatment with IL-12. As shown in Fig. 1, repetitive treatment of mice with recombinant IL-12 kept all mice alive during an experimental period of 19 weeks, whereas all untreated mice died between 3 and 10 weeks after infection with C. neoformans. However, termination of the repetitive treatment at day 134 led to rapidly occurring mortality (Fig. 1). Within 3 weeks after termination of the IL-12 treatment, two mice of a group of five mice died. The other three mice showed viable fungi in their lungs and brains, whereas only the lungs but not the brains from IL-12-treated mice carried detectable levels of viable cryptococci (data not shown). In another experiment, IL-12 treatment resulted in reduced levels of cryptococci in the brain but did not prevent dissemination of C. neoformans to the brain (data not shown). This demonstrates that long-term treatment with IL-12 is able to protect mice from death but unable to cure the mice of infection with C. neoformans.
Early mortality of IL-12-deficient mice but prolonged survival of IL-4-deficient mice infected with C. neoformans.
For a definitive analysis of the role of endogenous IL-12 in resistance to C. neoformans, mice deficient for the p35 or p40 subunit of IL-12 were experimentally infected (30, 33). In more than 10 experiments, significantly earlier mortality was reproducibly found in either IL-12 knockout strain compared to the wild-type (P < 0.0001). Table 1 shows that both types of IL-12-deficient mice died significantly earlier than wild-type mice (median survival time, 56 days for wild-type mice). Infected mice with a targeted disruption of the gene for the IL-12p35 subunit (IL-12p35−/− mice) had a median survival time of 32 days, whereas mice with a targeted disruption of the gene for the IL-12p40 subunit (IL-12p40−/− mice) had a median survival time of 26 days (Table 1). Most interestingly, the targeted disruption of the gene for the p40 subunit of IL-12 was associated with even earlier mortality than disruption of the gene for the p35 subunit of IL-12. A series of additional experiments in the same genetic background (C57BL/6 × 129/Sv/Ev) as well as five experiments using IL-12p35−/− and IL-12p40−/− mice in other genetic backgrounds (BALB/c, C57BL/6, and 129/Sv/Ev) reproduced the observed difference in the survival times of the two types of IL-12-deficient mouse strains. The statistical significance between the survival time of infected IL-12p35−/− and IL-12p40−/− mice was high (P < 0.0001). Treating IL-12p35−/− and IL-12p40−/− mice with exogenous IL-12 increased survival rates of infected IL-12-deficient mice to the level of those of wild-type mice (data not shown).
In contrast to the IL-12-deficient mice, IL-4−/− mice survived an experimental cryptococcal infection significantly longer than wild-type mice (Table 1). In two experiments, IL-4-deficient mice survived the entire experiments (100 and 77 days), whereas wild-type mice lived only 56 days (median value).
Organ load in mice infected with C. neoformans.
We assessed the fungal burden of Cryptococcus-infected mice in the lung, liver, spleen, and brain. Figure 2 shows a representative analysis of lungs from mice which had been infected for 21 days. Treatment of wild-type mice with IL-12 for 3 weeks decreased the lung load 100-fold from that in untreated mice (P = 0.014). The fungal load and organ distribution of C. neoformans found in IL-12p35−/− mice were similar to those in wild-type mice (2.7 × 105 and 5.2 × 105 CFU/lung, respectively), suggesting that there is no strict correlation of lung burden (at day 21 of the infection) with later mortality (see Table 1 for mortality data). Interestingly, IL-12p40−/− mice had a cryptococcal load about 10-fold higher than that of wild-type (P = 0.035) or IL-12p35−/− mice (P = 0.006). This suggests an important biological function of IL-12p40 in resistance to C. neoformans as already indicated by the observed prolonged survival time of IL-12p35−/− mice over IL-12p40−/− mice (Table 1). The mean cryptococcal burden in lungs of IL-4-deficient mice was about 10-fold lower than in wild-type mice (P = 0.041) but not as low as in IL-12-treated wild-type mice. Treatment with IL-12 reduced the fungal burden in brain as well as in the other organs analyzed but did not lead to sterile elimination (data not shown). The quantative pattern in fungal burden of the other organs from both IL-12-deficient mouse strains was similar to the lung data presented in Fig. 2. These data could be confirmed in the histopathological analysis.
Histopathological examination of organs from mice infected with C. neoformans.
C. neoformans elicits a variety of cellular responses, ranging from neutrophilic exudate to epitheloid cell granulomas with or without central caseous necrosis or “cystic” formations of extracellular fungi (35, 45). The organs most frequently studied are the lung and brain, but with disseminated disease, any organ may be infected. The immune status of the host affects the inflammatory cell response against C. neoformans. In addition, there is some evidence that the histological pattern of cryptococcosis depends not only on the age of the lesions but also on the organ infected. Granulomatous reactions are frequently larger and better developed in organs rich in sessile macrophages or lymphocytes and in more advanced stages of the infection. The granulomatous reaction is characterized by epitheloid and giant cells but also by infiltration with plasma cells, lymphocytes, and neutrophils. “Cystic” lesions occur in organs poor in macrophages and lymphocytes, such as brain. “Cystic” lesions consist of a gelatinous agglomeration of fungal cells often sharply demarcated from the surrounding parenchyma without inflammatory cell responses. Mixed forms composed of a granulomatous reaction with smaller intracellular yeasts and masses of “cystic” formations of large capsulated fungus cells can be found in the same lesions in organs such as the lung. Fibrotic changes and scar formation of granulomatous lesions can occur in older lesions which are in the process of healing.
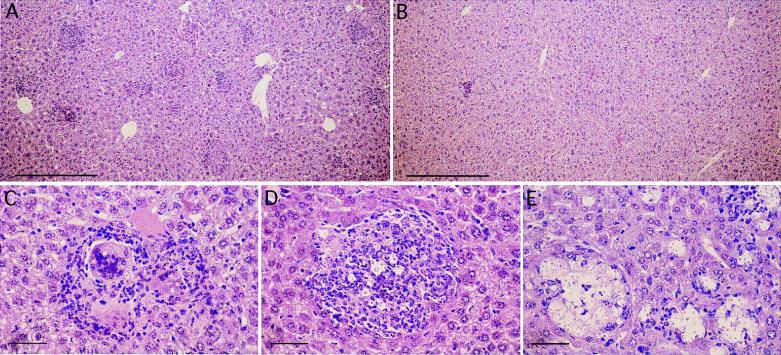
We analyzed formalin-fixed and paraffin-embedded organs from Cryptococcus-infected mice at various time points after the infection. The major histopathological features found were formation of granulomatous lesions and “cysts”. Figure 3 shows sections from livers of infected wild-type, IL-12p35−/−, and IL-12p40−/− mice. We chose liver for histopathological analysis because the inflammatory response associated with granulomatous reactions and “cystic” foci is easily visible and countable on a background of a homogenous parenchymal structure. In principle, we obtained similar data from lungs which were also studied (data not shown). In the livers of wild-type mice infected for 21 days, granulomas were formed at multiple sites (Fig. 3A). Treatment of wild-type mice with IL-12 led to a profound reduction in the number of granulomas (Fig. 3B). Typical granulomas were found in wild-type mice with epitheloid giant cells containing cryptococci and with lymphocytes and granulocytes (Fig. 3C). Similarly, in liver sections of IL-12p35−/− mice, granuloma formation could be detected (Fig. 3D). In IL-12p40−/− mice, however, a striking difference could be observed at day 21 of infection (Fig. 3E). The liver lesions found in those mice at day 21 consisted mainly of multiple “cysts” (Fig. 3E). Almost no cyst-like formations could be observed in liver sections of wild-type and IL-12p35−/− mice.
FIG. 3.
Histopathological analysis of livers from IL-12-treated or untreated mice 21 days after infection with C. neoformans. Liver sections of wild-type mice (A, B, and C), IL-12p35−/− mice (D), and IL-12p40−/− mice (E) 21 days after the infection with C. neoformans are shown. The livers were fixed in 10% phosphate-buffered formalin. Tissues were embedded in paraffin, and sections 5 μm thick were stained with hematoxylin and eosine. Microphotographs A and B demonstrate representative liver sections from animals treated with either IL-12 (B) or buffer (A) for 3 weeks. The bars in panels A and B represent 0.3 mm, while the bars in panels C, D, and E represent 0.05 mm. Photographs C, D, and E demonstrate individual lesions typically found. A representative granuloma with phagocytosed cryptococci in epitheloid giant cells is seen in wild-type mice (C) and IL-12p35−/− mice (D). In IL-12p40−/− mice (E), primarily “cystic” lesions can be found. The figure shows two of five experiments analyzed histologically.
Treatment of infected wild-type mice with recombinant IL-12 over a period of 21 days resulted in significantly decreased numbers of liver granulomas from that of untreated mice (Fig. 3A and B and 4) (P = 0.001). Reduction of the number of granulomas upon treatment with exogenous IL-12 was also found in IL-12p35−/− mice (P = 0.002) and IL-12p40−/− mice (P = 0.014) (Fig. 4). This was paralleled by prolonged survival of infected wild-type (Fig. 1) and IL-12-deficient mice (data not shown) as described above.
Functional characteristics of the CD4+ T cells induced in IL-12p35−/− and IL-12p40−/− mice by infection with C. neoformans.
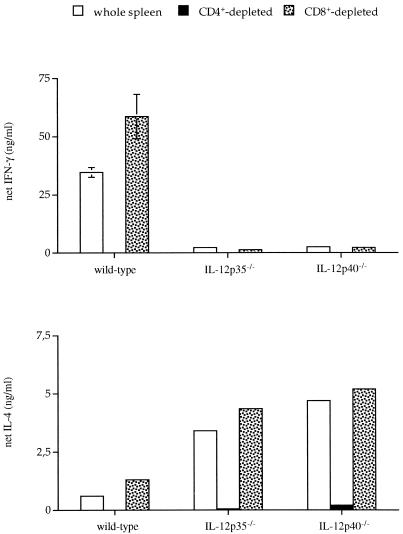
IL-12 which, as shown above, plays a key role in resistance to C. neoformans is known to be fundamentally involved in induction of Th1 differentiation (19, 30, 33). Therefore, we were interested in characterizing the Th phenotype in C. neoformans-infected wild-type and IL-12-deficient mice. As shown in Fig. 5, IFN-γ and IL-4 produced ex vivo in response to heat-killed cryptococci were completely derived from the CD4+ T-cell population, indicating that T-helper cells are indeed involved in immunity to cryptococcosis (14, 15). Spleen cells from uninfected wild-type, IL-12p35−/−, and IL-12p40−/− mice did not produce a detectable level of IFN-γ or IL-4 when stimulated with a series of different numbers of heat-inactivated cryptococci excluding nonspecific activation. Whereas total splenocytes (i.e., CD4+ T cells) from wild-type mice infected for 21 days produced considerable amounts of IFN-γ, splenocytes (i.e., CD4+ T cells) from IL-12-deficient IL-12p35−/− and IL-12p40−/− mice produced only marginal levels of IFN-γ. IL-4, however, was mainly produced by splenocytes (i.e., CD4+ T cells) from both types of IL-12-deficient mice, indicating Th2 polarization in the absence of IL-12. We found no evidence for antigen-specific secretion of IFN-γ or IL-4 from CD8+ T cells. However, upon polyclonal stimulation using anti-CD3 MAb, CD8+ T cells produced massive amounts of IFN-γ (data not shown).
FIG. 5.
Net cytokine secretion of total spleen cells from mice infected with C. neoformans. Spleens were taken from three mice per group 21 days after infection with 104 CFU of C. neoformans. Spleen cells were depleted of CD4+ or CD8+ T cells by using specific antibodies and magnetic beads. As assessed by subsequent fluorescence-activated cell sorter analysis, depletion of CD4+ or CD8+ T cells was complete (100%). The graph shows cytokine secretion after specific stimulation with heat-killed cryptococci with background values (medium controls) subtracted. The levels of IFN-γ and IL-4 in splenocyte cultures incubated with medium for 48 h were less than 5 and 10%, respectively. Polyclonal stimulation with anti-CD3 was similar between different groups (not shown). Levels of IFN-γ and IL-4 were quantified in 48-h culture supernatants by an enzyme-linked immunosorbent assay. Since the concentration of cells was kept constant at 2.5 × 106 cells/ml in total and depleted samples, bars representing the CD8+-depleted samples reflect CD4+-enriched cultures with elevated cytokine production. This figure demonstrates the results of one representative experiment of nine similar experiments with cytokine analyses of lymphocytes cultured ex vivo.
DISCUSSION
While other studies showed partial protection of C. neoformans-infected mice upon treatment with IL-12 (3, 25), our study for the first time demonstrates that continuous treatment with IL-12 is able to protect infected mice throughout a prolonged period (180 days) of treatment. Although long-term treatment did not result in sterile elimination, infected mice survived for the entire experimental period and the fungal load in the lung and other organs such as the brain was reduced. The latter finding indicates that IL-12 treatment did not affect dissemination of C. neoformans but the growth of already seeded foci. Similar data were obtained by Hill and Aguirre, who demonstrated that CD4+ T cells are able to protect the brain against C. neoformans by restricting the growth of cryptococci rather than preventing colonization (15). At this point, it remains open whether the number of cryptococci in brain was reduced directly by a local (brain) T-cell response or indirectly by T cells acting in peripheral tissues.
The results of our study point to a central role of IL-12-induced differentiation of Th1 cells in the course of infection with C. neoformans. In the absence of Th1 cells, infected IL-12-deficient mice die earlier and develop higher organ burdens than wild-type mice. The central role of the Th1 phenotype is further underlined by enhanced resistance against cryptococcal infection observed in IL-4-deficient mice. Three weeks after infection, CD4+ T cells from IL-4−/− mice produced fourfold more IFN-γ than CD4+ T cells from wild-type mice upon ex vivo activation with anti-CD3 MAb (data not shown). This result indicates a polarized Th1 response in IL-4−/− mice which might correlate with enhanced resistance to C. neoformans infection in the absence of IL-4. However, even in wild-type mice, the course of the infection with C. neoformans is eventually fatal unless IL-12 is given continuously during the infection period. In accordance with data described for Leishmania major infection where resistance is critically dependent on the Th1 response (12, 44), Kawakami et al. showed that treatment with IL-12 is protective only when done within the first week of infection with C. neoformans (25). Treatment in the second week of the infection did not protect mice from early death (25). The importance of a Th1 response for protection against C. neoformans has also been demonstrated in a study by Hoag et al. (17). Hoag et al. (18) were able to correlate resistance and susceptibility in different inbred mouse strains with Th1 and Th2 responses, respectively. In a pulmonary model of C. neoformans infection, resistant C.B-17 mice switched from a Th1 response to a Th2 response upon treatment with anti-IL-12 MAb. This treatment ultimately resulted in higher lung burden (17).
The in vivo importance of Th1 cytokines such as IFN-γ and tumor necrosis factor for immunity against cryptococcosis was shown by several groups (1, 21). In another study, the protective activity of IL-12 treatment could be correlated with expression of tumor necrosis factor alpha, IL-12p40, IFN-γ-inducing factor (now referred to as IL-18), and inducible nitric oxide synthase (24). Since IFN-γ activated macrophages are important in host resistance against cryptococcosis (3), this effect can be easily explained by IL-12-dependent induction of IFN-γ during therapy with IL-12. Especially for IL-18, a synergistic action with IL-12 has been described in stimulating fungicidal activity of macrophages against C. neoformans by induction of IFN-γ synthesis in natural killer cells (48). Our data from C. neoformans-infected IL-12-deficient mice indicate that endogenous IL-18 is unable to compensate for the lack of IL-12 production as published for infection with L. major (31) and Mycobacterium tuberculosis (4). However, recently it was demonstrated that exogenous IL-18 was able to protect mice against pulmonary and disseminated infection with C. neoformans by inducing IFN-γ (23).
For the first time in a model of infectious diseases, we found earlier mortality and higher organ burdens in IL-12p40−/− mice than in IL-12p35−/− mice. Since the IL-12p40 subunit can be secreted in its free form independently of the p35 subunit (7), the higher resistance of IL-12p35−/− mice might be mediated by an as yet unknown agonistic biological function of the free p40 chain. This might explain why p40 is physiologically overproduced by antigen-presenting cells activated by microbial antigens (7). Differential regulation of IFN-γ production from T cells by p40 as reported for mixed lymphocyte cultures (38) could not be found in our murine model of C. neoformans infection (Fig. 5 shows similar amounts of IFN-γ by splenocytes from infected IL-12p35−/− and IL-12p40−/− mice). In addition, splenocytes from infected IL-12p35−/− and IL-12p40−/− mice did not differ in ex vivo production of IL-10 (data not shown). Also, no difference in production of nitric oxide from spleen cells derived from IL-12p40−/− and IL-12p35−/− mice could be observed, which rules out a potential role for the p40 subunit in nitric oxide-dependent effector mechanisms (data not shown). Attempts to protect infected IL-12p40−/− mice by administering exogenous recombinant monomeric or dimeric IL-12p40 during the first week or during the first 3 weeks of infection failed (data not shown). However, administration of homodimeric IL-12p40 to wild-type mice led to elevated organ loads and earlier mortality (data not shown), demonstrating the known antagonistic activity of IL-12p40 in the presence of bioactive IL-12 as published for other systems (9, 32, 34). This suggests that recombinant p40 most likely was administered adequately to favor a potential biological activity. Therefore, it is conceivable that endogenous IL-12p40 (but not exogenously added IL-12p40) associates with an as yet unknown molecule different from p35 to become functional. Whatever the p40-dependent agonistic mechanism is, its protective activity as shown for survival (Table 1) and organ burden (Fig. 2) is highly significant. The aberrant histological pattern of the inflammatory reaction without pronounced granulomatous lesions and an increase in “cyst” formation with areactive clusters of cryptococci (Fig. 3E) found in infected IL-12p40−/− mice may point to a function of p40 in cellular recruitment and granuloma formation.
Recently it was found in alloimmunity that homodimeric p40 accentuates alloreactive CD8+ Th function by enhancing IFN-γ production (38). Moreover, endogenous p40 produced by IL-12p35−/− mice (but not by IL-12p40−/− mice) may stimulate alloreactive Th1 differentiation in vivo (39). Indeed, preliminary experiments in C. neoformans-infected IL-12-deficient mice suggest a defective delayed-type hypersensitivity response in IL-12p40−/− mice but not in IL-12p35−/− and wild-type mice (data not shown). It will be interesting to define the underlying mechanism for a potential role of endogenous p40 in cellular immune responses such as delayed-type hypersensitivity and granuloma formation.
REFERENCES
- 1.Aguirre K M, Havell E A, Gibson G W, Johnson L L. Role of tumor necrosis factor and gamma interferon in acquired resistance to Cryptococcus neoformans in the central nervous system of mice. Infect Immun. 1995;63:1725–1731. doi: 10.1128/iai.63.5.1725-1731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherwinski H M, Schumacher J H, Brown K D, Mosmann T R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clemons K V, Brummer E, Stevens D A. Cytokine treatment of central nervous system infection: efficacy of interleukin-12 alone and synergy with conventional antifungal therapy in experimental cryptococcosis. Antimicrob Agents Chemother. 1994;38:460–464. doi: 10.1128/aac.38.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper A M, Magram J, Ferrante J, Orme I M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin-10 inhibits human lymphocyte IFN-g production by suppressing natural killer cell stimulatory factor (NKSF/IL-12) J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Andrea A, Rengaraju M, Valiante N M, Chehimi J, Kubin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E, Chizzonite R, Wolf S F, Trinchieri G. Role of the production of natural killer cell stimulatory factor (NKSF/IL-12) in the ability of B cell lines to stimulate T and NK cell proliferation. Cell Immunol. 1992;145:187–198. doi: 10.1016/0008-8749(92)90322-g. [DOI] [PubMed] [Google Scholar]
- 7.D’Andrea A, Rengaraju M, Valiante N M, Chehimi J, Kubin M, Aste-Amezaga M, Chan S H, Kobayashi M, Young D, Nickbarg E, Chizzonite R, Wolf S F, Trinchieri G. Production of natural killer cell stimulatory factor (NKSF/IL-12) induces Th1-type specific immune responses and inhibits the development of IL-4 producing Th cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Hermoso D, Mathoulin-Pelissier S, Couprie B, Ronin O, Dupont B, Dromer F. DNA typing suggests pigeon droppings as a source of pathogenic Cryptococcus neoformans serotype D. J Clin Microbiol. 1997;35:2683–2685. doi: 10.1128/jcm.35.10.2683-2685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillessen S, Carvajal D, Ling P, Podlaski F J, Stremlo D L, Familetti P C, Gubler U, Presky D H, Stern A S, Gately M K. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;10:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 10.Granger D L, Perfect J R, Durack D T. Macrophage-mediated fungistasis in vitro: requirements for intracellular and extracellular cytotoxicity. J Immunol. 1986;136:672–680. [PubMed] [Google Scholar]
- 11.Graybill J R, Drutz D J. Host defense in cryptococcosis. II. Cryptococcosis in the nude mouse. Cell Immunol. 1978;40:263–274. doi: 10.1016/0008-8749(78)90334-9. [DOI] [PubMed] [Google Scholar]
- 12.Heinzel F P, Schoenhaut D S, Rerko R M, Rosser L E, Gately M K. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hidore M R, Murphy J W. Natural cellular resistance of beige mice against Cryptococcus neoformans. J Immunol. 1986;137:3624–3631. [PubMed] [Google Scholar]
- 14.Hill J O. CD4+ T cells cause multinucleated giant cells to form around Cryptococcus neoformans and confine the yeast within the primary site of infection in the respiratory tract. J Exp Med. 1992;175:1685–1695. doi: 10.1084/jem.175.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill J O, Aguirre K M. CD4+ T cell-dependent acquired state of immunity that protects the brain against Cryptococcus neoformans. J Immunol. 1994;152:2344–2350. [PubMed] [Google Scholar]
- 16.Hill J O, Harmsen A G. Intrapulmonary growth and dissemination of an avirulent strain of Cryptococcus neoformans in mice depleted of CD4+ and CD8+ T cells. J Exp Med. 1991;173:755–758. doi: 10.1084/jem.173.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoag K A, Lipscomb M F, Izzo A A, Street N E. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1997;17:733–739. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 18.Hoag K A, Street N E, Huffnagle G B, Lipscomb M F. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am J Respir Cell Mol Biol. 1995;13:487–495. doi: 10.1165/ajrcmb.13.4.7546779. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O’Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 20.Huffnagle G B, Boyd M B, Street N E, Lipscomb M F. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6) J Immunol. 1998;160:2393–2400. [PubMed] [Google Scholar]
- 21.Huffnagle G B, Toews G B, Burdick M D, Boyd M B, McAllister K S, McDonald R A, Kunkel S L, Strieter R M. Afferent phase production of TNF-alpha is required for the development of protective T cell immunity to Cryptococcus neoformans. J Immunol. 1996;157:4529–4536. [PubMed] [Google Scholar]
- 22.Huffnagle G B, Yates J L, Lipscomb M F. Immunity to pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T-cells. J Exp Med. 1991;173:793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawakami K, Qureshi M H, Zhang T, Okamura H, Kurimoto M, Saito A. IL-18 protects mice against pulmonary and disseminated infection with Cryptococcus neoformans by inducing IFN-gamma production. J Immunol. 1997;159:5528–5534. [PubMed] [Google Scholar]
- 24.Kawakami K, Tohyama M, Qifeng X, Saito A. Expression of cytokines and inducible nitric oxide synthase mRNA in the lungs of mice infected with Cryptococcus neoformans: effects of interleukin-12. Infect Immun. 1997;65:1307–1312. doi: 10.1128/iai.65.4.1307-1312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawakami K, Tohyama M, Xie Q, Saito A. IL-12 protects mice against pulmonary and disseminated infection caused by Cryptococcus neoformans. Clin Exp Immunol. 1996;104:208–214. doi: 10.1046/j.1365-2249.1996.14723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi M, Fitz L, Ryan M, Hewick R M, Clark S C, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biological effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopf M, Le Gros G, Bachmann M, Lamers M C, Blüthmann H, Köhler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 28.Lovchik J, Lipscomb M, Lyons C R. Expression of lung inducible nitric oxide synthase protein does not correlate with nitric oxide production in vivo in a pulmonary immune response against Cryptococcus neoformans. J Immunol. 1997;158:1772–1778. [PubMed] [Google Scholar]
- 29.Lovchik J, Lyons C R, Lipscomb M. A role for gamma interferon-induced nitric oxide in pulmonary clearance of Cryptococcus neoformans. Am J Respir Cell Mol Biol. 1995;13:116–121. doi: 10.1165/ajrcmb.13.1.7598935. [DOI] [PubMed] [Google Scholar]
- 30.Magram J, Connaughton S E, Warrier R R, Carvajal D M, Wu C Y, Ferrante J, Stewart C, Sarmiento U, Faherty D A, Gately M K. IL-12-deficient mice are defective in IFN-γ production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 31.Mattner F, Di Padova K, Alber G. Interleukin-12 is indispensable for protective immunity against Leishmania major. Infect Immun. 1997;65:4378–4383. doi: 10.1128/iai.65.11.4378-4383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattner F, Fischer S, Guckes S, Jin S, Kaulen H, Schmitt E, Rüde E, Germann T. The interleukin-12 subunit IL-12 p40 specifically inhibits effects of the interleukin-12 heterodimer. Eur J Immunol. 1993;23:2202–2208. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- 33.Mattner F, Magram J, Ferrante J, Launois P, Di Padova K, Behin R, Gately M K, Louis J A, Alber G. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur J Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- 34.Mattner F, Ozmen L, Podlaski F J, Wilkinson V L, Presky D H, Gately M K, Alber G. Treatment with homodimeric interleukin-12 (IL-12) p40 protects mice from IL-12-dependent shock but not from tumor necrosis factor alpha-dependent shock. Infect Immun. 1997;65:4734–4737. doi: 10.1128/iai.65.11.4734-4737.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyaji M, Nishimura K. Studies on organ specificity in experimental murine cryptococcosis. Mycopathologia. 1981;76:145–154. doi: 10.1007/BF00437195. [DOI] [PubMed] [Google Scholar]
- 36.Murphy E E, Terres G, Macatonia S E, Hsieh C, Mattson J, Lanier L, Wysocka M, Trinchieri G, Murphy K, O’Garra A. B7 and IL-12 cooperate for proliferation and IFN-γ production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med. 1994;180:223–231. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohara J, Paul W E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985;315:333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- 38.Piccotti J R, Chan S Y, Li K, Eichwald E J, Bishop D K. Differential effects of IL-12 receptor blockade with IL-12 p40 homodimer on the induction of CD4+ and CD8+ IFN-gamma-producing cells. J Immunol. 1997;158:643–648. [PubMed] [Google Scholar]
- 39.Piccotti J R, Li K, Chan S Y, Ferrante J, Magram J, Eichwald E J, Bishop D K. Alloantigen-reactive Th1 development in IL-12-deficient mice. J Immunol. 1998;160:1132–1138. [PubMed] [Google Scholar]
- 40.Prat M, Gribaudo G, Comoglio P M, Cavallo G, Landolfo S. Monoclonal antibodies against murine gamma interferon. Proc Natl Acad Sci USA. 1984;81:4515–4519. doi: 10.1073/pnas.81.14.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Randhawa H S, Clayton Y M, Riddell R W. Isolation of Cryptococcus neoformans from pigeon habitats in London. Nature. 1965;208:801. doi: 10.1038/208801a0. [DOI] [PubMed] [Google Scholar]
- 42.Sethi K K, Randhawa H S. Survival of Cryptococcus neoformans in the gastrointestinal tract of pigeons following ingestion of the organism. J Infect Dis. 1968;118:135–138. doi: 10.1093/infdis/118.2.135. [DOI] [PubMed] [Google Scholar]
- 43.Stern A S, Podlaski F J, Hulmes J D, Pan Y E, Quinn P M, Wolitzky A G, Familetti P C, Stremlo D L, Truitt T, Chizzonite R, Gately M K. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci USA. 1990;87:6808–6812. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sypek J P, Chung C L, Mayor S E, Subramanyam J M, Goldman S J, Sieburth D S, Wolf S F, Schaub R G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watabe T, Miyaji M, Nishimura K. Studies on relationship between cysts and granulomas in murine cryptococcosis. Mycopathologia. 1984;86:113–120. doi: 10.1007/BF00436496. [DOI] [PubMed] [Google Scholar]
- 46.Wolf S, Seiburth D, Perussia B, Yetz-Adalpe J, D’Andrea A, Trinchieri G. Cell sources of natural killer cell stimulatory factor (NKSF/IL-12) transcripts and subunit expression. FASEB J. 1992;6:A1335. [Google Scholar]
- 47.Yuan R R, Casadevall A, Oh J, Scharff M D. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc Natl Acad Sci USA. 1997;94:2483–2488. doi: 10.1073/pnas.94.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang T, Kawakami K, Qureshi M H, Okamura H, Kurimoto M, Saito A. Interleukin-12 (IL-1) and IL-18 synergistically induce fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594–3599. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuger A, Louie E, Holzman R S, Simberkoff M S, Rahal J J. Cryptococcal disease in patients with the acquired immunodeficiency syndrome. Diagnostic features and outcome of treatment. Ann Intern Med. 1986;104:234–240. doi: 10.7326/0003-4819-104-2-234. [DOI] [PubMed] [Google Scholar]