Abstract
Objectives
Anthracycline-induced cardiotoxicity is a debilitating cardiac dysfunction for which there are no effective treatments, making early prevention of anthracycline-induced subclinical cardiotoxicity (AISC) crucial. High-density lipoprotein cholesterol (HDL-C) plays a role in cardioprotection, but its impact on AISC remains unclear. Our study aims to elucidate the protective capacity of HDL-C in AISC in patients with diffuse large B-cell lymphoma (DLBCL) treated with R-CHOP (cyclophosphamide, vincristine, doxorubicin, prednisone and rituximab).
Design
Prospective observational study.
Setting
Conducted in China from September 2020 to September 2022.
Participants
70 chemotherapy-naïve patients newly diagnosed with DLBCL who were scheduled to receive the standard dose of R-CHOP; 60 participants included in a case–control study (DOI: 10.1186/s12885-022-10085-6).
Primary outcome measures
Serum biomarkers, 2D speckle tracking echocardiography and conventional echocardiography were measured at baseline, at the end of the third and sixth cycles of R-CHOP and 6 and 12 months after chemotherapy.
Results
24 patients experienced AISC, while 10 did not. 36 patients were lost to follow-up and death. Cox regression analysis showed that higher levels of HDL-C were associated with a significantly lower risk of AISC (unadjusted HR=0.24, 95% CI 0.09 to 0.67, p=0.006; adjusted HR=0.27, 95% CI 0.09 to 0.79, p=0.017). Patients without AISC had a more stable and higher HDL-C level during the follow-up period. HDL-C levels significantly decreased from the end of the third cycle of chemotherapy to the end of the sixth cycle of chemotherapy in all patients (p=0.034), and particularly in the AISC group (p=0.003). The highest level of HDL-C was significantly higher in patients without AISC than in those with AISC (1.52±0.49 vs 1.22±0.29, p=0.034).
Conclusions
Our study suggests that higher HDL-C levels may associate with lower AISC risk in patients with DLBCL treated with R-CHOP. HDL-C could be a cardioprotective target, but further research is needed to confirm its benefits and limitations.
Study registration number
Study registration number: ChiCTR2100054721
Keywords: Lymphoma, CHEMOTHERAPY, CARDIOLOGY, Echocardiography
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This prospective observational study contributes to our understanding of the association between high-density lipoprotein cholesterol (HDL-C) and anthracycline-induced subclinical cardiotoxicity, offering a foundation for the development of early intervention and prevention strategies.
The study used advanced imaging techniques (2D speckle tracking echocardiography) to assess the subclinical cardiac dysfunction in the patients, which can provide more sensitive and accurate results compared with traditional echocardiography.
The study only included patients with diffuse large B-cell lymphoma who received R-CHOP (cyclophosphamide, vincristine, doxorubicin, prednisone and rituximab), which may limit the generalisability of the findings to patients with other types of cancer or chemotherapy regimens.
The relatively small sample size in this study may potentially impact the robustness and generalisability of our findings. Additional comprehensive studies, including both clinical and basic research, are necessary to fully evaluate the benefits and limitations of HDL-C as a cardioprotective strategy in anthracycline-treated patients with cancer.
Introduction
The improved management of cancer has led to a significant increase in the survival rate of cancer survivors.1 However, anthracycline, one of the most effective chemotherapeutic agents used to treat various cancers, is associated with potentially life-threatening and severe cardiovascular diseases.2 Studies have shown a significant increase in mortality in patients with cancer with cardiovascular disease.3 4 As advances in cancer treatment and an ageing population continue, the number of patients with both conditions is rising.5 As a result, the field of cardio-oncology has become increasingly important in recent years.
Non-Hodgkin’s lymphoma (NHL) is the seventh most common cancer in the USA and the most frequent haematological malignancy globally, accounting for about 3% of cancer cases and deaths.6 Among NHLs, diffuse large B-cell lymphoma (DLBCL) is the most prevalent type, representing approximately one-third of all cases.7 The combination of cyclophosphamide, vincristine, doxorubicin and prednisone with rituximab (R-CHOP) is a standard first-line therapy that has substantially improved survival outcomes in patients with DLBCL.8 Nonetheless, anthracycline-containing chemotherapy agents are associated with cardiotoxicity, a major long-term adverse effect that significantly affects the quality of life and survival of cancer survivors.
Anthracycline-induced cardiotoxicity (AIC) is a devastating consequence of successful cancer treatment, often leading to hypokinetic cardiomyopathy and ultimately heart failure. AIC is an irreversible form of cardiac dysfunction for which no guidelines or accepted therapies for cardioprotection currently exist.9 10 Therefore, early prevention and detection of AIC are crucial for providing opportunities for early intervention. Anthracycline-induced subclinical cardiotoxicity (AISC) is an early stage of AIC, characterised by abnormal echocardiography index without clinical symptoms.11 Early intervention is recommended by the 2022 International Cardio-Oncology Society (IC-OS) consensus statement once AISC is detected.12 Global longitudinal strain (GLS) measured by 2D speckle tracking echocardiography can reliably identify most early myocardial deformation variations. In our study, we used early measurement of GLS to identify AISC.13 14
High-density lipoprotein (HDL) is the sole lipoprotein with protective attributes among the five types of lipoproteins. Its salutary effects include antioxidant, anti-inflammatory and antiapoptotic properties. Numerous preclinical investigations have suggested that HDL may have direct and indirect protective effects against AIC.15–17 The roles of HDL cholesterol (HDL-C) and apolipoprotein A1 (ApoA1) in providing cardiovascular protection of HDL have been the subject of recent debate. Therefore, further investigation is warranted to explore the clinical data pertaining to the association between HDL and anthracycline-related cardiotoxicity.
We undertook a prospective observational study to investigate the potential impact of HDL-C on AISC. Using 2D speckle tracking echocardiography, we identified AISC and sought to establish any correlation between HDL-C and AISC. Additionally, we assessed the fluctuations in HDL-C levels during R-CHOP chemotherapy in chemotherapy-naïve patients recently diagnosed with DLBCL. Subsequently, our team conducted a case–control study, revealing that HDL-C serves as a predictive factor for AISC in patients with DLBCL treated with three cycles of (R)-CHOP.18 Both the case–control study and the present study are from the same database of the registered study, ‘Study of Antineoplastic Drugs Induced Early Cardiotoxicity in Patients with Lymphoma’ (ChiCTR2100054721). Even though this case–control study was analysed with data from different parts of the same database for different objectives, the result underscores the significance of further investigating the relationship between HDL-C and AISC.
Methods
Study population
We recruited chemotherapy-naïve patients newly diagnosed with DLBCL who were scheduled to receive the standard dose of R-CHOP chemotherapy regimen at our institution from 1 September 2020 to 1 September 2022. Our inclusion criteria were as follows: newly diagnosed DLBCL, age between 18 and 80 years, Eastern Cooperative Oncology Group (ECoG) performance status ≤2, left ventricular ejection fraction (LVEF) ≥50%, and acceptable bone marrow, renal and hepatic functions for chemotherapy. Conversely, our exclusion criteria were symptomatic heart failure, a history of myocardial ischaemia, myocarditis, myocardial infarction, clinical or subclinical pericardial effusion, arrhythmia requiring medical intervention, a history of other cancers, under lipid-lowering treatment and severe active infections such as syphilis, hepatitis or HIV infection. This study shares its database with a case–control study previously conducted by our group, as referenced earlier.18 The patients enrolled in the two study were not identical because of a few differences in specific objectives, inclusion criteria and exclusion criteria. In brief, the case–control study specifically included patients with DLBCL who received the standard dose of (R)-CHOP chemotherapy regimen (CHOP with or without rituximab combination), and patients undergoing lipid-lowering therapy were not excluded. In the current study, 10 of the 70 enrolled participants were not enrolled in the case–control study. More details can be seen in this case–control study.18
Treatment
Patients received a total of six cycles of standard R-CHOP (cyclophosphamide at 750 mg/m2 on D1, doxorubicin at 50 mg/m2 on D1, vincristine at 1.4 (maximum 2) mg/m2 on D1 and 100 mg prednisone on D1–5, with rituximab at 375 mg/m2 on D1 in each cycle), with or without two cycles of rituximab maintenance (rituximab at 375 mg/m2 on D1 in each cycle).
Definition of subclinical cardiotoxicity
According to the IC-OS consensus statement, the definition of subclinical cardiotoxicity was a relative GLS decrease from baseline ((baseline−current GLS)/baseline GLS) of >12%, but with a normal LVEF.12
Study protocol
We defined ‘baseline’ as the initial assessment conducted before the initiation of the first cycle of chemotherapy. At baseline, the end of the third cycle of R-CHOP, the end of the sixth cycle of R-CHOP and 6 and 12 months after chemotherapy completion, all enrolled patients underwent conventional echocardiography, 2D speckle tracking echocardiography and blood sampling. Every patient received electrocardiography (ECG) examination before every cycle of chemotherapy to ensure the safety of the treatment. Demographic data and clinical variables, including age, gender, body mass index (BMI), ECoG performance status, diabetes mellitus, hypertension, drinking history (an adult who has consumed more than 20 drinks in their lifetime, with each drink considered to have an average alcohol content of 12 g) and smoking history (an adult who has smoked at least 100 cigarettes in their lifetime), were collected at the time of enrolment. Left ventricular systolic dysfunction was measured by LVEF, fractional shortening, left ventricular mass index, left ventricular diastolic dimension, E, e′, E/e′ and GLS. HDL-C, low-density lipoprotein cholesterol, cardiac troponin T, high-sensitivity C reactive protein, N-terminal prohormone of brain natriuretic peptide, total cholesterol (TC) and total triglyceride (TG) were measured. We used the baseline HDL-C level as a surrogate marker for HDL quantity. The patients were categorised into two groups based on the average HDL-C value for males and females in the modified criteria of the National Cholesterol Educated Program Adult Treatment Panel III.19 High HDL-C was defined as a serum HDL-C ≥1.16 mmol/L, while low HDL-C was defined as a serum HDL-C <1.16 mmol/L. We determined the sample size using an online sample size calculator, which indicated a total requirement of 23 events.20
Statistical analysis
The study was conducted with two aims: first, to evaluate the relationship between HDL-C and AISC; and second, to conduct a preliminary exploration of the differences in HDL-C and the variability of HDL-C changes between patients with and without AISC during the follow-up period. Continuous variables were expressed as mean±SD and compared using the t-test. Non-normally distributed variables were presented as median (Q1–Q3) and compared with the Wilcoxon-Mann-Whitney test. Categorical variables were expressed as n (%) and compared using the χ2 or Fisher’s exact test, as appropriate. Correlation analysis was conducted to investigate the associations of change in HDL-C with change in GLS. The probabilities of survival were calculated using Kaplan-Meier methods and compared using log-rank tests. Cox proportional hazards regression models were conducted to assess the association between variables and AISC. Covariates for multivariable Cox regression models included age, sex and variables that had a p value <0.15 in the univariable Cox regression analysis (GLS was excluded as it is the factor that defines AISC). Two multivariable Cox regression models were constructed: the first model included age and sex; and the second model included age, sex, hypertension, BMI and E. Statistical analysis and visualisation were performed using IBM SPSS V.22.0 and GraphPad Prism V.8. Statistical tests were two sided, with a p value <0.05 being considered statically significant.
Patient and public involvement
None.
Results
Assessment of the association between HDL-C and AISC
Study population and baseline characteristics
This investigation enrolled a total of 70 patients with chemotherapy-naïve DLBCL and were planned to be treated with the standard R-CHOP regimen. Based on the baseline HDL-C level, we segregated the patients into two groups: the high-level group (HDL-C≥1.16 mmol/L, n=28) and the low-level group (HDL-C<1.16 mmol/L, n=42). Patients with drinking history had a greater chance of having a high HDL-C level (p=0.034). The patients with high HDL-C showed substantially higher TC (p=0.011) and lower total TG (p=0.002). The baseline characteristics of the patients in both groups were well balanced (online supplemental table S1).
bmjopen-2023-074541supp003.pdf (112.1KB, pdf)
High HDL-C was an independent protective target of AISC
The clinical endpoint was defined as the first detection of AISC, and the median survival time of the whole cohort was 16 months. The median survival time of patients with low HDL-C was 4 months, while that of patients with high HDL-C was not reached. The median follow-up time of the cohort was 10 months. During the follow-up period, 24 patients experienced AISC, while 10 did not. Approximately half of the patients (n=36) were lost to follow-up and death. A flow chart detailing the patients enrolled in the study can be found in online supplemental figure S1.
bmjopen-2023-074541supp001.pdf (152.1KB, pdf)
The log-rank test revealed that patients with higher HDL-C were less likely to experience AISC (p=0.001, HR=0.26, 95% CI 0.12 to 0.58) (online supplemental figure S2).
bmjopen-2023-074541supp002.pdf (73.8KB, pdf)
According to the results of the univariable Cox regression analysis, variables had a p value <0.15 including age, BMI, hypertension, GLS, E and HDL-C group. Increasing age was significantly associated with a decreased HR of 0.97 (95% CI 0.943 to 0.998, p=0.034) per 1-year increase. BMI showed an HR of 1.09 (95% CI 0.97 to 1.22, p=0.139) per 1 kg/m2 increase. Similarly, hypertension had an HR of 0.22 (95% CI 0.03 to 1.62, p=0.136) for yes versus no. A lower GLS was significantly associated with an increased HR of 1.46 (95% CI 1.20 to 1.77, p<0.001) per −1% decrease. E velocity showed an HR of 1.03 (95% CI 1.00 to 1.06, p=0.075) per 1 cm/s increase. The HDL-C group (high vs low) had a significantly lower HR of 0.24 (95% CI 0.09 to 0.67, p=0.006). Further details about other variables can be found in online supplemental table S2.
bmjopen-2023-074541supp004.pdf (85.4KB, pdf)
The results of the multivariable Cox regression analysis showed that high HDL-C was significantly associated with a lower risk of AISC after adjusting for age and sex (model 1) (HR=0.28, 95% CI 0.10 to 0.84, p=0.018). Similarly, after adjusting for age, sex and variables with p<0.15 in the univariable Cox regression analysis (excluding GLS as it defines AISC) (model 2), the same association was observed (HR=0.27, 95% CI 0.09 to 0.79, p=0.017) (table 1).
Table 1.
Outcomes of study participants
| HR (95% CI) (Unadjusted) |
P value | HR (95% CI) (Adjusted*) |
P value | HR (95% CI) (Adjusted†) |
P value | |
| Low HDL-C | Ref | Ref | Ref | |||
| High HDL-C | 0.24 (0.09 to 0.67) | 0.006 | 0.28 (0.10 to 0.80) | 0.018 | 0.27 (0.09 to 0.79) | 0.017 |
The endpoint was defined as the first detection of anthracycline-induced subclinical cardiotoxicity.
Low HDL-C: <1.16 mmol/L. High HDL-C: ≥1.16 mmol/L.
*Adjusted for age and sex.
†Adjusted for age, sex, hypertension, body mass index and E.
HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio.
Preliminary exploration of the difference of HDL-C between patients with and without AISC
Study population and baseline characteristics
In this analysis, we selectively included 34 of the enrolled patients who were not lost to follow-up and death. The patients who exhibited AISC at any time during the follow-up period were segregated into the AISC group (n=24), while those who did not demonstrate AISC were classified into the No-AISC group (n=10). Patients within the AISC group were comparatively younger (50±12.45 vs 59.7±9.67, p=0.035) and exhibited a higher baseline GLS (22.0 (21.0, 22.8) vs 18.0 (17.0, 20.0), p<0.001). More baseline information can be seen in online supplemental table S3.
bmjopen-2023-074541supp005.pdf (117.4KB, pdf)
Timeline of HDL-C level in patients with and without AISC
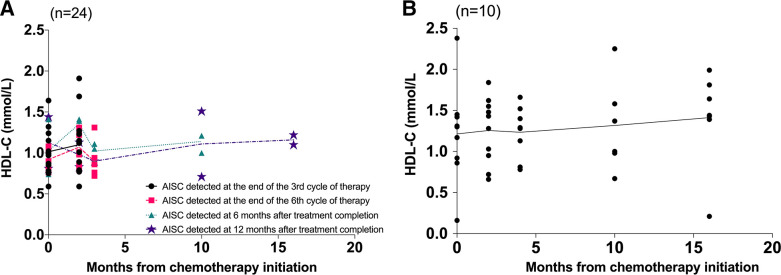
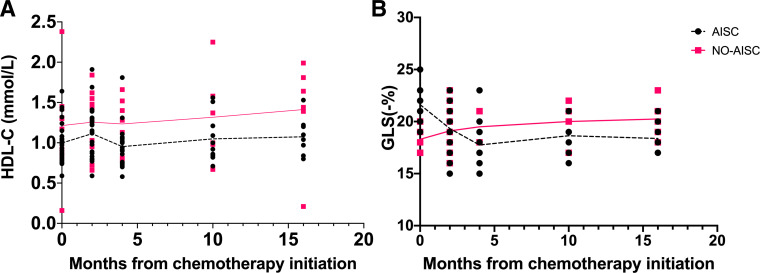
Figure 1 displays the timeline of HDL-C levels in patients with and without AISC. In figure 1A, the patient population was categorised into four groups based on the time of AISC detection. Among the groups, 12 patients were identified with AISC at the end of the third cycle of chemotherapy, 7 patients at the end of the sixth cycle, 3 patients at 6 months after treatment completion and 2 patients at 12 months after treatment completion. With the exception of the group in which patients detected AISC at the end of the third cycle of chemotherapy, all other groups exhibited a reduction in HDL-C values from the end of the third cycle of chemotherapy to the end of the sixth cycle of chemotherapy. Figure 1B portrays the HDL-C level in patients without AISC, indicating that the HDL-C level was more stable than in patients with AISC. Moreover, the overall HDL-C level was higher in patients without AISC than in patients with AISC throughout the follow-up period (figure 2A). In figure 2B, there was a significant decrease in GLS during the chemotherapy period (from 0 to 4 months), which remained stable after completion of chemotherapy (after 4 months) in patients with AISC.
Figure 1.
(A) Timeline of high-density lipoprotein cholesterol (HDL-C) levels in patients detected anthracycline-induced subclinical cardiotoxicity (AISC) at four time points. 12 patients were detected AISC at the end of the third cycle of chemotherapy. 7 patients were detected AISC at the end of the sixth cycle of chemotherapy. 3 patients were detected AISC at 6 months after treatment completion. 2 patients were detected AISC at 12 months after treatment completion. (B) Timeline of HDL-C levels of patients without AISC.
Figure 2.
Timeline of high-density lipoprotein cholesterol (HDL-C) levels (A) and global longitudinal strain (GLS) (B) in patients with and without anthracycline-induced subclinical cardiotoxicity (AISC) during the whole follow-up period.
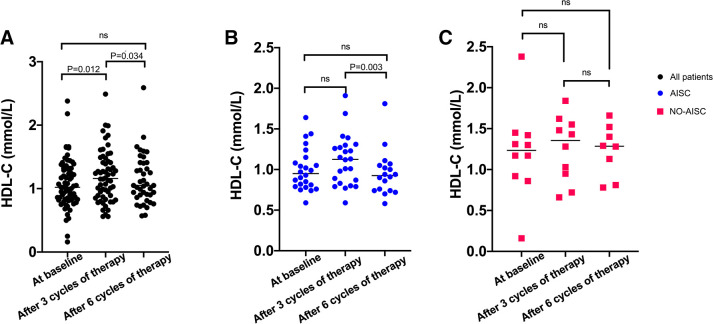
Based on figure 2, we observed that the fluctuations in HDL-C and GLS were most pronounced during the chemotherapy period. The fluctuations in HDL-C levels of patients with DLBCL during R-CHOP chemotherapy were presented in figure 3. The levels of HDL-C significantly increased for all patients from baseline to the end of the third cycle of chemotherapy (p=0.012) and significantly decreased from the end of the third cycle to the end of the sixth cycle of chemotherapy (p=0.034) (figure 3A). Patients with AISC showed a significant decrease in HDL-C levels during R-CHOP chemotherapy from the end of the third cycle to the end of the sixth cycle (p=0.003) (figure 3B). However, no significant difference was observed in HDL-C levels for patients without AISC during R-CHOP chemotherapy (figure 3C). We conducted correlation analysis separately for the change in HDL-C and GLS from baseline to after three cycles of chemotherapy, from baseline to after six cycles of chemotherapy and from after three cycles to after six cycles of chemotherapy. However, we found no statistically significant differences in the associations between changes in HDL-C and GLS (p=0.965, 0.087, 0.449).
Figure 3.
(A) Changes in high-density lipoprotein cholesterol (HDL-C) in all patients from baseline to the end of the sixth cycle of chemotherapy. (B) Changes in HDL-C in patients with anthracycline-induced subclinical cardiotoxicity (AISC). (C) Changes in HDL-C in patients without AISC.
Contrasting values of HDL-C parameters between patients with and without AISC
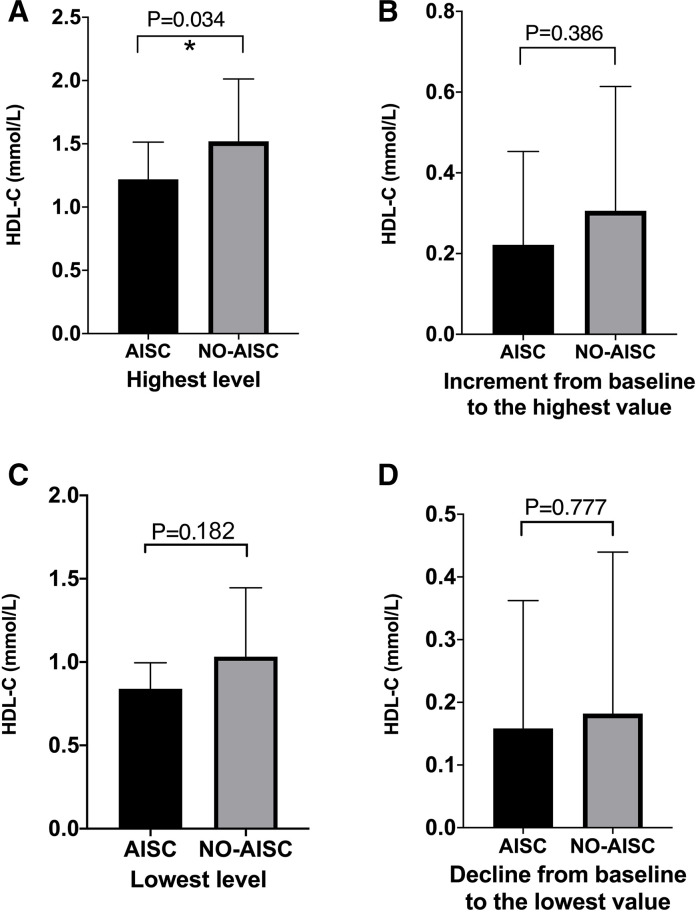
Figure 4 presents the contrasting values between patients with AISC and those without in terms of four parameters, namely the highest and lowest levels of HDL-C during chemotherapy, the increment and decline in HDL-C values from baseline. Patients without AISC showed significantly higher values in the highest level of HDL-C (1.52±0.49 vs 1.22±0.29, p=0.034, figure 4A). However, no significant differences were observed between the two groups in terms of HDL-C increment from baseline to the highest value (0.31±0.31 vs 0.22±0.23, p=0.386, figure 4B). While the lowest level of HDL-C was lower in patients with AISC, the difference was not statistically significant (0.84±0.16 vs 1.03±0.41, p=0.182, figure 4C). Furthermore, there were no significant differences in HDL-C decline between patients with AISC and those without (0.16±0.20 vs 0.18±0.26, p=0.777, figure 4D).
Figure 4.
High-density lipoprotein cholesterol (HDL-C) differences between anthracycline-induced subclinical cardiotoxicity (AISC) and No-AISC. (A) Highest level of HDL-C during chemotherapy. (B) The HDL-C value increment from baseline to the highest value. (C) The lowest level of HDL-C during chemotherapy. (D) The HDL-C value declined from baseline to the lowest.
Discussion
This prospective observational study investigated the relationship between HDL-C and incidence of AISC in 70 patients with DLBCL who were receiving anthracycline-containing chemotherapy. The study found that higher levels of HDL-C were associated with a lower incidence of AISC. Moreover, patients without AISC had more stable and higher levels of HDL-C than those with AISC during the follow-up period. The results also showed that HDL-C levels significantly decreased from the end of the third cycle of chemotherapy to the end of the sixth cycle of chemotherapy in all patients, especially in the AISC group, indicating that anthracycline-containing chemotherapy has adverse effects on HDL-C levels. Notably, the highest level of HDL-C was significantly higher in patients without AISC compared with those with AISC. These findings suggest that HDL-C may have a protective role against AISC in patients with DLBCL undergoing anthracycline-containing chemotherapy, and maintaining a relatively high level of HDL-C may be more effective in managing cardioprotection than monitoring changes in HDL-C levels over time. The results of this study highlight the importance of early serum lipid management in these patients.
Lipoproteins are classified into five categories, namely chylomicron, very-low-density lipoprotein, intermediate-density lipoprotein, low-density lipoprotein and HDL, based on their size, density and lipid composition (cholesterol and TG).21 Among these, HDL exhibits distinctive cytoprotective actions and triggers antioxidative, anti-inflammatory and antiapoptotic effects. The protective roles of HDL in cardiovascular disease have been controversial in recent years, and that the quality of HDL (cholesterol efflux capacity, antioxidant activity, anti-inflammatory activity, endothelial function, etc) rather than the quantity of HDL has been proposed as the true cardioprotective effect. The Framingham Heart Study, as early as 1988, reported a correlation between HDL-C and cardiovascular mortality.22 Recent studies have challenged the HDL-C hypothesis by revealing that HDL-C level is not inversely correlated with cardiovascular diseases.23 24 In our study, we used the baseline HDL-C level as a surrogate marker for HDL quantity, but we did not directly measure the quality of HDL. Measuring the level of HDL-C in serum is a commonly used method to assess the effect of HDL on cardiovascular health. HDL facilitates the transportation of cholesterol from the body tissues back to the liver, and higher levels of HDL-C are generally associated with a lower risk of heart disease. Nevertheless, it is crucial to note that HDL-C levels may not accurately reflect the functional properties of HDL. ApoA1, the most abundant protein in HDL, is associated with several beneficial effects of HDL.15 25 The function and abundance of ApoA1 are reported to play a dominant role in HDL quality.26 In the context of AIC, several studies have indicated that HDL can protect against anthracycline-induced cardiomyocyte apoptosis and atrophy in isolated cardiomyocytes27 28 and animal models.16 28 Based on these earlier trials, HDL-C and ApoA1 could serve as protective factors against anthracycline-related cardiovascular disease. The case–control study conducted by our team, using the same database, demonstrated that both HDL-C and ApoA1 act as predictive factors in patients undergoing three cycles of anthracycline-containing chemotherapy.18 Both the present study and the case–control study are derived from a registered research (ChiCTR2100054721) as mentioned before. The objective of this registered study was to explore the correlation between cardiotoxicity occurrence and lymphoma, antitumour drugs and cardiovascular risk factors. This registered study included patients with clinically diagnosed lymphoma who were evaluated by a haematologist as requiring chemotherapy. The study collected the demographic data and clinical variables of enrolled patients. Enrolled patients underwent conventional echocardiography, 2D speckle tracking echocardiography and blood sampling at baseline, the end of the third cycle of chemotherapy, the end of the sixth cycle of chemotherapy and 6 and 12 months after completing chemotherapy. In our case–control study, according to the changes in GLS at baseline and after the third cycle of chemotherapy, patients were divided into the AISC and No-AISC groups. Then demographic data, clinical variables and biochemical variables were compared between the two groups. In contrast to the current study, this case–control study specifically included patients with DLBCL who received the standard dose of (R)-CHOP chemotherapy regimen (CHOP with or without rituximab combination), and patients undergoing lipid-lowering therapy were not excluded. The aim of this case–control study was to analyse the influencing factors of AISC in patients with DLBCL treated with three cycles of (R)-CHOP chemotherapy regimen, and results indicated that both HDL-C and ApoA1 act as predictive factors against AISC. However, our present study did not focus on the investigation of the impact of ApoA1 on AISC. Even when ApoA1 was included in the Cox regression model, no significant association with AISC in patients with DLBCL treated with R-CHOP was observed (p>0.05, data not shown), probably because the number of events in our study was insufficient to support a robust ApoA1 analysis. Therefore, the role of ApoA1, the most abundant protein in HDL, in the context of AISC warrants further investigation in future research.
As far as we know, few clinical studies have investigated the association between HDL-C and AISC. This study is the first clinical research that uses the IC-OS consensus statement12 to define subclinical cardiotoxicity, with univariate and multivariable analyses being used to identify the influential factors of AISC in patients with DLBCL in this cohort. Kaplan-Meier methods and log-rank tests reveal that patients with high HDL-C levels were less likely to develop AISC. After subjecting it to univariate and multivariable Cox regression methods, high HDL-C levels still showed statistically significant differences. These results suggest that high HDL-C could be a potentially independent protective factor for AISC in patients with DLBCL and provide an opportunity for investigators to develop a tool for early intervention and prevention of AISC. Further research is necessary to confirm our findings.
Several studies have demonstrated that serum lipid levels are altered during anthracycline-containing chemotherapy in patients with cancer.29 30 Huxley et al and Averina et al have shown that imbalanced serum lipid distribution is a risk factor for cardiovascular disease.31 32 As a result, anthracycline-containing treatment can induce dyslipidaemia and facilitate the occurrence and development of cardiovascular diseases in patients with cancer. In a study of 394 patients with breast cancer, Li et al found that HDL-C levels after chemotherapy were significantly lower than those before chemotherapy.33 Similarly, Lu et al and Kalábová et al found that HDL-C levels significantly decreased during anthracycline-containing chemotherapy in patients with breast cancer.34 35 In our study, we specifically assessed the changes in HDL-C levels over time during follow-up. Except for the group of patients who experienced AISC at 12 months after treatment completion, HDL-C levels in all other groups increased from baseline to the third cycle of chemotherapy. This phenomenon may be due to the fact that antitumour drugs require cholesterol to cross cell membranes.36 However, HDL-C levels significantly decreased from the end of the third cycle of chemotherapy to the end of the sixth cycle of chemotherapy in all patients, especially in the AISC group, which is consistent with previous research results,33–35 and further confirmed that anthracycline-containing chemotherapy has adverse effects on HDL-C levels in patients with DLBCL. The HDL-C level in patients without AISC was more stable than that in patients with AISC. Therefore, anthracycline-containing chemotherapy may promote the occurrence and development of cardiotoxicity in patients with DLBCL by inducing HDL-C turbulence.
Besides, the findings of our study indicate a significant decrease in GLS during the chemotherapy period in patients with AISC. This result is consistent with previous research, which has reported that doxorubicin dose at the range of 100–150 mg/m2 can cause cardiotoxicity.37 Notably, we also observed that GLS remained stable after completion of chemotherapy, suggesting that the cardiac effects of anthracycline-based chemotherapy may be dose related. These findings have important implications for the monitoring and management of cardiotoxicity in patients undergoing anthracycline-based chemotherapy, as early detection of cardiac dysfunction during treatment may improve patient outcomes. We investigated the associations of change in HDL-C with change in GLS; no statistically significant differences were found.
The analysis of HDL-C levels should consider the changes over time and the absolute values. In our study, patients without AISC had significantly higher absolute HDL-C levels than those with AISC, while the lowest absolute HDL-C levels did not differ significantly between the two groups. The alterations from HDL-C extremes to baseline did not exhibit any variation between the groups either. This suggests that the highest absolute HDL-C value was a preferable indicator of AISC protection than the change in HDL-C from baseline to the extremum value. Maintaining a relatively high level of HDL-C may be more effective in managing the cardioprotection of anthracycline-treated patients with cancer than monitoring changes in HDL-C levels over time.
In our investigation, we observed that among the four patients with pre-existing hypertension, one patient experienced AISC during the follow-up (online supplemental table 3). Multivariable Cox regression analysis showed that hypertension did not have a significant impact on AISC (p>0.05). Hypertension, a common risk factor for both cancer and cardiovascular diseases, was also recognised as a risk factor for cardiotoxicity. Studies have reported that pre-existing hypertension was associated with anthracycline and trastuzumab-induced LVEF decline in a retrospective study38 and early left ventricular systolic dysfunction in patients with lymphoma receiving (R)-CHOP in a prospective study.39 We noted that all patients with hypertension in our study were under a single antihypertensive drug regimen (beta blockers or ACE inhibitor/angiotensin receptor blocker (ACEI/ARB)) to manage their blood pressure. Two meta-analyses have demonstrated that beta blockers and ACEI can prevent cardiotoxicity caused by chemotherapy.40 41 We speculate that the protective effects of beta blockers and ACEI/ARB may have contributed to the result observed in our study regarding the relationship between hypertension and AISC.
There are several limitations to our study that must be acknowledged. First, while our study highlights the potential importance of HDL-C in managing AISC, additional studies are necessary to fully evaluate the benefits and limitations of HDL-C as a cardioprotective strategy in anthracycline-treated patients with cancer. Second, this is a single-centre prospective observational study with a medium sample size. To confirm our findings, a larger sample size study conducted at multiple centres is needed. Third, previous studies have suggested that there may be a reversed U-shaped relationship between HDL-C levels and cardiovascular diseases.42 Due to the small sample size of this study, we did not further investigate the influence of extremely high levels of HDL-C on cardiotoxicity, and further clinical studies should be done to verify it. Besides, the measurement of GLS was only taken at baseline and at several points throughout the chemotherapy treatment and follow-up period. It is crucial to extend the duration of follow-up in future research to obtain a more comprehensive understanding of the long-term effects of anthracycline treatment on cardiovascular health.
Conclusions
In conclusion, our prospective observational study suggests that higher levels of HDL-C may be associated with a lower risk of AISC in patients with DLBCL treated with R-CHOP chemotherapy. HDL-C levels remained stable and consistently higher in patients without AISC compared with those with AISC. Additionally, the highest absolute HDL-C value was found to be a preferable indicator of AISC protection. These findings suggest that HDL-C may be a potential cardioprotective target for managing AISC in this patient population. However, further research is needed to confirm and expand on these findings, including determining the optimal HDL-C level for cardioprotection and the potential benefits of early serum lipid management.
Supplementary Material
Acknowledgments
The authors thank all the nurses, other medical workers and patients' families who assisted in this study.
Footnotes
QD and XT contributed equally.
Contributors: Conceptualisation: WO, TJ, NZ, KL, YW, XZ, DW, QD, XT. Data curation: WO, TJ, YW, XZ. Formal analysis: WO, TJ. Funding acquisition: WO, NZ, DW, XT. Investigation: DW, QD, XT. Methodology: WO, TJ, NZ, KL, YW, XZ, DW, QD, XT. Resources: DW, QD, XT. Supervision: QD, XT. Validation: NZ, KL. Visualisation: WO, TJ. Writing—original draft: WO. Writing—review and editing: WO, TJ, NZ, KL, YW, XZ, DW, QD, XT. QD and XT contributed equally to this work and are considered as co-corresponding authors. XT is responsible for the overall content as the guarantor.
Funding: This research was funded by the Science-Health Joint Medical Scientific Research Project of Chongqing ‘Study of antineoplastic drugs induced early cardiotoxicity in patients with Lymphoma’ (grant number: 2021MSXM276), the Natural Science Foundation of Chongqing ‘The mechanism of doxorubicin promoting atherosclerosis in lymphoma patients through NF-κB/miR-33 signaling pathway’ (grant number: cstc2019jcyj-msxmX0043), the Science-Health Joint Medical Scientific Research Project of Chongqing ‘Evaluation of cardiac function in patients with anthracycline chemotherapy by a new strain imaging technique’ (grant number: 2019ZDXM033) and the Chongqing Postgraduate Scientific Research and Innovation Project ‘Study on the role of high-density lipoprotein in anthracycline-induced subclinical cardiotoxicity in patients with lymphoma’ (grant number: CYS22340).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
This study involves human participants and was approved by the ethics committee of the First Affiliated Hospital of Chongqing Medical University (approval number: 2018-016) and conducted in accordance with the Declaration of Helsinki. Participants gave informed consent to participate in the study before taking part.
References
- 1.Rosen MR, Myerburg RJ, Francis DP, et al. Translating stem cell research to cardiac disease therapies: pitfalls and prospects for improvement. J Am Coll Cardiol 2014;64:922–37. 10.1016/j.jacc.2014.06.1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minotti G, Menna P, Salvatorelli E, et al. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 2004;56:185–229. 10.1124/pr.56.2.6 [DOI] [PubMed] [Google Scholar]
- 3.Hasin T, Gerber Y, McNallan SM, et al. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol 2013;62:881–6. 10.1016/j.jacc.2013.04.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Qadir H, Austin PC, Lee DS, et al. A Population-Based Study of Cardiovascular Mortality Following Early-Stage Breast Cancer. JAMA Cardiol 2017;2:88–93. 10.1001/jamacardio.2016.3841 [DOI] [PubMed] [Google Scholar]
- 5.Stoltzfus KC, Zhang Y, Sturgeon K, et al. Fatal heart disease among cancer patients. Nat Commun 2020;11:2011. 10.1038/s41467-020-15639-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thandra KC, Barsouk A, Saginala K, et al. Epidemiology of Non-Hodgkin’s Lymphoma. Medical Sciences;9:5. 10.3390/medsci9010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 2016;66:443–59. 10.3322/caac.21357 [DOI] [PubMed] [Google Scholar]
- 8.Salles G, Barrett M, Foà R, et al. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv Ther 2017;34:2232–73. 10.1007/s12325-017-0612-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGowan JV, Chung R, Maulik A, et al. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc Drugs Ther 2017;31:63–75. 10.1007/s10557-016-6711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell RR, Alexander J, Jain D, et al. The role and clinical effectiveness of multimodality imaging in the management of cardiac complications of cancer and cancer therapy. J Nucl Cardiol 2016;23:856–84. 10.1007/s12350-016-0538-8 [DOI] [PubMed] [Google Scholar]
- 11.Curigliano G, Lenihan D, Fradley M, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 2020;31:171–90. 10.1016/j.annonc.2019.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrmann J, Lenihan D, Armenian S, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J 2022;43:280–99. 10.1093/eurheartj/ehab674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fallah-Rad N, Walker JR, Wassef A, et al. The Utility of Cardiac Biomarkers, Tissue Velocity and Strain Imaging, and Cardiac Magnetic Resonance Imaging in Predicting Early Left Ventricular Dysfunction in Patients With Human Epidermal Growth Factor Receptor II–Positive Breast Cancer Treated With Adjuvant Trastuzumab Therapy. Journal of the American College of Cardiology 2011;57:2263–70. 10.1016/j.jacc.2010.11.063 [DOI] [PubMed] [Google Scholar]
- 14.Negishi K, Negishi T, Haluska BA, et al. Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. Eur Heart J Cardiovasc Imaging 2014;15:324–31. 10.1093/ehjci/jet159 [DOI] [PubMed] [Google Scholar]
- 15.Kluck GEG, Durham KK, Yoo JA, et al. High Density Lipoprotein and Its Precursor Protein Apolipoprotein A1 as Potential Therapeutics to Prevent Anthracycline Associated Cardiotoxicity. Front Cardiovasc Med 2020;7:65. 10.3389/fcvm.2020.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durham KK, Chathely KM, Mak KC, et al. HDL protects against doxorubicin-induced cardiotoxicity in a scavenger receptor class B type 1-, PI3K-, and Akt-dependent manner. Am J Physiol Heart Circ Physiol 2018;314:H31–44. 10.1152/ajpheart.00521.2016 [DOI] [PubMed] [Google Scholar]
- 17.Van Linthout S, Frias M, Singh N, et al. Therapeutic potential of HDL in cardioprotection and tissue repair. Handb Exp Pharmacol 2015;224:527–65. 10.1007/978-3-319-09665-0_17 [DOI] [PubMed] [Google Scholar]
- 18.Dong Q, Ou W, Wang M, et al. Study on influencing factors of anthracycline-induced subclinical cardiotoxicity in DLBCL patients administered (R)-CHOP. BMC Cancer 2022;22:988. 10.1186/s12885-022-10085-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97. 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 20.MA K, J S. Sample size calculators [Website]: UCSF CTSI. 2021. Available: https://www.sample-size.net/
- 21.Mahley RW, Innerarity TL, Rall SC, et al. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res 1984;25:1277–94. [PubMed] [Google Scholar]
- 22.Wilson PW, Abbott RD, Castelli WP. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis 1988;8:737–41. 10.1161/01.atv.8.6.737 [DOI] [PubMed] [Google Scholar]
- 23.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012;367:2089–99. 10.1056/NEJMoa1206797 [DOI] [PubMed] [Google Scholar]
- 24.Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N Engl J Med 2017;376:1933–42. 10.1056/NEJMoa1609581 [DOI] [PubMed] [Google Scholar]
- 25.Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest 2006;116:3090–100. 10.1172/JCI30163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith JD. Dysfunctional HDL as a diagnostic and therapeutic target. Arterioscler Thromb Vasc Biol 2010;30:151–5. 10.1161/ATVBAHA.108.179226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brinck JW, Thomas A, Brulhart-Meynet M-C, et al. High-density lipoprotein from end-stage renal disease patients exhibits superior cardioprotection and increase in sphingosine-1-phosphate. Eur J Clin Invest 2018;48. 10.1111/eci.12866 [DOI] [PubMed] [Google Scholar]
- 28.Durham KK, Kluck G, Mak KC, et al. Treatment with apolipoprotein A1 protects mice against doxorubicin-induced cardiotoxicity in a scavenger receptor class B, type I-dependent manner. Am J Physiol Heart Circ Physiol 2019;316:H1447–57. 10.1152/ajpheart.00432.2018 [DOI] [PubMed] [Google Scholar]
- 29.Bicakli DH, Varol U, Degirmenci M, et al. Adjuvant chemotherapy may contribute to an increased risk for metabolic syndrome in patients with breast cancer. J Oncol Pharm Pract 2016;22:46–53. 10.1177/1078155214551315 [DOI] [PubMed] [Google Scholar]
- 30.Rzymowska J. Effect of cytotoxic chemotherapy on serum lipid levels in breast cancer patients. Pathobiology 1999;67:129–32. 10.1159/000028062 [DOI] [PubMed] [Google Scholar]
- 31.Huxley R, Lewington S, Clarke R. Cholesterol, coronary heart disease and stroke: a review of published evidence from observational studies and randomized controlled trials. Semin Vasc Med 2002;2:315–23. 10.1055/s-2002-35402 [DOI] [PubMed] [Google Scholar]
- 32.Averina M, Nilssen O, Brenn T, et al. Factors behind the increase in cardiovascular mortality in Russia: apolipoprotein AI and B distribution in the Arkhangelsk study 2000. Clin Chem 2004;50:346–54. 10.1373/clinchem.2003.023853 [DOI] [PubMed] [Google Scholar]
- 33.Li X, Liu Z-L, Wu Y-T, et al. Status of lipid and lipoprotein in female breast cancer patients at initial diagnosis and during chemotherapy. Lipids Health Dis 2018;17:91. 10.1186/s12944-018-0745-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalábová H, Melichar B, Ungermann L, et al. Intima-media thickness, myocardial perfusion and laboratory risk factors of atherosclerosis in patients with breast cancer treated with anthracycline-based chemotherapy. Med Oncol 2011;28:1281–7. 10.1007/s12032-010-9593-1 [DOI] [PubMed] [Google Scholar]
- 35.Lu Q, Wu X, Zhu Y, et al. Effects of Chemotherapy on Serum Lipids in Chinese Postoperative Breast Cancer Patients. Cancer Manag Res 2020;12:8397–408. 10.2147/CMAR.S253397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Bennett WFD, Zheng T, et al. Effect of Cholesterol on Cellular Uptake of Cancer Drugs Pirarubicin and Ellipticine. J Phys Chem B 2016;120:3148–56. 10.1021/acs.jpcb.5b12337 [DOI] [PubMed] [Google Scholar]
- 37.Blanco JG, Sun C-L, Landier W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes--a report from the Children’s Oncology Group. J Clin Oncol 2012;30:1415–21. 10.1200/JCO.2011.34.8987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamirani Y, Fanous I, Kramer CM, et al. Anthracycline- and trastuzumab-induced cardiotoxicity: a retrospective study. Med Oncol 2016;33:82. 10.1007/s12032-016-0797-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szmit S, Jurczak W, Zaucha JM, et al. Pre-existing arterial hypertension as a risk factor for early left ventricular systolic dysfunction following (R)-CHOP chemotherapy in patients with lymphoma. J Am Soc Hypertens 2014;8:791–9. 10.1016/j.jash.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 40.Shah P, Garris R, Abboud R, et al. Meta-Analysis Comparing Usefulness of Beta Blockers to Preserve Left Ventricular Function During Anthracycline Therapy. The American Journal of Cardiology 2019;124:789–94. 10.1016/j.amjcard.2019.05.046 [DOI] [PubMed] [Google Scholar]
- 41.Dong H, Yao L, Wang M, et al. Can ACEI/ARB prevent the cardiotoxicity caused by chemotherapy in early-stage breast cancer?-a meta-analysis of randomized controlled trials. Transl Cancer Res 2020;9:7034–43. 10.21037/tcr-20-1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng M, Darabi M, Tubeuf E, et al. Free cholesterol transfer to high-density lipoprotein (HDL) upon triglyceride lipolysis underlies the U-shape relationship between HDL-cholesterol and cardiovascular disease. Eur J Prev Cardiol 2020;27:1606–16. 10.1177/2047487319894114 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-074541supp003.pdf (112.1KB, pdf)
bmjopen-2023-074541supp001.pdf (152.1KB, pdf)
bmjopen-2023-074541supp002.pdf (73.8KB, pdf)
bmjopen-2023-074541supp004.pdf (85.4KB, pdf)
bmjopen-2023-074541supp005.pdf (117.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request.