Abstract
Background
Arthritis is thought to be closely related to serum uric acid. The study aims to assess the association between asymptomatic hyperuricemia (AH) and arthritis.
Methods
A multistage, stratified cluster was used to conduct a cross-sectional study of adult US civilians aged≥20 years from the 2007–2018 National Health and Nutrition Examination Survey. Participants with hyperuricemia and without hyperuricemia prior to gout were included. A questionnaire was used to determine whether participants had arthritis and the type of arthritis. Logistic regression was used to investigate the association between hyperuricemia and arthritis.
Result
During the past 12 years, the percentage of participants with arthritis changed from 25.95% (22.53%–29.36%) to 25.53% (21.62%–29.44%). The prevalence of osteoarthritis (OA) increased from 8.70% (95% CI: 6.56% to 10.85%) to 12.44% (95% CI: 9.32% to 15.55%), the prevalence of AH changed from 16.35% (95% CI: 14.01% to 18.40%) to 16.39% (95% CI: 13.47% to 19.30%). Participants with AH were associated with onset of arthritis (OR=1.34, 95% CI: 1.07 to 1.69), but the association was muted after adjusting demographic and socioeconomic factors. For participants aged 40–49 years, AH is associated with incident arthritis (OR=1.96, 95% CI: 1.23 to 2.99) and the relationship remained after adjusting for education level, income to poverty ratio, body mass index, diabetes, hypertension and smoking (OR=2.00, 95% CI: 1.94 to 3.36). Compared with male, female participants with AH are more likely to develop arthritis, especially in OA (OR=1.35, 95% CI: 1.14 to 1.60).
Conclusion
Our data identified AH as the risk factor for incident arthritis, especially for OA, which might be exaggerated in aged population and female population.
Keywords: Rheumatology, Knee, Risk management
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The study comprehensively assessed the association between asymptomatic hyperuricemia (AH) and arthritis in the US population aged≥20 years during 2007–2018.
The respondents were intricately weighted, and adjustments were made for different covariates to reduce the likelihood of interference from confounding factors.
Due to the observational study design, future randomised control study or longitudinal study should be conducted to validate the causal relationship between AH and onset of arthritis other than gouty arthritis.
Our findings report trends through 2007–2018, it will be important to continue to examine how the COVID-19 pandemic has potentially influenced such trends when data from 2019 and 2022 become available.
Introduction
More than one in five adults in the USA had doctor-diagnosed arthritis, and arthritis-attributable activity limitations significantly increased over time independent of the population ageing.1 By 2040, the adults with doctor-diagnosed arthritis are projected to increase 49% to 78.4 million (1 in 4 US adults), and the arthritis-attributable activity limitation will increase 52% to 34.6 million (1 in 9 adults).2 High medical care expenditures and earnings losses attributable to arthritis signalling the need for identification of disease and risk factors that are in most need for interventions.3 Osteoarthritis (OA) as the most common form of arthritis, involves structural changes in the articular cartilage, subchondral bones, ligaments, bursae, synovium and muscles surrounding the joint.4 From 1990 to 2019, the global age standardised incidence rate of OA increased from 474 to 492 per 100 000 population and expected to increase due to global population ageing.5 6 About 20% of the general population affected by hyperuricemia, which might be more prominent in male and aged population.7 Prior research has consistently shown a significant correlation between arthritis, particularly OA and rheumatoid arthritis (RA), and hypertension.8 The intricate relationship between metabolic processes and arthritis, alongside the interplay between metabolic and immunological factors, is garnering heightened attention. Metabolic syndrome’s implication in various forms of arthritis, such as OA, is increasingly recognised.9 10
In 2007–2016, the prevalence of hyperuricemia, gout and the urate-lowering therapy among patients with gout remained stable.11 The true significance of asymptomatic hyperuricemia (AH) as a risk factor for incident gout becomes apparent when considering that only half of patients with longstanding hyperuricemia develop clinically evident gout over a 15-year period.12 13 Advanced imaging, including ultrasonography or dual-energy CT, demonstrated approximately 15%–40% of patients with chronic hyperuricemia have silent monosodium urate crystal deposition.14 As the crystallisation of monosodium urate marks the progression of hyperuricemia towards gout, it remains uncertain whether hyperuricemia contributes to other forms of arthritis.15
Both hyperuricemia and OA are influenced by common risk factors such as obesity and ageing. This shared relationship between risk factors suggests a potential connection between the hyperuricemia and OA, with intra-articular urate contributing to crystallisation and cartilage disruption in the context of these shared risk factors.16 The predilection for both OA and gout occur in the same joints strongly suggest that OA may predispose to the localised deposition of monosodium urate crystals, which influence structural joint damage.17–19 Monosodium urate crystals have been shown to inhibit the viability and function of human chondrocytes in vitro with a dose–dependent manner.20 Death of chondrocytes can lead to an increase in urate, which may even promote crystal deposition on the cartilage, further aggravating OA progression.16 Monosodium urate crystals inhibit osteocyte viability and, through interactions with macrophages, indirectly promote a shift in osteocyte function that favours bone resorption and inflammation.21 Uric acid is a danger signal of increasing risk of OA through inflammasome activation.22 Therefore, we hypothesised that hyperuricemia prior to gout was associated with OA. The aim of this study was to (1) ascertain the association between AH and arthritis, (2) determine the association between AH and OA and (3) investigate the effect of age and gender on such association.
Patients and methods
Patient and public involvement
The National Health and Nutrition Examination Survey (NHANES) is an ongoing longitudinal survey conducted by the National Center for Health Statistics (NCHS) to assess the health and nutritional status of the USA through a series of interviews and examination items. The NHANES is conducted biennially in a nationally representative, non-institutionalised civilian population and use a hierarchical multistage probabilistic clustering design to select a representative sample of over-sampled participants. The sampling methods and examination information used in this study have been described in detail elsewhere.23
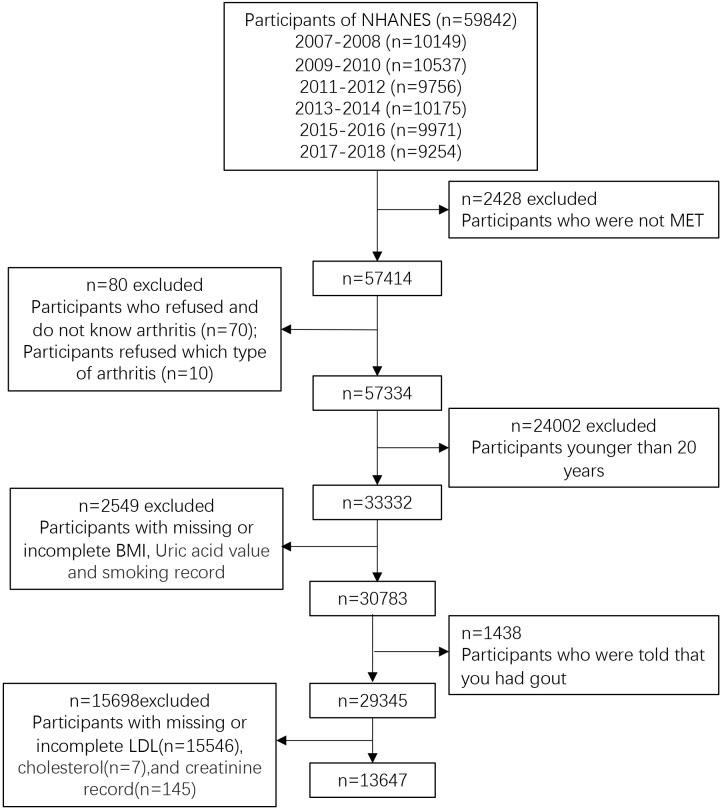
The study used data from NHANES database for the 2007–2018 study cycle (n=59 842) and excluded those who did not participate in the examination (n=2428). We excluded participants who refused and do not know ever had or had not arthritis, refused to answer which type of arthritis (n=80), who are younger than 20 years old (n=24 002), who have missing and incomplete body mass index (BMI), uric value and smoking record (n=2549). We also excluded participants who were told that you had gout (n=1438) and participants with missing or incomplete low-density lipoprotein (LDL), cholesterol and creatinine record. In the end, this study consisted of 13 647 eligible participants (figure 1), which is representative of the population size of 87 901 487.
Figure 1.
Flow chart of sample selection from the NHANES 2007–2018. MET, mobile examination center test; BMI, body mass index; LDL, low-density lipoprotein; NHANES, The National Health and Nutrition Examination Survey.
Conditions of arthritis
The status of arthritis was classified using questionnaires. Participants aged 20 years and older were asked ‘Has a doctor or other health professional ever said that you had arthritis?’. If the participants gave a positive answer, they were further asked ‘Which type of arthritis was it?’. Participants’ responses included RA, OA, other do not know type and refuse to answer. Individuals were excluded from the current analysis if their self-reported type of arthritis declined to answer. A consistent relationship between self-reports of arthritis and a clinical diagnosis of arthritis has been demonstrated in previous reports.24
Hyperuricemia
Hyperuricemia is an elevated level of uric acid in the blood. The normal upper limit for serum uric acid (SUA) at physiological levels is 6.8 mg/dL. This is the saturation point at which urate may precipitate under physiological conditions.25 26 Hyperuricemia is defined as SUA> 6.8 mg/dL, while the normal state is defined as SUA≤ 6.8 mg/dL.
Covariates
Covariates are identified in statistical models by means of interview responses and examinations. Covariates that could confound the association between OA and AH were selected based on the results of interviews and examinations in the NHANES database. These factors were chosen to screen for variables that might be associated with OA risk and/or could be associated with AH. This selection aimed to minimise potential confounding variables in the association between OA and AH. The chosen covariates included self-reported demographic characteristics, such as gender, age, race, education level, BMI, blood pressure, poverty income ratio (PIR), smoking, physical activity level (PAL) and diabetes.
Age is divided into seven groups: 20–29, 30–39, 40–49, 50–59, 60–69 and 70+ years. Race is divided into four groups: non-Hispanic white, non-Hispanic black, Hispanic and other races. Education is grouped as high school or below, some college and college graduate or above. BMI is calculated from measured weight and height determined by standard NHANES protocols.27 BMI is categorised as three groups: normal (<18.5 kg/m2), overweight (18.5–24.9 kg/m2) and obesity (≥25 kg/m2). Participants with systolic blood pressure≥130 mm Hg or diastolic blood pressure≥80 mm Hg are defined as hypertension.28 PIR as a socioeconomic indicator is stratified into three levels: low income (PIR<1.3), middle income (1.3≤PIR<3.5) and high income (PIR≥3.5).
Smoking status is categorised according to interview results as current (smoked more than 100 cigarettes in the lifetime and currently still smoked), before (smoked more than 100 cigarettes in the lifetime but did not currently smoke) and never (smoked less than 100 cigarettes in the lifetime). PAL is divided into two categories, moderate activity, which includes moderate work activity, walking or cycling, moderate recreational activity and vigorous activity, which includes vigorous work activity and vigorous recreational activity. Participants with self-reported diabetes had either a diabetes physician’s diagnosis of diabetes or an elevated fasting plasma glucose level or an elevated oral glucose tolerance, or/and glycated haemoglobin≥6.5%. Laboratory data included cholesterol, LDL, High-density lipoprotein (HDL), triglycerides, creatinine and albumin.
Statistical analysis
Design factors involving complex weighting, clustering and stratification in the NHANES database. Statistical analysis was conducted using STATA (V.16). Complex stratification designs were considered using appropriate sample weights in accordance with NHANES analytical reporting guidelines. In baseline study characteristics, means and SEs were used for continuous variables. Categorical variables were expressed as numbers and percentages. Χ2 test and t-test were used for categorical and continuous variables, respectively. A weighted logistic regression was used to assess the association between OA and AH and to control for confounding factors. Finally, subgroup analysis was performed using hierarchical multivariate regression. The 95% CIs and p values were calculated. A two-tailed test with p values less than 0.05 are considered significant.
Results
The characteristics of study participants
A total of 13 647 participants were eligible and included in the analysis from 2007–2008 to 2017–2018 (online supplemental sTable 1). Between 2007–2008 and 2017–2018, the proportion of participants in the 60–69 age group increased from 11.44% (95% CI: 9.42% to 13.46%) to 14.81% (95% CI: 11.45% to 18.16%). In addition, the proportion of Hispanics increased from 5.09% (95% CI: 2.60% to 7.58%) to 6.86% (95% CI: 5.00% to 8.72%), while the proportion of non-Hispanic whites decreased from 70.37% (95% CI: 63.63% to 77.11%) to 61.96% (95% CI: 57.22% to 66.69%). Between 2007–2008 and 2017–2018, the proportion of high school or below decreased, which is from 43.02% (95% CI: 37.88% to 48.16%) to 39.61% (95% CI: 36.03% to 43.19%), while the proportion of college graduate or above increased, which was from 28.53% (95% CI: 24.06% to 33.00%) to 30.47% (95% CI: 24.72% to 36.23%) (online supplemental sTable 1).
bmjopen-2023-074391supp001.pdf (120.2KB, pdf)
During the past 12 years, the percentage of participants with arthritis changed from 25.95% (22.53%–29.36%) to 25.53% (21.62%–29.44%). The prevalence of RA increased from 3.57% (95% CI: 2.87% to 4.27%) in 2007–2008 to 4.04% (95% CI: 2.82% to 5.25%) in 2017–2018, while the proportion of those who do not know arthritis decreased from 10.13% (95% CI: 8.22% to 12.05%) to 6.02% (95% CI: 4.66% to 7.37%). There was also a little decrease in other arthritis 3.54% (95% CI: 2.56% to 4.52%) and 3.04% (95% CI: 1.78% to 4.30%). The prevalence of OA showed a clear upward trend during the 12 years, from 8.70% (95% CI: 6.56% to 10.85%) in 2007–2008 to 12.44% (95% CI: 9.32% to 15.55%) in 2017–2018 (p<0.01) (online supplemental sTable 1).
The 50–59 age group displayed the highest percentage of individuals with AH (19.39% (95% CI: 17.17% to 21.61%)) among all the age groups that were examined. A larger proportion of men (77.17% (95% CI: 74.96% to 79.37%)) had AH compared with women (22.83% (95% CI: 20.63% to 25.04%)) (table 1). There are significant differences in race between participants with and without AH (p<0.01). Participants in the AH group had higher levels of obesity, hypertension, diabetes, LDL, triglycerides and creatinine than those in the normal state group (table 1).
Table 1.
Baseline characteristics of high uric acid group versus the normal state group
| Characteristics | Normal state | AH | P value |
| N* | 11 387 | 2260 | |
| Gender | <0.01 | ||
| Male | 41.66 (40.55–42.76) | 77.17 (74.96–79.37) | |
| Female | 58.34 (57.24–59.45) | 22.83 (20.63–25.04) | |
| Age | <0.01 | ||
| 20–29 | 18.86 (17.56–20.15) | 17.30 (15.07–19.53) | |
| 30–39 | 18.03 (16.98–19.07) | 15.15 (12.83–17.48) | |
| 40–49 | 19.44 (18.21–20.67) | 17.39 (14.72–20.05) | |
| 50–59 | 19.05 (17.98–20.12) | 19.39 (17.17–21.61) | |
| 60–69 | 14.01 (12.95–15.07) | 15.66 (13.36–17.95) | |
| 70+ | 10.62 (9.91–11.32) | 15.11 (13.21–17.00) | |
| Race | <0.01 | ||
| Other races | 17.22 (15.50–18.93) | 14.33 (12.20–16.47) | |
| Hispanic | 6.32 (5.21–7.44) | 5.39(4.02–6.76) | |
| Non-Hispanic white | 66.30 (63.54–69.05) | 32 (64.74–71.91) | |
| Non-Hispanic black | 10.16 (8.80–11.53) | 11.96 (9.82–14.09) | |
| Education level | 0.137 | ||
| High school or below | 38.72 (36.58–40.86) | 40.33 (37.04–43.63) | |
| Some college | 30.44 (28.96–31.92) | 31.18 (28.20–34.17) | |
| College graduate or above | 30.84 (28.51–33.16) | 28.49 (25.66–31.31) | |
| BMI | <0.01 | ||
| Normal | 34.09 (32.60–35.58) | 13.41 (11.57–15.25) | |
| Overweight | 33.07 (32.07–34.08) | 33.41 (30.59–36.23) | |
| Obesity | 32.83 (31.48–34.19) | 53.18 (49.93–56.43) | |
| Blood pressure | <0.01 | ||
| Hypertension | 40.84 (39.20–42.49) | 59.45 (56.85–62.06) | |
| Normal | 59.16 (57.51–60.80) | 40.55 (37.94–43.15) | |
| PIR | 0.03 | ||
| Low income | 22.36 (20.66–24.06) | 18.98 (17.00–20.97) | |
| Middle income | 35.84 (34.22–37.47) | 36.73 (33.94–39.53) | |
| Elevated income | 41.79 (39.48–44.11) | 44.28 (40.87–47.69) | |
| Smoking | <0.01 | ||
| Current | 19.72 (18.32–21.11) | 17.51 (15.41–19.62) | |
| Before | 23.23 (21.86–24.60) | 31.37 (28.28–34.47) | |
| Never | 57.06 (55.32–58.79) | 51.11 (48.07–54.16) | |
| PAL | 0.744 | ||
| Moderate activities | 59.26 (57.72–60.81) | 58.20 (55.43–60.97) | |
| Vigorous activities | 40.74 (39.19–42.28) | 41.80 (39.03–44.57) | |
| Diabetes | <0.01 | ||
| Yes | 9.85 (8.99–10.70) | 15.68 (14.06–17.30) | |
| No | 90.15 (89.30–91.01) | 84.32 (82.70–85.94) | |
| Arthritis | <0.01 | ||
| No arthritis | 75.13 (73.67–76.59) | 73.29 (70.69–75.89) | |
| OA | 11.54 (10.55–12.53) | 11.40 (9.56–13.24) | |
| RA | 3.54 (3.11–3.97) | 4.62 (3.56–5.69) | |
| Other | 3.24 (2.69–3.79) | 3.14 (2.12–4.17) | |
| Unspecified | 6.55 (5.88–7.22) | 7.54 (6.03–9.05) | |
| Cholesterol (mg/dL)† | 191.01±40.22 | 192.23±41.65 | 0.1924 |
| LDL (mg/dL)† | 113.39±35.07 | 115.48±36.82 | 0.0088 |
| HDL (mg/dL)† | 55.58±15.89 | 48.87±15.10 | <0.01 |
| Triglycerides (mg/dL)† | 110.45±62.12 | 139.39±73.24 | <0.01 |
| Creatinine (mg/dL)† | 122.00±75.58 | 144.47±82.80 | <0.01 |
| Albumin (mg/dL)† | 35.52±320.96 | 97.29±565.77 | <0.01 |
*N represents unweighted number, and the remaining values are weighted values using The National Health and Nutrition Examination Survey mobile examination center weight.
†Figures are expressed as mean±SE, other figures are expressed as per cent (95% CIs).
AH, asymptomatic hyperuricemia (serum urate>6.8 mg/dL without gout); BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OA, osteoarthritis; PAL, physical activity level; PIR, poverty income ratio; RA, rheumatoid arthritis.
The prevalence of patients with OA and AH (11.40% (95% CI: 9.56% to 13.24%)) is considerably higher than that of other three types of arthritis (RA: 4.62% (95% CI: 3.56% to 5.69%), other: 3.14% (95% CI: 2.12% to 4.17%) and unspecified: 7.54% (95% CI: 6.03% to 9.05%)) (p<0.01) (table 1).
The characteristics of hyperuricemia and arthritis
The higher frequency of participants with arthritis, including OA, RA, other forms and those who were unaware of having arthritis, among individuals aged over 50 years, suggests that age may be a contributing factor to the prevalence of arthritis in this population (table 2). The characteristics of the 13 647 participants included in our study with self-reported OA, RA, other and unspecified are presented using weighted statistics (table 1). The prevalence of the four types of arthritis was higher among female participants than among male participants, which was most notable in OA (female: 65.94% vs male 34.06%) (table 2).
Table 2.
Baseline characteristics of arthritis group versus the non-arthritis group
| Characteristics | No arthritis | OA | RA | Other | Unspecified | P value |
| N* | 10 089 | 1402 | 662 | 408 | 1086 | |
| Gender | <0.01 | |||||
| Male | 50.40 (49.20–51.61) | 34.06 (31.15–6.97) | 40.89 (35.23–46.54) | 40.90 (34.65–47.15) | 42.48 (38.96–46.01) | |
| Female | 49.60 (48.39–50.80) | 65.94 (63.03–68.85) | 59.11 (53.46–64.77) | 59.10 (52.85–65.35) | 57.52 (53.99–61.04) | |
| Age | <0.01 | |||||
| 20–29 | 24.10 (22.69–25.52) | 1.15 (0.57–1.73) | 2.62 (0.30–4.94) | 3.98 (1.36–6.60) | 3.17 (1.79–4.55) | |
| 30–39 | 21.40 (20.16–22.64) | 5.15 (3.81–6.49) | 6.11 (3.79–8.43) | 9.40 (5.76–13.05) | 6.35 (4.47–8.24) | |
| 40–49 | 20.74 (19.35–22.14) | 11.11 (9.08–13.14) | 15.63 (11.52–19.73) | 22.38 (17.09–27.67) | 15.00 (11.89–18.10) | |
| 50–59 | 16.53 (15.39–17.66) | 25.56 (22.65–28.48) | 26.90 (20.60–33.20) | 29.34 (23.07–35.60) | 27.60 (23.49–31.71) | |
| 60–69 | 10.22 (9.19–11.25) | 29.91 (26.73–33.09) | 24.48 (19.59–29.36) | 21.26 (14.97–27.56) | 23.61 (20.04–27.19) | |
| 70+ | 7.01 (6.40–7.61) | 27.11 (24.11–30.12) | 24.26 (20.40–28.12) | 13.64 (9.48–17.79) | 24.27 (21.09–27.45) | |
| Race | <0.01 | |||||
| Other races | 18.98 (17.18–20.77) | 8.68 (6.70–10.65) | 15.21 (10.33–20.09) | 7.59 (4.42–10.75) | 11.13 (8.86–13.39) | |
| Hispanic | 6.83 (5.62–8.05) | 3.21 (2.36–4.06) | 5.11 (3.68–6.54) | 4.82 (2.82–6.81) | 5.14 (3.69–6.59) | |
| Non-Hispanic white | 63.36 (60.50–66.21) | 82.00 (79.15–84.86) | 63.62 (57.67–69.57) | 78.92 (73.97–83.87) | 72.33 (68.49–76.18) | |
| Non-Hispanic black | 10.83 (9.39–12.27) | 6.11 (4.65–7.57) | 16.06 (12.29–19.83) | 8.67 (5.89–11.46) | 11.40 (9.14–13.65) | |
| Education level | <0.01 | |||||
| High school or below | 37.86 (35.61–40.10) | 34.05 (30.26–37.84) | 52.13 (45.41–58.85) | 44.35 (38.06–50.64) | 50.09 (45.46–54.73) | |
| Some college | 29.76 (28.19–31.34) | 34.45 (31.27–37.18) | 32.25 (27.08–37.42) | 31.04 (24.03–38.06) | 31.61 (27.66–35.57) | |
| College graduate or above | 32.38 (30.03–34.73) | 31.50 (27.68–35.33) | 15.62 (9.94–21.30) | 24.61 (17.82–31.39) | 18.29 (14.06–22.52) | |
| BMI | <0.01 | |||||
| Normal | 33.47 (31.83–35.12) | 22.68 (19.75–25.60) | 27.88 (22.90–32.85) | 21.88 (16.37–27.40) | 20.66 (17.17–24.15) | |
| Overweight | 33.79 (32.56–35.03) | 32.36 (28.93–35.80) | 28.46 (23.46–33.46) | 29.37 (23.76–34.98) | 31.40 (27.98–34.83) | |
| Obesity | 32.74 (31.13–34.35) | 44.96 (41.35–48.57) | 43.66 (38.74–48.58) | 48.75 (42.23–55.26) | 47.93 (43.56–52.31) | |
| Blood pressure | <0.01 | |||||
| Hypertension | 36.92 (35.28–38.56) | 66.37 (62.74–70.00) | 63.74 (57.17–70.31) | 58.84 (51.88–65.81) | 63.77 (59.68–67.86) | |
| Normal | 63.08 (61.44–64.72) | 33.63 (30.00–37.26) | 36.26 (29.69–42.83) | 41.16 (34.19–48.12) | 36.23 (32.14–40.32) | |
| PIR | <0.01 | |||||
| Low income | 22.04 (20.42–23.65) | 16.37 (13.61–19.13) | 29.76 (23.51–36.01) | 22.45 (16.84–28.06) | 24.27 (19.78–28.76) | |
| Middle income | 35.83 (34.13–37.52) | 36.00 (32.49–39.50) | 36.22 (30.28–42.16) | 37.89 (30.75–45.02) | 36.74 (32.08–41.40) | |
| Elevated income | 42.14 (39.89–44.38) | 47.63 (43.03–52.24) | 34.02 (27.68–40.36) | 39.66 (31.66–47.67) | 38.98 (32.91–45.05) | |
| Smoking | <0.01 | |||||
| Current | 18.69 (17.41–19.97) | 18.21 (15.47–20.95) | 27.50 (22.36–32.63) | 27.31 (21.50–33.12) | 20.56 (17.17–23.95) | |
| Before | 21.77 (20.30–23.24) | 32.89 (29.41–36.36) | 33.14 (26.79–39.49) | 29.07 (23.55–34.59) | 34.03 (30.01–38.05) | |
| Never | 59.54 (57.76–61.32) | 48.90 (45.48–52.32) | 39.36 (33.15–45.58) | 43.62 (37.28–49.96) | 45.41 (41.18–49.65) | |
| PAL | <0.01 | |||||
| Moderate activities | 54.56 (53.24–55.88) | 73.88 (70.67–77.08) | 72.08 (66.44–77.72) | 67.01 (59.61–74.40) | 73.28 (68.99–77.58) | |
| Vigorous activities | 45.44 (44.12–46.76) | 26.12 (22.92–29.33) | 27.92 (22.28–33.56) | 32.99 (25.60–40.39) | 26.72 (22.42–31.01) | |
| Diabetes | <0.01 | |||||
| Yes | 8.50 (7.63–9.36) | 15.81 (13.36–18.27) | 22.68 (19.11–26.24) | 16.27 (10.90–21.63) | 18.40 (15.33–21.48) | |
| No | 91.51 (90.64–92.37) | 84.19 (81.73–86.64) | 77.32 (73.76–80.89) | 83.73 (78.37–89.10) | 81.60 (78.52–84.67) | |
| Uric acid | <0.01 | |||||
| AH | 84.35 (83.33–85.38) | 84.18 (81.78–86.59) | 80.11 (75.97–84.25) | 84.42 (79.68–89.17) | 82.04 (78.71–85.36) | |
| Normal state | 15.65 (14.62–16.67) | 15.82 (13.41–18.22) | 19.88 (15.75–24.03) | 15.58 (10.83–20.32) | 17.96 (14.64–21.29) | |
| Cholesterol (mg/dL)† | 190.59±40.19 | 194.85±42.44 | 190.37±39.50 | 192.29±39.04 | 192.42±41.20 | 0.0041 |
| LDL (mg/dL)† | 113.95±35.17 | 113.39±36.73 | 111.54±35.07 | 112.34±34.79 | 113.58±35.80 | 0.256 |
| HDL (mg/dL)† | 54.09±15.69 | 57.40±17.82 | 54.48±15.65 | 54.68±16.01 | 54.09±15.71 | <0.01 |
| Triglycerides (mg/dL)† | 112.74±65.21 | 120.35±63.20 | 121.79±63.47 | 126.30±70.36 | 123.75±62.26 | 0.015 |
| Creatinine (mg/dL)† | 129.07±79.51 | 111.90±67.15 | 118.38±72.13 | 122.80±68.54 | 118.03±71.54 | <0.01 |
| Albumin (mg/L)† | 38.98±354.61 | 54.86±421.95 | 67.42±349.01 | 46.01±258.35 | 83.60±558.69 | <0.01 |
*N represents unweighted number, and the remaining values are weighted values using The National Health and Nutrition Examination Survey mobile examination center weight.
†Figures are expressed as mean±SE, other figures are expressed as per cent (95% CIs).
AH, asymptomatic hyperuricemia (serum urate>6.8 mg/dL without gout); BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OA, osteoarthritis; PAL, physical activity level; PIR, poverty income ratio; RA, rheumatoid arthritis.
Participants with OA are higher in non-Hispanic white (82.00% (95% CI: 79.15% to 84.86%)), hypertension (66.37% (95% CI: 62.74% to 70.00%)), elevated income (47.63% (95% CI: 43.03% to 52.24%)), moderate activities (73.88% (95% CI: 70.67% to 77.08%)), cholesterol (194.85±42.44) and HDL (57.40±36.73) than those without arthritis. Similar trends are observed in participants with RA, OA, other types of arthritis and those who responded with ‘don’t know’ when asked about the type of arthritis (table 2).
And the proportion of participants who self-reported OA was the highest in arthritis. The proportion of AH is higher in participants with OA (84.18% (95% CI: 81.78% to 85.59%)) than in those with RA (80.11% (95% CI: 75.97% to 84.25%)) and unspecified (82.04% (95% CI: 78.71% to 85.36%)) arthritis types. But it is slightly lower than no arthritis (84.35% (95% CI: 83.33% to 85.38%)) and other arthritis (84.42% (95% CI; 79.68% to 89.17%)) (p<0.01) (table 2).
The association between AH and arthritis
Overall, AH was associated with onset of arthritis (OR=1.34, 95% CI: 1.07 to 1.69) (table 3). However, the association muted in different models after adjusting for demographic, socioeconomic factors, etc.
Table 3.
Association between asymptomatic hyperuricemia and total arthritis
| Unadjusted model | Model 1 | Model 2 | Model 3 | |
| Control (reference) | 1 | 1 | 1 | 1 |
| Total arthritis | ||||
| OR (95% CI) | 1.34 (1.07 to 1.69) | 1.14 (0.87 to 1.49) | 1.11 (0.83 to 1.48) | 1.07 (0.80 to 1.41) |
| P value | 0.012 | <0.01 | <0.01 | <0.01 |
Model 1: adjusted for age, gender and race.
Model 2: adjusted for age, gender, education level, income to poverty ratio, race, body mass index (BMI), physical activity level (PAL), diabetes, hypertension and smoking record.
Model 3: adjusted for age, gender, education level, income to poverty ratio, race, BMI, PAL, hypertension, smoking, cholesterol, low-density lipoprotein, high-density lipoprotein, triglyceride, creatinine and albumin.
For participants aged 40–49 years, AH is significantly associated with incident arthritis (OR=1.96, 95% CI: 1.23 to 2.99). The association remained after adjusted for education level, income to poverty ratio, BMI, diabetes, hypertension and smoking (OR=2.00, 95% CI: 1.94 to 3.36) (table 4).
Table 4.
The total arthritis was analysed stratified by gender, age and race
| Model 1 (OR, 95% CI, P value) | Model 2 (OR, 95% CI, P value) | Model 3 (OR, 95% CI, P value) | |
| Gander | |||
| Male | 1 | 1 | 1 |
| Female | 0.753 (0.633 to 0.896) 0.002 | 0.730 (0.608 to 0.877) 0.001 | 0.712 (0.582 to 0.872) 0.001 |
| Age | |||
| 20–29 | 1 | 1 | 1 |
| 30–39 | 1.788 (1.078 to 2.966) 0.025 | 1.718 (1.003 to 2.940) 0.048 | 1.181 (0.635 to 2.199) 0.595 |
| 40–49 | 1.957 (1.285 to 2.981) 0.002 | 2.002 (1.941 to 3.358) 0.009 | 1.324 (0.721 to 2.432) 0.362 |
| 50–59 | 1.409 (0.989 to 2.008) 0.057 | 1.472 (0.963 to 2.251) 0.074 | 0.975 (0.582 to 1.632) 0.932 |
| 60–69 | 1.034 (0.718 to 1.489) 0.856 | 1.076 (0.700 to 1.653) 0.737 | 0.721 (0.436 to 1.192) 0.200 |
| 70+ | 1.106 (0.789 to 1.549) 0.556 | 1.122 (0.725 to 1.737) 0.602 | 0.739 (0.426 to 1.282) 0.278 |
| Race | |||
| Other race | 1 | 1 | 1 |
| Hispanic | 1.604 (1.136 to 2.264) 0.008 | 1.582 (1.056 to 2.371) 0.027 | 1.456 (0.962 to 2.203) 0.075 |
| Non-Hispanic white | 0.895 (0.696 to 1.150) 0.381 | 1.040 (0.786 to 1.376) 0.780 | 0.971 (0.732 to 1.288) 0.839 |
| Non-Hispanic black | 2.017 (1.471 to 2.765) 0.000 | 2.305 (1.622 to 3.276) 0.000 | 2.203 (1.536 to 3.160) 0.000 |
Model 1: adjusted for age, gender and race.
Model 2: adjusted for age, gender, race, education level, income to poverty ratio, body mass index (BMI), diabetes, hypertension and smoking record.
Model 3: adjusted for age, gender, race, education level, income to poverty ratio, BMI, hypertension, smoking, cholesterol, low-density lipoprotein, high-density lipoprotein, triglyceride, creatinine and albumin.
Among non-Hispanic black participants, AH was significantly associated with arthritis. (OR=2.02, 95% CI: 1.47 to 2.77). The results kept significant adjusting for education level, income to poverty ratio, BMI, diabetes, hypertension and smoking (OR=2.31, 95% CI: 1.62 to 3.28) and for cholesterol, LDL, HDL, triglyceride, creatinine and albumin (OR=2.20, 95% CI: 1.55 to 3.16) (table 4).
Compared with male participants, female participants with AH showed a higher likelihood of OA (OR=1.35, 95% CI: 1.14 to 1.60). However, for RA (OR: 1.08, 95% CI: 0.83 to 1.41), other forms of arthritis (OR: 1.00, 95% CI: 0.78 to 1.29) and the ‘unspecified’ category (OR: 0.99, 95% CI: 0.82 to 1.20), the observed associations were not statistically significant. Notably, this trend was more prominent within the OA subgroup (online supplemental sTable 2). Among participants aged>50 years, there is a significant association between AH and different types of arthritis (including OA, RA, other, unspecified). More importantly, the strength of this association increased with age, specifically for 50–59, 60–69 and 70+ years.
Discussion
Based on 12 years of nationally representative data from NHANES, our findings indicated an association between AH and the arthritis, with a notable focus on OA. The correlation was present before adjusting the model. However, after adjusting for additional variables such as cholesterol and creatinine, the correlation weakened, suggesting that the relationship between AH and arthritis (including OA) might not be independent and could be influenced by metabolic and physiological factors like cholesterol and creatinine.29 Our research findings suggest a significant correlation between AH and arthritis among non-Hispanic black individuals, possibly due to metabolic syndrome-related metabolic abnormalities being less sensitive in identifying elevated uric acid levels in non-Hispanic black populations.30
Although hyperuricemia is a major contributor to the development of gouty arthritis, accumulating evidence suggest that AH may increase the risk of developing RA, psoriatic arthritis and spondylarthritis.31–33 In vitro studies on synoviocytes from healthy and RA subjects revealed that monosodium urate crystals could increase the release of the inflammatory cytokine interleukin 6 (IL-6), the chemokine CXCL8 and the matrix metalloproteinase-1.34 The injection of urate crystals in vivo leads to produce main mediators in the pathogenesis of Psoriatic arthritis (PsA), such as IL-17.35 The hyperuricemia not only play an important role in the development and progression of psoriatic arthritis, but also affect severity of clinical manifestations and degree of inflammation.36 Monosodium urate crystals interact with articular tissues to influence the development of axial spondyloarthritis as monosodium urate crystal deposition associated with the progress of radiographic grade at the sacroiliac joint.18 37
Our data indicate that AH may serve as a marker for potential risk in relation to OA.22 An increasing body of evidence suggests that AH, characterised by elevated SUA levels without any symptoms of gout or kidney stone disease, may be associated with an increased risk of OA, particularly in weight-bearing joints such as the knee.16 38 39 The relationship between AH and arthritis is complex and multifaceted, and the exact nature of this relationship is not yet clear. Hyperuricemia may promote the development of arthritis via deposition of urate crystals in the joints, promoting chronic low-grade inflammation and exacerbating oxidative stress.20 22 40 However, it is also possible that the association between hyperuricemia and arthritis is partially due to common risk factors such as obesity and metabolic syndrome.41 42 Further research is needed to better understand the relationship between these two conditions and to identify potential therapeutic targets for the prevention or treatment of arthritis in patients with hyperuricemia.
The intimate relationship between hyperuricemia and OA may repurpose FDA-approved urate-lowering therapy drugs in the treatment of OA. Currently, the drugs used to treat OA mainly include non-steroidal anti-inflammatory drugs, corticosteroids.43 However, these drugs could only use to relieve the clinical symptoms but not decrease the onset of arthritis. In accordance with our findings, another study also supports the significant association between arthritis and hypertension.44 In recent years, there has been growing interest in exploring the role of urate-lowering therapy in the treatment of OA.45 Urate crystal deposition can directly damage cartilage, stimulate the production of proinflammatory cytokines and lead to inflammation and cartilage degradation.46 Urate-lowering therapy drugs such as allopurinol and febuxostat have been shown to have anti-inflammatory properties, inhibit the production of reactive oxygen species, reduce the expression of proinflammatory cytokine.47–49 Our results raise the possibility that pharmacological treatment of AH via a treat-to-target (T2T) strategy may decrease incident of arthritis, especially for OA. The T2T strategy involves targeting specific uric acid levels and adjusting drug therapy accordingly to achieve this goal.50 51
Our findings highlight those female participants with AH are more likely to develop arthritis, especially for OA, than male participants, and ageing may exaggerate this trend. Among adults in the USA, serum urate was 6.0 mg/dL in men and 4.8 mg/dL in women, and hyperuricemia prevalence rates were 20.2% and 20.0%, respectively.11 Studies have also shown that hyperuricemia is more common in men over 30 years and women over 50 years.52 The gender and age associated increase in SUA levels may be explained by menopause in women and alcohol consumption in men.53 Menopause can lead to an increase in SUA levels, while postmenopausal hormone replacement therapy may be associated with a decrease in SUA levels.54 The difference in SUA levels between men and women is due to the increased renal uric acid clearance caused by oestrogen in women before menopause.55 Serum urate levels were significantly associated with knee OA as determined by osteophytosis in women but not in men.56 Women typically have a higher prevalence of hand and knee arthritis than men, women also tend to have more severe knee OA, particularly after menopausal age.57
The strength of our study was the use of data from a large, nationally representative sample. However, results should be interpreted with caution with inherent limitation. First, it is not possible to interpret the findings from a causal point of view due to the cross-sectional approach. Prospective study and Mendelian randomization study are needed to further investigate the relationship between the AH and arthritis, especially OA. Second, recall bias may affect the accuracy of prevalence estimates, although this study used CDC-recommended self-reported and physician-diagnosed arthritis as case definitions.24 58 Third, our result might be charged with choosing a single number to represent the prevalent of arthritis in the US population as it only included adults in the national non-institutionalised population of the country.59 Fourth, medication use for the participants was not included in this study. Finally, we had limited information on the involvement of OA in each participant, such as imaging and treatment procedures.
In summary, our study results suggest that AH patients may benefit from close monitoring for the development of arthritis, understanding the relationship between hyperuricemia and arthritis and identifying factors that contribute to their increased risk of these diseases, which may be of great significance for the prevention and management of these conditions.
Supplementary Material
Footnotes
ZL, DW and HZ contributed equally.
Contributors: JG is the guarantor of the study and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. ZL, DW and JG conceived and designed the study, performed the analysis and wrote the paper. HZ participated in the revision and refinement of the content. All authors read and commented on the manuscript and approved the final version of the manuscript.
Funding: Project funded by Guangdong Clinical Research Center of Immune Disease (2020B1111170008), Scientific Research Fund of Sichuan Academy of Medical Sciences (N/A) and Sichuan Provincial People's Hospital (2022QN38).
Disclaimer: The funder was not involved in the preparation of this manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. The data used for the analyses are publicly available from the National Center for Health Statistics (NCHS), which is part of the Centers for Disease Control and Prevention (CDC) in the USA (https://www.cdc.gov/nchs/nhanes/https://www.cdc.gov/nchs/nhanes/).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The data released from the National Health and Nutrition Examination Survey did not require informed patient consent. This study used an anonymised publicly available data set with no identifiable information on the survey participants, and thus did not require ethics approval.
References
- 1.Barbour KE, Helmick CG, Boring M, et al. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation - United States, 2013-2015. MMWR Morb Mortal Wkly Rep 2017;66:246–53. 10.15585/mmwr.mm6609e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hootman JM, Helmick CG, Barbour KE, et al. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015-2040. Arthritis Rheumatol 2016;68:1582–7. 10.1002/art.39692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy LB, Cisternas MG, Pasta DJ, et al. Medical expenditures and earnings losses among US adults with arthritis in 2013. Arthritis Care Res (Hoboken) 2018;70:869–76. 10.1002/acr.23425 [DOI] [PubMed] [Google Scholar]
- 4.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019;393:1745–59. 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 5.Vos T, Lim SS, Abbafati C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature 2018;561:45–56. 10.1038/s41586-018-0457-8 [DOI] [PubMed] [Google Scholar]
- 7.Kuo C-F, Grainge MJ, Zhang W, et al. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol 2015;11:649–62. 10.1038/nrrheum.2015.91 [DOI] [PubMed] [Google Scholar]
- 8.Liang X, Chou OHI, Cheung CL, et al. Is hypertension associated with arthritis? the United States national health and nutrition examination survey 1999-2018. Ann Med 2022;54:1767–75. 10.1080/07853890.2022.2089911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bortoluzzi A, Furini F, Scirè CA. Osteoarthritis and its management - epidemiology, nutritional aspects and environmental factors. Autoimmun Rev 2018;17:1097–104. 10.1016/j.autrev.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 10.Mobasheri A, Rayman MP, Gualillo O, et al. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2017;13:302–11. 10.1038/nrrheum.2017.50 [DOI] [PubMed] [Google Scholar]
- 11.Chen-Xu M, Yokose C, Rai SK, et al. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the national health and nutrition examination survey, 2007-2016. Arthritis Rheumatol 2019;71:991–9. 10.1002/art.40807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalbeth N, Phipps-Green A, Frampton C, et al. Relationship between serum urate concentration and clinically evident incident gout: an individual participant data analysis. Ann Rheum Dis 2018;77:1048–52. 10.1136/annrheumdis-2017-212288 [DOI] [PubMed] [Google Scholar]
- 13.Lioté F, Pascart T. From Hyperuricaemia to gout: what are the missing links Nat Rev Rheumatol 2018;14:448–9. 10.1038/s41584-018-0040-6 [DOI] [PubMed] [Google Scholar]
- 14.Dalbeth N, Stamp L. Hyperuricaemia and gout: time for a new staging system? Ann Rheum Dis 2014;73:1598–600. 10.1136/annrheumdis-2014-205304 [DOI] [PubMed] [Google Scholar]
- 15.Dalbeth N, Gosling AL, Gaffo A, et al. Gout. Lancet 2021;397:1843–55. 10.1016/S0140-6736(21)00569-9 [DOI] [PubMed] [Google Scholar]
- 16.Neogi T, Krasnokutsky S, Pillinger MH. Urate and osteoarthritis: evidence for a reciprocal relationship. Joint Bone Spine 2019;86:576–82. 10.1016/j.jbspin.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roddy E, Zhang W, Doherty M. Are joints affected by gout also affected by osteoarthritis Ann Rheum Dis 2007;66:1374–7. 10.1136/ard.2006.063768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalbeth N, Aati O, Kalluru R, et al. Relationship between structural joint damage and Urate deposition in gout: a plain radiography and dual-energy CT study. Ann Rheum Dis 2015;74:1030–6. 10.1136/annrheumdis-2013-204273 [DOI] [PubMed] [Google Scholar]
- 19.Yokose C, Chen M, Berhanu A, et al. Gout and osteoarthritis: associations, pathophysiology, and therapeutic implications. Curr Rheumatol Rep 2016;18:65. 10.1007/s11926-016-0613-9 [DOI] [PubMed] [Google Scholar]
- 20.Chhana A, Callon KE, Pool B, et al. The effects of monosodium urate monohydrate crystals on chondrocyte viability and function: implications for development of cartilage damage in gout. J Rheumatol 2013;40:2067–74. 10.3899/jrheum.130708 [DOI] [PubMed] [Google Scholar]
- 21.Chhana A, Pool B, Callon KE, et al. Monosodium urate crystals reduce osteocyte viability and indirectly promote a shift in osteocyte function towards a proinflammatory and Proresorptive state. Arthritis Res Ther 2018;20:208. 10.1186/s13075-018-1704-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denoble AE, Huffman KM, Stabler TV, et al. Uric acid is a danger signal of increasing risk for osteoarthritis through Inflammasome activation. Proc Natl Acad Sci U S A 2011;108:2088–93. 10.1073/pnas.1012743108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Z, Liao H, Scheetz J, et al. The association between retinopathy and arthritis: findings from a US national survey 2005-2008. Curr Eye Res 2020;45:1543–9. 10.1080/02713683.2020.1760306 [DOI] [PubMed] [Google Scholar]
- 24.March LM, Schwarz JM, Carfrae BH, et al. Clinical validation of self-reported osteoarthritis. Osteoarthritis and Cartilage 1998;6:87–93. 10.1053/joca.1997.0098 [DOI] [PubMed] [Google Scholar]
- 25.Martillo MA, Nazzal L, Crittenden DB. The crystallization of Monosodium Urate. Curr Rheumatol Rep 2014;16:400. 10.1007/s11926-013-0400-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.FitzGerald JD, Dalbeth N, Mikuls T, et al. American college of rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken) 2020;72:744–60. 10.1002/acr.24180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plan and operation of the third national health and nutrition examination survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat 1994:1–407. [PubMed] [Google Scholar]
- 28.Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/Apha/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/American heart Association task force on clinical practice guidelines. Hypertension 2018;71:1269–324. 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 29.Kerekes G, Nurmohamed MT, González-Gay MA, et al. Rheumatoid arthritis and metabolic syndrome. Nat Rev Rheumatol 2014;10:691–6. 10.1038/nrrheum.2014.121 [DOI] [PubMed] [Google Scholar]
- 30.DeBoer MD, Gurka MJ. Low sensitivity for the metabolic syndrome to detect uric acid elevations in females and non-Hispanic-black male adolescents: an analysis of NHANES 1999-2006. Atherosclerosis 2012;220:575–80. 10.1016/j.atherosclerosis.2011.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiou A, England BR, Sayles H, et al. Coexistent hyperuricemia and gout in rheumatoid arthritis: associations with comorbidities, disease activity, and mortality. Arthritis Care Res (Hoboken) 2020;72:950–8. 10.1002/acr.23926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuruta N, Imafuku S, Narisawa Y. Hyperuricemia is an independent risk factor for psoriatic arthritis in psoriatic patients. J Dermatol 2017;44:1349–52. 10.1111/1346-8138.13968 [DOI] [PubMed] [Google Scholar]
- 33.Ho H-H, Yu K-H, Chen J-Y, et al. Coexisting ankylosing spondylitis and gouty arthritis. Clin Rheumatol 2007;26:1655–61. 10.1007/s10067-007-0563-8 [DOI] [PubMed] [Google Scholar]
- 34.Chen DP, Wong CK, Tam LS, et al. Activation of human fibroblast-like synoviocytes by uric acid crystals in rheumatoid arthritis. Cell Mol Immunol 2011;8:469–78. 10.1038/cmi.2011.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raucci F, Iqbal AJ, Saviano A, et al. IL-17A neutralizing antibody regulates monosodium urate crystal-induced gouty inflammation. Pharmacol Res 2019;147:104351. 10.1016/j.phrs.2019.104351 [DOI] [PubMed] [Google Scholar]
- 36.Tripolino C, Ciaffi J, Ruscitti P, et al. Hyperuricemia in psoriatic arthritis: epidemiology, pathophysiology, and clinical implications. Front Med (Lausanne) 2021;8:737573. 10.3389/fmed.2021.737573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J, Li A, Jia E, et al. Monosodium urate crystal deposition associated with the progress of radiographic grade at the sacroiliac joint in axial SpA: a dual-energy CT study. Arthritis Res Ther 2017;19:83. 10.1186/s13075-017-1286-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S, Pillinger MH, Krasnokutsky S, et al. The association between asymptomatic hyperuricemia and knee osteoarthritis: data from the third national health and nutrition examination survey. Osteoarthritis Cartilage 2019;27:1301–8. 10.1016/j.joca.2019.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao L, Lin S, Zhan F. The association between serum uric acid level and changes of MRI findings in knee osteoarthritis: a retrospective study (A STROBE-compliant article). Medicine (Baltimore) 2019;98:e15819. 10.1097/MD.0000000000015819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joosten LAB, Crişan TO, Bjornstad P, et al. Asymptomatic hyperuricaemia: a silent activator of the innate immune system. Nat Rev Rheumatol 2020;16:75–86. 10.1038/s41584-019-0334-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zurita-Cruz J, Villasis-Keever M, Manuel-Apolinar L, et al. Resistin/uric acid index as a prognostic factor in adolescents with obesity after lifestyle intervention. J Pediatr 2020;219:38–42. 10.1016/j.jpeds.2019.12.006 [DOI] [PubMed] [Google Scholar]
- 42.Musumeci G, Aiello FC, Szychlinska MA, et al. Osteoarthritis in the Xxist century: risk factors and Behaviours that influence disease onset and progression. Int J Mol Sci 2015;16:6093–112. 10.3390/ijms16036093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Neogi T, Chen C, et al. Low-dose aspirin use and recurrent gout attacks. Ann Rheum Dis 2014;73:385–90. 10.1136/annrheumdis-2012-202589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krasnokutsky S, Oshinsky C, Attur M, et al. Serum urate levels predict joint space narrowing in non-gout patients with medial knee osteoarthritis. Arthritis Rheumatol 2017;69:1213–20. 10.1002/art.40069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bardin T, Richette P. Impact of Comorbidities on gout and Hyperuricaemia: an update on prevalence and treatment options. BMC Med 2017;15:123. 10.1186/s12916-017-0890-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schett G, Schauer C, Hoffmann M, et al. Why does the gout attack stop? A roadmap for the immune pathogenesis of gout. RMD Open 2015;1(Suppl 1):e000046. 10.1136/rmdopen-2015-000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geng Q, Zhang H, Cui Y, et al. Febuxostat mitigates IL-18-induced inflammatory response and reduction of extracellular matrix gene. Am J Transl Res 2021;13:979–87. [PMC free article] [PubMed] [Google Scholar]
- 48.Nasi S, Castelblanco M, Chobaz V, et al. Xanthine oxidoreductase is involved in chondrocyte mineralization and expressed in osteoarthritic damaged cartilage. Front Cell Dev Biol 2021;9:612440. 10.3389/fcell.2021.612440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Zhang Z, Huang X. L-arginine and allopurinol supplementation attenuates inflammatory mediators in human osteoblasts-osteoarthritis cells. Int J Biol Macromol 2018;118(Pt A):716–21. 10.1016/j.ijbiomac.2018.06.047 [DOI] [PubMed] [Google Scholar]
- 50.Kiltz U, Smolen J, Bardin T, et al. Treat-to-target (T2t) recommendations for gout. Ann Rheum Dis 2017;76:632–8. 10.1136/annrheumdis-2016-209467 [DOI] [PubMed] [Google Scholar]
- 51.Perez-Ruiz F, Moreno-Lledó A, Urionagüena I, et al. Treat to target in gout. Rheumatology (Oxford) 2018;57(suppl_1):i20–6. 10.1093/rheumatology/kex442 [DOI] [PubMed] [Google Scholar]
- 52.Miao Z, Li C, Chen Y, et al. Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong Coastal cities of Eastern China. J Rheumatol 2008;35:1859–64. [PubMed] [Google Scholar]
- 53.Lin KC, Lin HY, Chou P. The interaction between uric acid level and other risk factors on the development of gout among asymptomatic hyperuricemic men in a prospective study. J Rheumatol 2000;27:1501–5. [PubMed] [Google Scholar]
- 54.Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women--the third national health and nutrition examination survey. Arthritis Res Ther 2008;10:R116. 10.1186/ar2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicholls A, Snaith ML, Scott JT. Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br Med J 1973;1:449–51. 10.1136/bmj.1.5851.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding X, Zeng C, Wei J, et al. The associations of serum uric acid level and hyperuricemia with knee osteoarthritis. Rheumatol Int 2016;36:567–73. 10.1007/s00296-015-3418-7 [DOI] [PubMed] [Google Scholar]
- 57.Srikanth VK, Fryer JL, Zhai G, et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis and Cartilage 2005;13:769–81. 10.1016/j.joca.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 58.El Miedany Y, El Gaafary M, Youssef SS, et al. Incorporating patient reported outcome measures in clinical practice: development and validation of a questionnaire for inflammatory arthritis. Clin Exp Rheumatol 2010;28:734–44. [PubMed] [Google Scholar]
- 59.Murphy LB, Cisternas MG, Greenlund KJ, et al. Defining arthritis for public health surveillance: methods and estimates in four US population health surveys. Arthritis Care Res (Hoboken) 2017;69:356–67. 10.1002/acr.22943 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-074391supp001.pdf (120.2KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. The data used for the analyses are publicly available from the National Center for Health Statistics (NCHS), which is part of the Centers for Disease Control and Prevention (CDC) in the USA (https://www.cdc.gov/nchs/nhanes/https://www.cdc.gov/nchs/nhanes/).