Abstract
Helicobacter pylori, an important etiologic agent in a variety of gastroduodenal diseases, produces large amounts of urease as an essential colonization factor. We have demonstrated previously that urease is located within the cytoplasm and on the surface of H. pylori both in vivo and in stationary-phase culture. The purpose of the present study was to assess the relative contributions of cytoplasmic and surface-localized urease to the ability of H. pylori to survive exposure to acid in the presence of urea. Toward this end, we compared the acid resistance in vitro of H. pylori cells which possessed only cytoplasmic urease to that of bacteria which possessed both cytoplasmic and surface-localized or extracellular urease. Bacteria with only cytoplasmic urease activity were generated by using freshly subcultured bacteria or by treating repeatedly subcultured H. pylori with flurofamide (1 μM), a potent, but poorly diffusible urease inhibitor. H. pylori with cytoplasmic and surface-located urease activity survived in an acid environment when 5 mM urea was present. In contrast, H. pylori with only cytoplasmic urease shows significantly reduced survival when exposed to acid in the presence of 5 mM urea. Similarly, Escherichia coli SE5000 expressing H. pylori urease and the Ni2+ transport protein NixA, which expresses cytoplasmic urease activity at levels similar to those in wild-type H. pylori, survived minimally when exposed to acid in the presence of 5 to 50 mM urea. We conclude that cytoplasmic urease activity alone is not sufficient (although cytoplasmic urease activity is likely to be necessary) to allow survival of H. pylori in acid; the activity of surface-localized urease is essential for resistance of H. pylori to acid under the assay conditions used. Therefore, the mechanism whereby urease becomes associated with the surface of H. pylori, which involves release of the enzyme from bacteria due to autolysis followed by adsorption of the enzyme to the surface of intact bacteria (“altruistic autolysis”), is essential for survival of H. pylori in an acid environment. The ability of H. pylori to survive exposure to low pH is likely to depend on a combination of both cytoplasmic and surface-associated urease activities.
Helicobacter pylori is a spiral, gram-negative bacterium which is the etiologic agent of chronic superficial gastritis. Infection with H. pylori is strongly associated with peptic ulcer disease, gastric carcinoma, and gastric lymphoma (4, 18, 34–36).
An important characteristic of H. pylori is its substantial urease activity, which appears to be essential for survival and pathogenesis of the bacterium. It is thought that hydrolysis of urea by urease generates ammonia to counterbalance gastric acidity, presumably by forming a neutral microenvironment surrounding the bacterium within the gastric lumen. Supporting this hypothesis, it has been shown that H. pylori survives at low pH in vitro in the presence of urea and functional urease activity (25, 27, 28). Furthermore, isogenic urease-negative mutants of H. pylori and of a related bacterium, Helicobacter mustelae, which lack urease activity are unable to colonize the gastric mucosa of mice, ferrets, and gnotobiotic piglets (1, 12, 41).
A unique feature of H. pylori is that a significant fraction of urease, which is found exclusively within the cytoplasm in all other bacteria and in plants (33), is associated with the outer membrane both in vitro and in vivo (11, 38). We have shown that urease becomes associated with the surface of H. pylori by a novel mechanism. Urease is released as a result of autolysis of a fraction of bacteria and becomes adsorbed to the surface of the remaining intact bacteria (38). We refer to this process as “altruistic autolysis,” since survival of the population in vitro and presumably in vivo is dependent upon the occurrence of autolysis within a fraction of the bacteria (11, 38).
In light of the demonstration that enzymatically active urease is present both within the cytoplasm and associated with the outer membrane (8, 19, 38), we sought to determine the relative contributions of urease activity in these two compartments to the ability of H. pylori to survive exposure to acid in vitro. In this report, we tested the hypothesis that H. pylori cells grown in vitro that contain cytoplasmic urease only are sensitive to low pH in the presence of physiologic concentrations of urea.
MATERIALS AND METHODS
Bacterial culture.
H. pylori wild-type strains N6 and 84-183 (both urease positive) (8, 9) and H. pylori urease-negative mutant strain N6ureB::Km (kindly provided by A. Labigne, Institut Pasteur, Paris, France) (17) were grown on Trypticase soy agar containing sheep blood (Remel, Lenexa, Kans.) at 37°C in an atmosphere containing 10% CO2, 5% O2, and 85% inert gas (N2). Cultures on agar plates were established in one of two ways, as described previously (38). Briefly, freshly subcultured (fresh sub) bacteria were prepared by directly culturing previously frozen stock preparations onto fresh agar plates immediately from storage vials for 24 h. A single subculture after 24 h onto another agar plate to increase the number of bacteria was employed. This agar plate was incubated for another 24 h before the bacteria were harvested for acid resistance. In our experience, bacterial autolysis is maximal under late-logarithmic growth conditions. Therefore, under fresh sub conditions, we avoided late-logarithmic (typically more than 48 h of growth) conditions. As determined by electron microscopic immunolocalization and biochemical analysis (37), in H. pylori 84-183 grown in this manner, 3 to 7% and 40 to 60% of the urease is surface associated or extracellular at 24 and 72 h, respectively (38). Cultures termed “repeat sub” were subcultured at least twice at 72-h (which is at the end of the logarithmic growth phase) intervals and then harvested for analysis. In H. pylori 84-183 grown in this manner, 30 to 50% and 70 to 90% of the urease is surface associated or extracellular at 24 and 72 h, respectively (38). Proteus mirabilis ATCC 7002 was grown on Trypticase soy agar containing sheep blood at 37°C in room air. Prior to use, P. mirabilis ATCC 7002 was grown for 2 h in Luria-Bertani broth containing 50 mM urea to induce urease activity (33). Escherichia coli SE5000(pHP808/pUEF202) carries the H. pylori urease gene cluster (21) and nixA encoding the Ni2+ transport protein (31) and expresses cytoplasmic urease activity at levels similar to those in wild-type H. pylori. E. coli SE5000(pHP808) carries the entire urease gene cluster; E. coli SE5000(pUEF202) produces NixA only (31). E. coli SE5000 and its transformants were all grown in M-9 minimal medium containing 1 μM NiCl (31).
Analytical methods.
Urease activity was measured with a coupled enzyme assay as described previously (8). One unit of urease activity was defined as the amount capable of hydrolyzing 1 μmol of urea per min. Protein concentrations were measured by the method of Bradford, as described previously (8). Urease specific activity was expressed as units of activity per milligram of protein. Urease specific activity was measured within intact cells and in bacteria disrupted in a French pressure cell at 20,000 lb/in2 in phosphate-buffered saline (pH 7.2) containing protease inhibitors (phenylmethylsulfonyl fluoride [0.1 μM] and leupeptin [1 μM]).
Acid resistance assay.
In general, methods described by McGowan et al. (27) were used to assess the resistance of bacteria to acid. Briefly, bacteria were harvested from culture plates and suspended in normal saline (150 mmol of NaCl per liter [pH 7.2]) to yield a final suspension of approximately 109 CFU/ml. Bacterial suspensions were then diluted 1:10 (final concentration, 15 mmol of NaCl per liter). The diluted suspensions were incubated in 100 mmol of citric acid-HCl buffer per liter (pH 2) with or without urea (5 to 50 mM) in a microaerobic environment. After 30 min of incubation, serial dilutions were made in normal saline (pH 7.2), and 0.1 ml of an appropriate dilution was plated onto blood agar plates and incubated at 37°C for 72 to 96 h in a microaerobic environment to enumerate viable bacteria (CFU per milliliter). In the case of P. mirabilis and E. coli SE5000, 0.1 ml of appropriate serial dilutions was plated onto blood agar plates maintained at 37°C in room air. E. coli SE5000(pHP808/pUEF202), E. coli SE5000(pHP808), and E. coli SE5000(pUEF202) were grown on L plates containing ampicillin (50 μg/ml) and chloramphenicol (20 μg/ml).
Effect of urease inhibitors.
Flurofamide (kindly provided by R. Leunk, Procter & Gamble, Cincinnati, Ohio), a potent urease inhibitor (50% inhibitory concentration [IC50] = 60 nM [30]), was used at a final concentration of 1 μM (approximately 17 times the reported IC50). Flurofamide stock (100 μM) was prepared in water. Acetohydroxamic acid, another urease inhibitor, was used at a final concentration of 7 mM. (The concentration was not 7 μM, as incorrectly reported previously due to a typographical error [38]; 7 mM is approximately twice the reported IC50 for H. pylori urease [30].) Briefly, whole-cell suspensions of H. pylori 84-183 were incubated with the inhibitor for 30 min in brucella broth containing 10% fetal bovine serum in a microaerobic environment prior to exposure of the culture to an acidic environment (100 mmol of citric acid-HCl buffer per liter [pH 2]) for an additional 30-min incubation. At the end of incubation, serial dilutions were made to enumerate viable bacteria as described above.
Time course of urease inhibition.
Suspensions of H. pylori 84-183 were incubated for various time intervals in a microaerobic environment in the presence of flurofamide (1 μM), acetohydroxamic acid (7 mM), or no inhibitor prior to assessment of urease activity in whole cells.
Ultrastructural localization of urease antigen in E. coli SE5000(pHP808/pUEF202).
To determine the location of urease antigen within E. coli SE5000(pHP808/pUEF202), we employed methods used previously to localize urease and HspB within H. pylori grown in vitro (38). Briefly, after growth in M-9 minimal medium containing 1 μM NiCl, bacteria were pelleted and fixed in 2% paraformaldehyde–0.2% glutaraldehyde–phosphate-buffered saline (pH 7.2) for 2 h at room temperature. Bacteria were then embedded in 10% gelatin, which was solidified on ice. Blocks for ultracryotomy were prepared and immunolabeled with 10% goat serum in blocking buffer (38). Immunolabeling with primary affinity-purified, polyclonal antiserum made in mouse ascites fluid (kindly supplied by H. Kleanthous, Orovax, Inc., Boston, Mass.) against H. pylori urease (diluted 1:250 to 1:500) was carried out for 2 h. Incubation with secondary antiserum (goat anti-mouse immunoglobulin G–colloidal gold [5-nm-diameter particles]; Jackson ImmunoResearch Labs, West Grove, Pa.), diluted 1:25, was carried out for 1 h. Sections were then stained with uranyl acetate (38). Controls for specificity of labeling included E. coli SE5000 (which does not carry urease genes), labeled as described above, and substitution of preimmune mouse ascites fluid for the primary antibody during immunolabelling of E. coli SE5000(pHP808/pUEF202).
RESULTS
Effects of exposure to acid on survival of H. pylori.
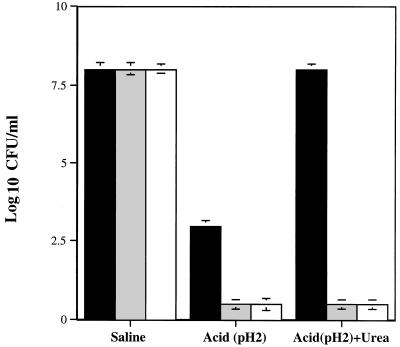
To determine the ability of H. pylori to survive exposure to acid, urease-positive H. pylori strain N6 (72-h repeat sub), urease-negative mutant H. pylori strain N6ureB::Km (72-h repeat sub), and urease-positive P. mirabilis strain ATCC 7002 (exposed to 50 mM urea to induce synthesis of urease) were exposed to acid in the presence or absence of 5 mM urea. At pH 7 in the absence of urea, all three strains survived; however, at pH 2 in the absence of urea, survival of H. pylori N6 was reduced 5 logs, while H. pylori N6ureB::Km and P. mirabilis did not survive (Fig. 1). At pH 2, in the presence of 5 mM urea, survival of H. pylori N6 was similar to that in the presence of saline (pH 7.2) alone (Fig. 1). Addition of 5 mM urea did not promote survival of either the urease-negative H. pylori mutant N6ureB::Km or P. mirabilis ATCC 7002 (Fig. 1). These results are similar to those obtained with other H. pylori strains, including 84-183 (25, 27). Since urease localization data were available for H. pylori 84-183, we chose strain 84-183 for further studies.
FIG. 1.
Effects of exposure to acid (pH 2, 30 min) on survival of H. pylori N6 (urease positive; 72-h repeat sub), H. pylori N6ureB::Km (urease-negative mutant; 72-h repeat sub), and P. mirabilis ATCC 7002 (urease positive) in the presence and absence of 5 mM urea. Solid bars represent H. pylori N6, shaded bars represent H. pylori N6ureB::Km (urease-negative mutant), and open bars represent P. mirabilis ATCC 7002. In this and subsequent graphs, bacterial survival is expressed as CFU per milliliter ± standard error.
Properties of urease inhibitors.
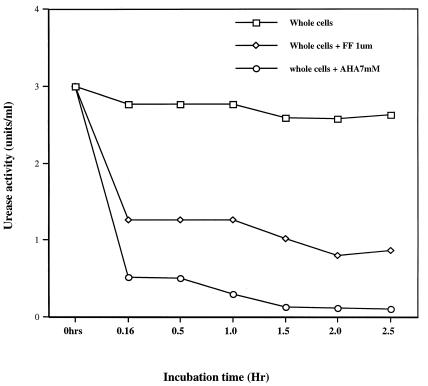
Flurofamide at a concentration of 1 μM diffuses slowly, if at all, across the membranes of H. pylori (38, 39). In contrast, acetohydroxamic acid diffuses rapidly across the membranes of H. pylori (30, 38). To indirectly characterize the time course of diffusion of flurofamide and acetohydroxamic acid across the membranes of H. pylori, we assessed urease activity after exposure of H. pylori 84-183 to these urease inhibitors for up to 150 min. In these experiments, H. pylori 84-183 cells (72-h fresh sub) were used to partially limit the amount of surface-adsorbed or extracellular urease (38). After incubation with 1 μM flurofamide for 10 min at pH 7, urease activity was reduced to approximately 50% of the initial activity. In contrast, the freely diffusible inhibitor acetohydroxamic acid inhibited over 95% of urease activity after 10 min (Fig. 2). Continued incubation of H. pylori whole cells for up to 60 min in the presence of flurofamide did not result in additional reduction in enzyme activity. Incubation for an additional 90 min resulted in slightly decreased urease activity (Fig. 2).
FIG. 2.
Effects of the urease inhibitors flurofamide (FF [1 μM]) and acetohydroxamic acid (AHA [7 mM]) on the time course of urease activity in whole-cell suspensions of H. pylori 84-183 (72-h fresh sub).
Effect of inhibition of external urease on acid resistance of H. pylori.
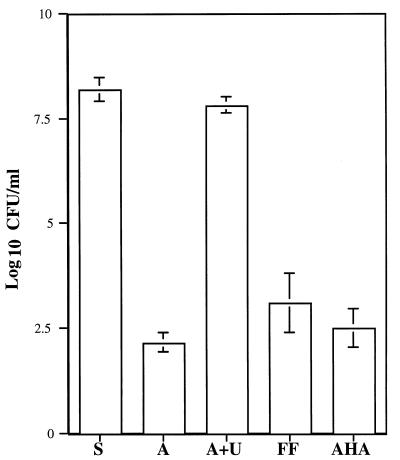
To characterize the relative contributions of cytoplasmic and surface-associated or extracellular urease to acid resistance, we subjected suspensions of 72-h fresh sub cultures of H. pylori 84-183 to either 1 μM flurofamide or 7 mM acetohydroxamic acid for 30 min. Subsequently, bacteria were exposed to an acidic environment (100 mmol of citric acid-HCl buffer per liter [pH 2]) in the presence or absence of urea. The rationale was that if flurofamide inhibits surface-associated and/or extracellular urease activity exclusively, then the relative contribution of cytoplasmic urease activity to survival of H. pylori in the presence of acid could be assessed. After 30 min of exposure to acetohydroxamic acid (7 mM), which inhibited >90% of urease activity (see Fig. 2), there was a 6-log reduction in bacterial survival (Fig. 3). Preincubation of H. pylori with flurofamide (1 μM) resulted in a 5- to 6-log reduction in bacterial survival upon exposure to acid (Fig. 3). Preincubation of H. pylori with flurofamide (1 μM) followed by 30 min of exposure to sterile saline (pH 7.2) had no adverse effect upon viability, as shown by other investigators (40 [data not shown]).
FIG. 3.
Effects of the urease inhibitors flurofamide and acetohydroxamic acid on resistance of H. pylori 84-183 (72-h fresh sub) to acid in the presence or absence of 5 mM urea. S, saline (pH 7.2); A, acid (pH 2.0); A+U, acid containing 5 mM urea; FF, bacteria preincubated in flurofamide (1 μM) for 30 min and then exposed to acid containing 5 mM urea for 30 min; AHA, bacteria preincubated in acetohydroxamic acid (7 mM) for 30 min then exposed to acid containing 5 mM urea for 30 min.
Role of cytoplasmic and surface-associated urease in survival in acid.
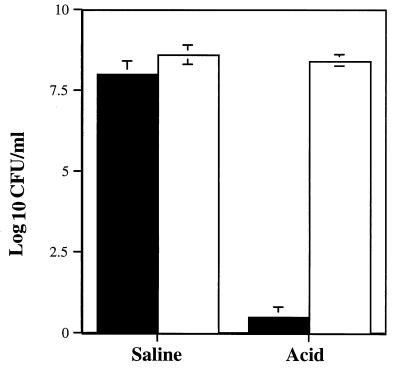
Previous cryoimmunolocalization studies in our laboratory have demonstrated that 24-h (fresh sub) preparations of H. pylori grown on blood agar plates possess little or no surface-associated urease; in contrast, 72-h (fresh and repeat sub) preparations demonstrate significant amounts of surface-associated and extracellular urease (38). We hypothesized that this difference in distribution of surface-associated urease would alter the survival of H. pylori exposed to acid in the presence of urea. Freshly subcultured (24 h) H. pylori 84-183 exposed to pH 2 for 30 min in the presence of 5 mM urea did not survive (Fig. 4). In marked contrast, exposure of 72-h fresh sub (Fig. 4) and 72-h repeat sub (Fig. 1) cultures of H. pylori 84-183 to pH 2 plus 5 mM urea did not significantly reduce bacterial viability.
FIG. 4.
Effects of culture age (hence urease distribution) on survival of H. pylori 84-183 in saline (pH 7.2) and in acid (pH 2.0) containing 5 mM urea. Solid bars represent survival of 24-h fresh sub bacteria (which contain cytoplasmic urease almost exclusively), while open bars represent survival of 72-h fresh sub bacteria (which possess both cytoplasmic and/or surface-associated or extracellular urease).
Ultrastructural localization of urease antigen in E. coli SE5000(pHP808/pUEF202).
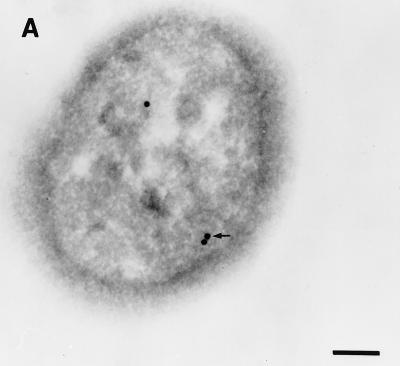
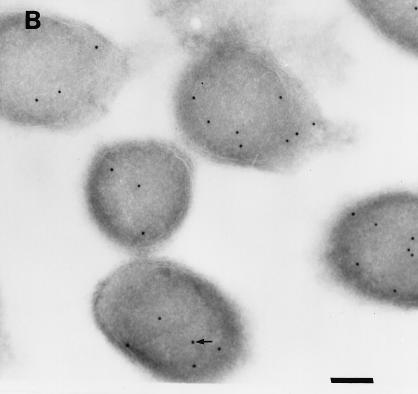
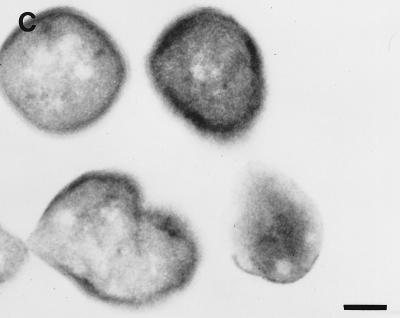
Colloidal gold particles representing the localization of urease were located exclusively within the cytoplasm of E. coli SE5000(pHP808/pUEF202) (Fig. 5A), at a density similar to that observed in cross-sections of H. pylori 84-183 (Fig. 5B). Immunolabeling of E. coli SE5000 (which does not carry urease genes) was not observed when antiurease was used as the primary antibody (data not shown) or when preimmune ascites fluid was substituted for the primary antibody during immunolabelling of E. coli SE5000(pHP808/pUEF202) (Fig. 5C).
FIG. 5.
Immunolocalization of H. pylori urease in E. coli SE5000(pHP808/pUEF202) and H. pylori 84-183. (A) Colloidal gold particles representing localization of H. pylori urease in E. coli SE5000(pHP808/pUEF202) are located exclusively within the cytoplasmic compartment (arrow). (B) Colloidal gold particles representing localization of urease in H. pylori 84-183 (24-h fresh sub) are located exclusively within the cytoplasmic compartment (arrow). (C) Immunolabeling was not observed when preimmune ascites fluid was substituted for primary antibody during immunolabelling of E. coli SE5000(pHP808/pUEF202). In all experiments, the concentration of primary antibody was 1:250. Bars, 120 nm.
Survival of E. coli expressing cytoplasmic H. pylori urease activity.
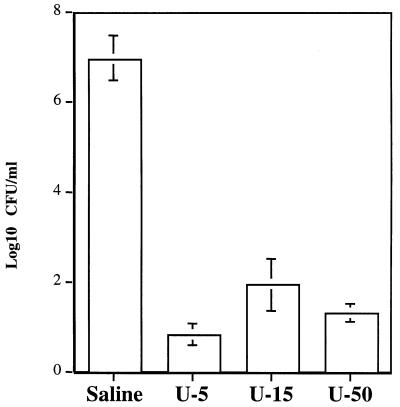
E. coli SE5000(pHP808/pUEF202), which carries the H. pylori urease gene cluster and nixA (encodes Ni2+ transport protein), expresses cytoplasmic urease activity at levels similar to those in wild-type H. pylori (31) and survived minimally upon exposure to pH 2 for 30 min in the presence of 5 to 50 mM urea (Fig. 6). In the presence of 50 mM urea (pH 2 for 30 min), survival was reduced 6 logs compared with survival of the same strain in saline (pH 7.2) (Fig. 6). At pH 2 for 30 min in the absence of urea, there was no survival. E. coli SE5000(pHP808), E. coli SE5000(pUEF202), and E. coli SE5000 did not survive at pH 2 for 30 min, even with the addition of 50 mM urea (data not shown).
FIG. 6.
Effects of urea concentration on survival of E. coli SE5000(pHP808/pUEF202) in acid (pH 2, 30 min). The concentrations of urea used were as follows: no urea present (U-0), 5 mM (U-5), 15 mM (U-15), and 50 mM (U-50).
Specific urease activity of bacterial preparations.
Specific urease activity data are summarized as follows. The specific urease activities of H. pylori (24-h fresh sub), H. pylori (72-h repeat sub), P. mirabilis ATCC 7002, and E. coli SE5000(pHP808/pUEF202) were 4.5 ± 0.35, 16.0 ± 1.9, 0.27 ± 0.04, and 11.6 ± 1.9 μmol of urea per min per mg of protein, respectively (means ± standard errors [n = 3]). The specific urease activity of French press lysates of 72-h repeat sub cultures of H. pylori 84-183 was 2.8 to 3.6 times greater than that in corresponding preparations of 24-h fresh sub bacteria. The specific urease activity of lysates of E. coli SE5000(pHP808/pUEF202) grown in M-9 medium containing 1 μM NiCl for 5 h to induce urease activity was similar to that measured in lysates of H. pylori 84-183 (72-h repeat sub). The specific urease activity of P. mirabilis ATCC 7002 lysates was very low compared with that of H. pylori 84-183 or the recombinant E. coli SE5000(pHP808/pUEF202). The specific urease activities of E. coli SE5000(pHP808), E. coli SE5000(pUEF202), and E. coli SE5000 were not determined, but have been shown previously to be negligible (21, 31).
DISCUSSION
A variety of evidence suggests that urease activity is essential for initiating colonization of the stomach of animal models by Helicobacter spp. (1, 12–14, 41). Urease is a common taxonomic characteristic among bacteria; in general, however, the enzyme is restricted to the cytoplasmic compartment (reviewed in references 32 and 33). In contrast, H. pylori is unique in possessing both cytoplasmic and surface-associated urease (5, 8, 11, 38). Using cryoimmunolocalization techniques, we have previously demonstrated that urease is located strictly within the cytoplasm of freshly subcultured, early-log-phase H. pylori (38). However, at the end of the log phase, a significant fraction of the enzyme becomes surface associated or extracellular in distribution. Using similar techniques, we have demonstrated that a significant fraction (∼30%) of urease is also associated with the surface of H. pylori in vivo (11). Both the surface-associated (or extracellular) and cytoplasmic forms of urease exhibit enzyme activity (38).
In the present study, we sought to determine the contribution of surface-associated and/or extracellular urease to acid resistance of H. pylori. We observed that H. pylori cells with surface-associated and/or extracellular and cytoplasmic urease activity survived in an acid environment, but only when urea was present at 5 mM, thus confirming observations of previous investigators (25, 27). In contrast, H. pylori cells with primarily cytoplasmic urease activity (24-h fresh sub and 72-h fresh sub cultures treated with 1 μM flurofamide) were unable to survive when exposed to acid in the presence of urea (5 mM). Similarly, E. coli cells carrying the H. pylori urease gene cluster and nixA (which encodes the Ni2+ transport protein), which expresses urease strictly within the cytoplasmic compartment, at activity levels similar to those in wild-type H. pylori (3, 31), survived minimally at urea concentrations of 5 to 50 mM.
The present study demonstrates that inhibition of surface-associated and/or extracellular urease activity in whole cells of H. pylori by flurofamide occurs within 10 min; thereafter, the relative amount of enzyme inhibition by 1 μM flurofamide does not increase significantly for up to 60 min. These results support the notion that flurofamide (1 μM) either does not diffuse or diffuses slowly across the cell membranes of H. pylori, as described previously (38), and thus for exposures limited to 30 min would be expected to inhibit surface-associated and/or extracellular urease activity almost exclusively. In contrast, acetohydroxamic acid reduces urease activity in whole cells almost completely within 10 min, demonstrating its ability to diffuse across the membranes of H. pylori (30), resulting in inhibition of both cytoplasmic and surface-associated and/or extracellular urease activity.
Taken together, the results of the present study demonstrate that intracellular urease alone is not sufficient to allow resistance to acid by H. pylori. In contrast, Scott and colleagues have proposed that intracellular urease is activated at low external pH and that it is the intracellular urease activity which is responsible for allowing resistance to external acid (39). The latter authors have confirmed two important observations from our previous work (11, 38). First, external urease is produced by lysis of H. pylori (39). Second, at a concentration of 1 μM, which is 17 times the IC50, flurofamide diffuses slowly, if at all, across the membranes of H. pylori (39). However, the latter authors have not used 1 μM flurofamide to selectively inhibit surface associated or extracellular urease activity, as we have done in the present experiments. A further distinction between these two studies is that different assays were used to assess the viability of H. pylori upon exposure to acid. While Scott et al. assessed proton motive force for short intervals in response to acidified urea as an indicator of bacterial viability (39), we have opted to measure viability directly by using methods established by previous investigators. One possible explanation for the contrasting conclusions of these two studies is that association with the outer membrane of H. pylori protects urease from inactivation by acid, although free urease is inactivated at low pH (2, 28). Such a protective niche would be accessible to urease inhibitors such as flurofamide. Scott et al. (39) have assumed that surface-associated urease is inactivated upon exposure to acid (similar to free urease), but they have not directly tested this assumption. Development of an autolysis-deficient mutant of H. pylori, in which urease is presumably located strictly within the cytoplasmic compartment, would be helpful to resolve discrepancies between the present study and that of Scott et al. (39).
Recent work by Vanet and Labigne has confirmed our earlier observation that urease is present in the cytoplasm and in the extracellular compartment of H. pylori cultures (42). However, the latter investigators propose that specific secretion of urease occurs either by ABC (16) or by type III (22) secretion systems. If a secretion system for H. pylori urease were to exist, it would have to meet a variety of unique requirements, as we have outlined previously (38). Furthermore, the observation that the UreA subunit is “secreted” at a much higher rate than is the UreB subunit is not supported by the observations of Scott et al. (39) and is not compatible with specific secretion of urease, which is composed of equimolar amounts of UreA and UreB subunits (8, 15, 19). Another important aspect of the work reported by Vanet and Labigne (42) is the proposal that H. pylori possesses a homolog of E. coli β-galactosidase that is restricted to the cytoplasm only. We are unable to find such a homolog or related proteins by sequence search of the published genome, by use of chromogenic substrates to detect E. coli β-galactosidase activity, or by testing affinity-purified β-galactosidase antiserum, including several monoclonal antibodies, from several commercial suppliers for cross-reactive peptides. Therefore, further characterization of the β-galactosidase homolog protein that is restricted to the cytoplasm alone is needed to allow verification of its presence.
Urease activity is not essential for growth of H. pylori on blood agar plates at neutral pH, as evidenced by growth of isogenic urease-negative mutants in culture (17, 37). However, cytoplasmic urease may function in the acquisition of nitrogen for protein synthesis, since urea nitrogen, following incubation with H. pylori, ultimately appears in protein (43).
Of interest, although P. mirabilis ATCC 7002 produces urease, it was susceptible to exposure to acid in the presence of urea (5 mM), confirming the results of Marshall et al. (25). There are several possible explanations for this observation. First, the Km for P. mirabilis urease (13 mM urea [23]) is too high for the enzyme to exhibit maximal activity at the low urea concentrations which protect H. pylori against acid in vitro. In contrast, the Km for H. pylori urease is 0.2 to 0.5 mM urea (8, 10, 15, 20). Second, the urease specific activity in P. mirabilis is too low to generate sufficient ammonia to counteract acid (pH 2). Finally, since urease is located strictly within the cytoplasm in P. mirabilis (23), the protection afforded by surface-associated and/or extracellular urease in H. pylori does not occur in P. mirabilis.
Beyond its role in mediating resistance to acid, surface-associated urease may play important roles in the pathogenesis and survival of H. pylori within the stomach. Our observation that a fraction of urease is surface associated in vivo helps to explain how urease can serve as a vaccine in animals and humans, since surface-associated urease would be directly accessible to components of both the humoral and cellular arms of the immune system (11, 38). However, our “altruistic autolysis” model also predicts that a subpopulation of H. pylori that contains only cytoplasmic urease exists deep within the protective gastric mucus or antral pits (11, 38). Because surface-associated urease is not present, such a subpopulation would be expected to evade surveillance by the immune system. Such Helicobacter strains with only cytoplasmic urease would also be expected to evade the effects of urease inhibitors and vaccines administered in vivo. In this regard, in studies with ferrets colonized with H. mustelae, inhibition of urease activity with flurofamide was insufficient to eradicate the bacteria (26). Finally, since urease is reportedly chemotactic for neutrophils (7, 24), continual release of urease may promote the low level of inflammation associated with all H. pylori strains, whether or not they possess other mechanisms for inducing gastric inflammation, such as the cagA pathogenicity island (6).
In this study, survival at pH 2.0 for 30 min is the only criterion assessed as a stringent indicator of acid resistance. The gastric environment presents various pH ranges (from pH 2 to 6) under normal conditions, and the roles of urease molecules present in the surface-associated and cytoplasmic compartments of H. pylori need to be defined under various pH conditions.
A special reference needs to be made to the role of urease in acid resistance in Yersinia enterocolitica. Recently, Young et al. (44) have shown that Y. enterocolitica possess a unique cytoplasmic urease with a pH optimum at 5.5 that is inactive at pH 7.0 but is activated by low pH. This enzyme exhibits full activity at pH 5.5. When extracellular pH is lowered (pH 2) and the cytoplasmic pH in intact bacteria reaches 5.5, urease activity is activated 785-fold over the activity at neutral pH. In the presence of urea, Y. enterocolitica urease is able to impart acid resistance to the bacterium. Furthermore, Y. enterocolitica urease cloned in E. coli is able to confer this ability to resist acid to E. coli. The situation in H. pylori is different. H. pylori urease exhibits a pH optimum of 8.3 and has little or no activity at pH 5.5, and free urease is rapidly inactivated by exposure to pH <5. Our observation that recombinant E. coli expressing H. pylori urease is unable to survive acid treatment also argues against acid activation (analogous to Yersinia) of H. pylori urease.
Eaton and Krakowka (13, 14) have attempted to cocolonize urease-positive H. pylori with an isogenic urease-negative mutant in gnotobiotic piglets. They report that under the conditions used, the urease-negative mutant was unable to colonize piglets, while the urease-positive parent strain was able to colonize. This experiment demonstrates that surface urease alone is not sufficient (presuming that the transfer of urease between two isogenic strains did occur as predicted) to allow survival of bacteria at normal gastric pH, most likely because both cytoplasmic and surface-associated urease activities are needed for acid resistance.
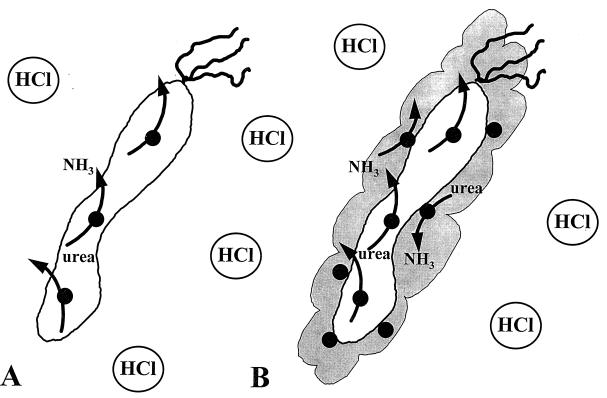
In vitro, free H. pylori urease becomes rapidly inactivated by short exposure to pH <5 (2, 39). Therefore, the fraction of urease that is released by autolysis and remains free is likely to be inactivated and of little consequence in acid resistance. A significant amount of urease activity in the older bacterial preparation is derived from this fraction. Therefore, much of the urease released by autolysis is likely to contribute very little to acid resistance before these urease molecules become irreversibly inactivated because of exposure to low pH. However, the fraction of the urease released by autolysis and associated with the surface may be stabilized and protected from acid denaturation, and it most likely contributes to acid resistance. This model of H. pylori urease localization, activity, and acid resistance is outlined in Fig. 7.
FIG. 7.
Model describing the roles of H. pylori urease activity in the cytoplasmic and surface-associated compartments. H. pylori urease activity exhibits a pH optimum of 8.3. Free urease is rapidly inactivated by exposure to pH <5 (2, 39) and likely does not contribute to acid resistance. (A) Most of the early-logarithmic-phase and some late-logarithmic-phase H. pylori cells contain cytoplasmic urease exclusively, with no surface-associated urease (38). H. pylori cells exhibiting cytoplasmic urease activity only are also generated by inhibition of surface-associated urease with 1 μM flurofamide (38). The cytoplasmic urease degrades urea to produce ammonia, which may be exported but is not sufficient to permit survival at pH 2 for 30 min. (B) In the late logarithmic phase of growth, H. pylori possesses both cytoplasmic and surface-associated urease activity (38), allowing quantitatively more urease activity per bacterium. The surface-associated urease activity is sensitive to flurofamide (38), a poorly diffusible urease inhibitor, and is protected from external low pH due to its association with the molecular chaperonin HspB (heat shock protein B, a groEL homolog) at the cell surface. Rapid external hydrolysis of urea helps to prevent entry of H+ into the bacterial cytoplasm, therefore maintaining neutral-to-alkaline cytoplasmic pH and allowing full urease activity in the cytoplasmic compartment. Internal urease in the absence of external urease is unable to maintain a neutral-to-alkaline cytoplasmic pH for optimum urease activity. It is important to note that the observations presented above are valid for an external pH of 2.0. It remains to be determined whether cytoplasmic urease alone is sufficient to allow survival of H. pylori at a higher external pH (3 to 5).
In summary, the present studies demonstrate that surface-associated or extracellular urease is essential for acid resistance of H. pylori. Therefore, the process whereby urease becomes associated with the surface of H. pylori, “altruistic autolysis,” is an essential mechanism contributing to the pathogenesis of H. pylori. We conclude that surface-associated urease enables H. pylori to adapt to its unique ecological niche in the acidic mammalian stomach.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grants CA67527 and DK39045 (B.E.D.) and National Institutes of Health grant AI25567 (H.L.T.M.).
REFERENCES
- 1.Andrutis K A, Fox J G, Schauer D B, Marini R P, Murphy J C, Yan L, Solnick J V. Inability of an isogenic urease-negative mutant strain of Helicobacter mustelae to colonize the ferret stomach. Infect Immun. 1995;63:3722–3725. doi: 10.1128/iai.63.9.3722-3725.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauerfeind P, Garner R, Dunn B E, Mobley H L T. Synthesis and activity of Helicobacter pylori urease and catalase at low pH. Gut. 1997;40:25–30. doi: 10.1136/gut.40.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauerfeind P, Garner R M, Mobley H L T. Allelic exchange mutagenesis of nixA in Helicobacter pylori results in reduced nickel transport and urease activity. Infect Immun. 1996;64:2877–2880. doi: 10.1128/iai.64.7.2877-2880.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser M J. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 5.Bode G, Malfertheiner P, Lehnhardt G, Nilius M, Ditschuneit H. Ultrastructural localization of urease of Helicobacter pylori. Med Microbiol Immunol. 1993;182:233–242. doi: 10.1007/BF00579622. [DOI] [PubMed] [Google Scholar]
- 6.Censini S, Lange C, Xiang Z Y, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. Cag, a pathogenicity island of Helicobacter pylori, encodes type i-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craig P M, Territo M C, Karnes W E, Walsh J H. Helicobacter pylori secretes a chemotactic factor for monocytes and neutrophils. Gut. 1992;33:1020–1023. doi: 10.1136/gut.33.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn B E, Campbell G P, Perez-Perez G I, Blaser M J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990;265:9464–9469. [PubMed] [Google Scholar]
- 9.Dunn B E, Perez-Perez G I, Blaser M J. Two-dimensional gel electrophoresis and immunoblotting of Campylobacter pylori proteins. Infect Immun. 1989;57:1825–1833. doi: 10.1128/iai.57.6.1825-1833.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn B E, Sung C-C, Taylor N S, Fox J G. Purification and characterization of Helicobacter mustelae urease. Infect Immun. 1991;59:3343–3345. doi: 10.1128/iai.59.9.3343-3345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn B E, Vakil N B, Schneider B G, Miller M M, Zitzer J B, Puetz T, Phadnis S H. Localization of Helicobacter pylori urease and heat shock protein in human gastric biopsies. Infect Immun. 1997;65:1181–1188. doi: 10.1128/iai.65.4.1181-1188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton K A, Brooks C L, Morgan D R, Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991;59:2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaton K A, Krakowka S. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect Immun. 1994;62:3604–3607. doi: 10.1128/iai.62.9.3604-3607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eaton K A, Krakowka S. Avirulent, urease-deficient Helicobacter pylori colonizes gastric epithelial explants ex vivo. Scand J Gastroenterol. 1995;30:434–437. doi: 10.3109/00365529509093303. [DOI] [PubMed] [Google Scholar]
- 15.Evans D J, Jr, Evans D G, Kirkpatrick S S, Graham D Y. Characterization of the Helicobacter pylori urease and purification of its subunits. Microb Pathog. 1991;10:15–26. doi: 10.1016/0882-4010(91)90062-f. [DOI] [PubMed] [Google Scholar]
- 16.Fath M J, Kolter R. ABC transporters: bacterial exporters. Microbiol Rev. 1993;57:995–1017. doi: 10.1128/mr.57.4.995-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrero R L, Cussac V, Courcoux P, Labigne A. Construction of isogenic urease-negative mutants of Helicobacter pylori by allelic exchange. J Bacteriol. 1992;174:4212–4217. doi: 10.1128/jb.174.13.4212-4217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forman D, Newell D G, Fullerton F, Yarnell J W, Stacey A R, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. Br Med J. 1991;302:1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawtin P R, Stacey A R, Newell D G. Investigation of the structure and localization of the urease of Helicobacter pylori using monoclonal antibodies. J Gen Microbiol. 1990;136:1995–2000. doi: 10.1099/00221287-136-10-1995. [DOI] [PubMed] [Google Scholar]
- 20.Hu L-T, Mobley H L T. Purification and N-terminal analysis of urease from Helicobacter pylori. Infect Immun. 1990;58:992–998. doi: 10.1128/iai.58.4.992-998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu L-T, Mobley H L T. Expression of catalytically active recombinant Helicobacter pylori urease at wild-type levels in Escherichia coli. Infect Immun. 1993;61:2563–2569. doi: 10.1128/iai.61.6.2563-2569.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones B D, Mobley H L T. Proteus mirabilis urease: genetic organization, regulation, and expression of structural genes. J Bacteriol. 1988;170:3342–3349. doi: 10.1128/jb.170.8.3342-3349.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mai U E, Perez-Perez G I, Allen J B, Wahl S M, Blaser M J, Smith P D. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J Exp Med. 1992;175:517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall B J, Barrett L J, Prakash C, McCallum R W, Guerrant R L. Urea protects Helicobacter (Campylobacter) pylori from the bactericidal effect of acid. Gastroenterology. 1990;99:697–702. doi: 10.1016/0016-5085(90)90957-3. [DOI] [PubMed] [Google Scholar]
- 26.McColm A A, Bagshaw J A, O’Malley C F. Development of a 14C-urea breath test in ferrets colonized with Helicobacter mustelae: effects of treatment with bismuth, antibiotics and urease inhibitors. Gut. 1993;34:181–186. doi: 10.1136/gut.34.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGowan C C, Cover T L, Blaser M J. The proton pump inhibitor omeprazole inhibits acid survival of Helicobacter pylori by a urease-independent mechanism. Gastroenterology. 1994;107:1573–1578. doi: 10.1016/0016-5085(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 28.Meyer-Rosburg K, Scott D R, Rex D, Melchers K, Sachs G. The effect of environmental pH on the proton motive force of Helicobacter pylori. Gastroenterology. 1996;111:886–900. doi: 10.1016/s0016-5085(96)70056-2. [DOI] [PubMed] [Google Scholar]
- 29.Mobley, H. L. T. 1996. The role of Helicobacter pylori urease in the pathogenesis of gastritis and peptic ulceration. Aliment. Pharmacol. Ther. 10(Suppl. 1):57–64. [DOI] [PubMed]
- 30.Mobley H L T, Cortesia M J, Rosenthal L E, Jones B D. Characterization of urease from Campylobacter pylori. J Clin Microbiol. 1988;26:831–836. doi: 10.1128/jcm.26.5.831-836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mobley H L, Garner R M, Bauerfeind P. Helicobacter pylori nickel-transport gene nixA: synthesis of catalytically active urease in Escherichia coli independent of growth conditions. Mol Microbiol. 1995;16:97–109. doi: 10.1111/j.1365-2958.1995.tb02395.x. [DOI] [PubMed] [Google Scholar]
- 32.Mobley H L T, Hausinger R P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989;53:85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mobley H L T, Island M D, Hausinger R P. Molecular biology of microbial ureases. Microbiol Rev. 1995;59:451–480. doi: 10.1128/mr.59.3.451-480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nomura A, Stemmermann G N, Chyou P H, Kato I, Perez-Perez G I, Blaser M J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 35.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 36.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Pérez G I, Olivares A Z, Cover T L, Blaser M J. Characteristics of Helicobacter pylori variants selected for urease deficiency. Infect Immun. 1992;60:3658–3663. doi: 10.1128/iai.60.9.3658-3663.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phadnis S H, Parlow M H, Levy M, Ilver D, Caulkins C M, Connors J B, Dunn B E. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect Immun. 1996;64:905–912. doi: 10.1128/iai.64.3.905-912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott D R, Weeks D, Hong C, Postius S, Melchers K, Sachs G. The role of internal urease in acid resistance of Helicobacter pylori. Gastroenterology. 1998;114:58–70. doi: 10.1016/s0016-5085(98)70633-x. [DOI] [PubMed] [Google Scholar]
- 40.Sjostrom J E, Larson H. Factors affecting growth and antibiotic susceptibility of H. pylori: effect of pH and urea on the survival of a wild-type strain and a urease-deficient mutant. J Med Microbiol. 1996;44:425–433. doi: 10.1099/00222615-44-6-425. [DOI] [PubMed] [Google Scholar]
- 41.Tsuda M, Karita M, Morshed M G, Okita K, Nakazawa T. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect Immun. 1994;62:3586–3589. doi: 10.1128/iai.62.8.3586-3589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanet A, Labigne A. Evidence for specific secretion rather than autolysis in the release of some Helicobacter pylori proteins. Infect Immun. 1998;66:1023–1027. doi: 10.1128/iai.66.3.1023-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams C L, Preston T, Hossack M, Slater C, McColl K E. Helicobacter pylori utilises urea for amino acid synthesis. FEMS Immunol Med Microbiol. 1996;13:87–94. doi: 10.1111/j.1574-695X.1996.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 44.Young G M, Amid D, Miller V L. A bifunctional urease enhances survival of pathogenic Yersinia enterocolitica and Morganella morganii at low pH. J Bacteriol. 1996;178:6487–6495. doi: 10.1128/jb.178.22.6487-6495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]