Abstract
DNA vaccination was evaluated with the experimental murine model of Trypanosoma cruzi infection as a means to induce antiparasite protective immunity, and the trypomastigote surface antigen 1 (TSA-1), a target of anti-T. cruzi antibody and major histocompatibility complex (MHC) class I-restricted CD8+ cytotoxic T-lymphocyte (CTL) responses, was used as the model antigen. Following the intramuscular immunization of H-2b and H-2d mice with a plasmid DNA encoding an N-terminally truncated TSA-1 lacking or containing the C-terminal nonapeptide tandem repeats, the antibody level, CTL response, and protection against challenge with T. cruzi were assessed. In H-2b mice, antiparasite antibodies were induced only by immunization with the DNA construct encoding TSA-1 containing the C-terminal repeats. However, both DNA constructs were efficient in eliciting long-lasting CTL responses against the protective H-2Kb-restricted TSA-1515–522 epitope. In H-2d mice, inoculation with either of the two TSA-1-expressing vectors effectively generated antiparasite antibodies and primed CTLs that lysed T. cruzi-infected cells in an antigen-specific, MHC class I-restricted, and CD8+-T-cell-dependent manner. When TSA-1 DNA-vaccinated animals were challenged with T. cruzi, 14 of 22 (64%) H-2b and 16 of 18 (89%) H-2d mice survived the infection. The ability to induce significant murine anti-T. cruzi protective immunity by immunization with plasmid DNA expressing TSA-1 provides the basis for the application of this technology in the design of optimal DNA multicomponent anti-T. cruzi vaccines which may ultimately be used for the prevention or treatment of Chagas’ disease.
Chagas’ disease, caused by the intracellular protozoan parasite Trypanosoma cruzi, is a lifelong health problem in Central and South America, where an estimated 18 million people are infected with this parasite and 90 million are at risk of infection (35, 65). Following a short-lived acute-phase illness characterized by fever and a patent parasitemia, infected individuals enter a nearly aparasitemic asymptomatic chronic phase, where most remain for the remainder of their lifetime. However, at 10 to 20 years postinfection nearly 30% of infected individuals develop severe cardiomyopathy, which is responsible for most of the 50,000 deaths caused by Chagas’ disease each year (45). Although reduviid vector control and blood bank screening measures have had a major impact in reducing transmission of T. cruzi (65), the operational costs to maintain such control programs, behavioral differences among vector species, existence of animal reservoirs, persistence of parasites in chronically infected patients, and lack of adequate chemotherapies to treat the infection will likely prevent these control measures alone from completely eradicating T. cruzi. An additional approach that could contribute significantly to control the transmission of Chagas’ disease is the development of anti-T. cruzi vaccines. To date, however, vaccine production for T. cruzi has been a low priority despite the current knowledge about the protective roles that antibodies, type 1 cytokines, and CD8+ T cells play in resistance to experimental T. cruzi infections (53).
During T. cruzi infection, both chagasic patients and experimental animals produce strong immune responses to molecules from the infective nonreplicative trypomastigote stage and the replicative amastigote forms (3, 4, 14, 29). Among these, trypomastigote surface antigen 1 (TSA-1) (15, 38), a major trypomastigote surface antigen and the first identified member of the trans-sialidase gene superfamily (48), is a target of protective immune responses in mice (61, 66). Immunization with an amino-proximal fragment of TSA-1 induces a strong antibody response and protects mice against an otherwise lethal challenge with T. cruzi (66). Our studies have recently identified TSA-1 as the first bona fide target of CD8+ cytotoxic T lymphocytes (CTL) in T. cruzi-infected mice and demonstrated that the adoptive transfer of TSA-1-specific gamma interferon (IFN-γ)- and tumor necrosis factor alpha-producing CTL lines protects naive animals against lethal T. cruzi infection (61). Moreover, we have recently determined that TSA-1 and amastigote surface protein-1 and -2 (33, 44), which are also recognized by murine CTL (32), represent three target molecules of T. cruzi-specific human CD8+ CTL (62). These studies demonstrated the validity of the mouse model to identify target antigens of protective anti-T. cruzi immune responses and provide a strong incentive for the development of vaccines as a potential control measure against Chagas’ disease. For this purpose, and given the success of plasmid DNA vaccination in specifically stimulating a broad spectrum of immune responses to the vector-encoded target antigen (12), we have chosen to investigate DNA-based immunization as a system to generate vaccine-induced resistance against T. cruzi and have used TSA-1 as a model antigen for its initial evaluation. In this report we document that intramuscular injection of BALB/c and C57BL/6J mice with TSA-1-encoding plasmid DNA induces antibodies, CTL, and significant protection against lethal challenge with T. cruzi.
MATERIALS AND METHODS
Mice and parasites.
Six- to 8-week-old female C57BL/6J (B6) and BALB/cByJ (BALB/c) mice (breeding pairs obtained from The Jackson Laboratory, Bar Harbor, Maine) were used in all experiments. The Brazil strain of T. cruzi was maintained in vivo by serial biweekly passage of 103 blood-form trypomastigotes (BFT) in C3H/HeSnJ mice (30) and by continuous in vitro passages of tissue culture-derived trypomastigotes (TCT) in monolayers of Vero cells (18). B6 mice were infected intraperitoneally with 103 BFT and challenged 3 months later with 105 TCT by subcutaneous injection at the base of the tail.
Cell lines and culture reagents.
P815 cells (H-2d; mastocytoma cells; ATCC TIB 64), J774 cells (H-2d; macrophages; ATCC TIB 67), 3T3 cells (H-2d; fibroblasts; ATCC CCL 163), and Vero cells (African Green monkey kidney cells; ATCC CCL 81) (all from the American Type Culture Collection, Rockville, Md.); RMA-S cells (peptide TAP.2 transporter-deficient, low H-2b expressor mutant of the RBL-5 Rauscher virus-induced T-cell lymphoma; provided by H.-G. Ljundggren, Karolinska Institute, Stockholm, Sweden); and 5A.Kb.α3 cells (H-2k fibroblasts stably transfected with the Kb gene; provided by S. Jameson, University of Minnesotta, Minneapolis) were maintained in complete RPMI 1640 (Mediatech, Herndon, Va.) medium (CR) containing 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah), 20 mM HEPES, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids and 50 μg of gentamicin per ml (all from Gibco BRL, Gaithersburg, Md.). COS-7 cells (simian virus 40-transformed African Green monkey kidney cells; ATCC CRL 1651) were grown in similarly supplemented Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech). T-cell medium (TCM) was prepared by supplementing CR with 50 μM 2-mercaptoethanol (Gibco BRL).
Peptides.
The peptide TSA-1515–522 (VDYNFTIV) (61), representing the H-2Kb-restricted T. cruzi TSA-1 CTL epitope, was produced by using 9-fluorenylmethoxycarbonyl-based solid-phase chemistry on an ACT MPS 350 peptide synthesizer (Advanced Chem Tech, Louisville, Ky.) by the Molecular and Genetic Instrumentation Facility at the University of Georgia (Athens). The H-2Kb-restricted OVA CTL peptide OVA257–264 (SIINFEKL) was used as a control (42). Lyophilized peptides were dissolved at 20 mg/ml in dimethyl sulfoxide and stored at −70°C. Before use, peptides were diluted with RPMI 1640. Peptides were not toxic to target cells or effector cell cultures.
Plasmid DNA constructs.
The genomic DNA fragments of the TSA-1 gene (15, 38) encoding amino acid residues 78 to 652 and 78 to 790, excluding and including, respectively, the (5)-nonapeptide tandem repeat unit, were amplified by PCR with pBluescript II SK (+)/TSA-1 (provided by David Fouts, University of California, Irvine) as a template. Forward and reverse primers were designed to incorporate, respectively, SalI and XbaI restriction sites (underlined below) for directional cloning. Primers were constructed on an Applied Biosystems (Foster City, Calif.) 394 DNA/RNA synthesizer at the Molecular Genetics Instrumentation Facility. The forward oligonucleotide primer 5′-AGTCGACGGATCCATGATTGCATTTGTCGAAGGC-3′ was used with reverse primers 5′-ATCTAGAAGCTTCATAGTTCACCGACACTCAGTGG-3′ and 5′-ATCTAGAAGCTTCATGCCGCAGCATTTGCTTCCCC-3′ to amplify a 1.7-kb (repeatless TSA-178–652) and a 2.1-kb (repeat-bearing TSA-178–790) product, respectively. The amplification products containing the A overhangs generated by Taq DNA polymerase during the PCR were cloned into the HincII site of the pUC19-T vector. Following digestion with SalI and XbaI, the 1.7- and 2.1-kb TSA-1 fragments were gel purified and cloned into the SalI and XbaI sites of the eukaryotic expression vector VR1012 (Vical Inc., San Diego, Calif.) (19) to generate VR1012 TSA1.7 and VR1012 TSA2.1. In the VR1012 vector, expression of the encoded gene is driven by a cytomegalovirus immediate-early gene promoter. Constructs were transformed into Escherichia coli DH5α competent cells and grown in Luria-Bertani broth with 70 μg of kanamycin per ml as described previously (43). Closed circular plasmid DNA was purified by anion-exchange chromatography with the Qiagen (Chatsworth, Calif.) maxi prep kit according to the manufacturer’s specifications. Plasmid DNA was sterilized by ethanol precipitation and dissolved in sterile phosphate-buffered saline (PBS).
In vitro expression.
Expression of VR1012 TSA1.7 and VR1012 TSA2.1 in COS-7 cells was assessed in vitro by transient transfection. COS-7 cells were seeded in six-well plates (Costar, Cambridge, Mass.) at 2 × 105 cells/well in 3 ml of complete DMEM and incubated overnight at 37°C and 6% CO2. In a final volume of 300 μl, 10 μg of plasmid DNA was mixed with 30 μg of Lipofectin reagent (Gibco BRL), and the mixture was incubated for 15 min at room temperature before being diluted with 1.7 ml of serum-free MEM. After the COS-7 monolayers (50 to 70% confluent) were washed with serum-free MEM, cells were overlaid with the mixture containing the DNA-Lipofectin complexes and incubated overnight at 37°C and 6% CO2. The cell culture medium was then replaced with 3 ml of complete DMEM and incubated for an additional day. Transiently transfected COS-7 cells were harvested by gentle trypsinization, washed in PBS, and seeded in eight-well Lab Tek chamber slides (Nunc Inc., Naperville, Ill.) at 104 cells/well. After overnight incubation at 37°C and 6% CO2, cells were washed with PBS, fixed in ice-cold methanol for 15 min at 4°C, and washed four more times before blocking with PBS–1% bovine serum albumin (BSA) for 1 h at 37°C. Cells were subsequently stained for 2 h at 37°C with a polyclonal anti-T. cruzi serum obtained from acutely infected C3H/HeSnJ mice or with normal mouse serum (1:200 dilution in PBS–1% BSA), washed three times, and finally incubated for 1 h at room temperature with fluorescein isothiocyanate-labeled F(ab′)2 goat anti-mouse immunoglobulin G (1:50 dilution in PBS–1% BSA) (Southern Biotechnology, Birmingham, Ala.). Slides were then rinsed four times with PBS–1% BSA and mounted in 10% glycerol–0.1 M sodium bicarbonate (pH 9)–2.5% 1,4-diazobicyclo[2,2,2]octane for visualization by laser scanning confocal microscopy (MRC-600) (Bio-Rad Laboratories, Hercules, Calif.).
Genetic immunizations and challenges.
Groups of B6 and BALB/c mice were injected intramuscularly into each tibialis anterior muscle with 50 μg of VR1012 TSA1.7, VR1012 TSA2.1, or control VR1012 suspended in 50 μl of PBS by using a 27-gauge needle. Mice were boosted 4 weeks later with an identical dose of plasmid (100 μg total) given by the same bilateral intramuscular injection. Tail blood samples were collected 3 and 2 weeks after the first and second doses, respectively, and sera were stored at −20°C until assayed for anti-T. cruzi antibody. Two weeks after the second dose, animals were infected by intraperitoneal injection of 105 (B6) or 103 (BALB/c) T. cruzi BFT. Parasitemias were monitored periodically by hemacytometer counts of 10 μl of tail vein blood in an ammonium chloride solution. Mortality was recorded daily.
Determination of serum antibody levels.
Antibody responses induced by the immunization of mice with plasmid DNA were evaluated by a solid-phase enzyme-linked immunosorbent assay (ELISA). In brief, capture antigen was prepared by sonication of 5 × 107 PBS-washed T. cruzi parasites (80% trypomastigotes, 20% amastigotes) in 50 mM carbonate-bicarbonate buffer (pH 9.6). Sonicated material was spun for 1 h at 100,000 × g at 4°C. Wells of flexible polyvinyl chloride 96-well plates (Falcon, Becton Dickinson & Co., Oxnard, Calif.) were coated overnight at 4°C with 100 μl of a predetermined optimal dilution (5 × 105 parasites/well) of the soluble antigen. Washed wells were blocked with 1% BSA in PBS–0.05% Tween 20 (PBST) for 1 h at 37°C. After blocking, 100 μl of pooled mouse sera (1:100 dilution in PBST) was added to the plates and incubated for 1 h at 37°C. Plates were washed six times with PBST and incubated for an additional hour with 100 μl of a horseradish peroxidase-labeled goat anti-mouse immunoglobulin (A, G, M) (1:1,000 dilution in PBST) (Cappel, Organon Teknika Corp., West Chester, Pa.). Washed wells were developed with 100 μl of the substrate 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid), and absorbance was read at 405 nm with an automated ELISA microplate reader (Bio-Tek Instruments, Winooski, Vt.).
Generation of effector cells.
Unless otherwise indicated, spleens from DNA-immunized mice were removed 2 weeks after the last dose, and immune spleen cell (SC) suspensions were prepared in TCM. In the case of B6 mice, SCs were cultured in 24-well plates at 5 × 106 cells/well. TSA-1515–522 peptide was included in each 2-ml culture at 1 μM (final concentration). In the case of BALB/c mice, 35 × 106 SCs in 10 ml of TCM were cultured in upright 25-cm2 tissue culture flasks containing irradiated monolayers of stimulator T. cruzi-infected J774 cells. After 2 days of incubation at 37°C and 6% CO2, cultures were made to 5% Rat T-STIM without concanavalin A (Collaborative Biomedical Products, Bedford, Mass.) and incubated for 4 additional days. Effector cells from BALB/c mice were also unstimulated immune SCs without secondary in vitro stimulation. SCs from B6 mice chronically infected with T. cruzi were obtained 6 months after parasite challenge and stimulated as described for SCs from DNA-immunized animals.
Preparation of peptide-pulsed target cells.
Peptide-pulsed targets were used to measure CTL activity of peptide-stimulated effector cells generated from plasmid DNA-immunized B6 mice. RMA-S (H-2b) cells preincubated for 24 h at 26°C and 6% CO2 were seeded into 24-well plates (Costar) at 106 cells/well in 2 ml of CR and incubated overnight under the same conditions in the presence of 0.05 μM TSA-1515–522 peptide or OVA257–264 negative control peptide and 100 μCi of a sterile Na251CrO4 solution (51Cr) (Amersham Life Science Corporation, Arlington, Heights, Ill.). Two hours prior to their processing for CTL assays, cells were shifted to 37°C and 6% CO2. P815 (H-2d) target cells were also prepared in 24-well plates by overnight incubation at 37°C and 6% CO2 with 51Cr and TSA-1515–522 peptide.
Preparation of T. cruzi-infected stimulator and target cells.
T. cruzi-infected cells were used to generate and measure the CTL activity of effector cells from plasmid DNA-immunized BALB/c mice. Monolayers of J774 cells (60% confluent) prepared in upright 25-cm2 tissue culture flasks (Corning, Corning, N.Y.) were infected overnight with T. cruzi TCT (50:1 parasite-to-host cell ratio). After extensive washing with serum-free RPMI 1640 to remove noninvading parasites, infected monolayers were irradiated (14 krads) (Gammacell 200; 60Co source) and then used as stimulators for immune SCs. To prepare T. cruzi-infected target cells used to ascertain the lytic activity of BALB/c-derived stimulated SCs, monolayers (50% confluent in horizontal 25-cm2 flasks) of major histocompatibility complex (MHC)-matched 3T3 (H-2d) and mismatched 5A.Kb.α3 (H-2k and H-2Kb) cells were incubated for 2 days at 37°C and 6% CO2 in CR supplemented with 1,000 U of IFN-α plus IFN-β (Lee Biomolecular Laboratories, Inc., San Diego, Calif.) per ml, washed, and then infected overnight with T. cruzi TCT (50:1 parasite-to-host cell ratio). After being washed, T. cruzi-infected monolayers were treated with PBS–1 mM EDTA to prepare single-cell suspensions and washed once more before a 1-h 51Cr labeling step at 37°C. To assess the lytic activity of unstimulated BALB/c-derived immune SCs, monolayers of untreated J774 cells were infected, and single-cell suspensions for 51Cr labeling were prepared by moderate pipetting of the cell monolayer. Under these conditions, stained (Leukostat; Fisher Scientific, Atlanta, Ga.) cytospin preparations of each culture indicated that 65 to 75% of the cells were infected.
CTL assay.
Cytolytic activity was measured by the 51Cr release assay, as previously described (63). In brief, 51Cr-labeled target cells were washed three times in CR and resuspended in TCM, and 5 × 103 target cells (100 μl) were added to effector cells (100 μl) at various effector cell-to-target cell (E/T) ratios in 96-well round-bottom plates (Corning). After a 5-h incubation at 37°C and 6% CO2, supernatants were harvested with the SCS System (Skatron, Sterling, Va.), and radioactivity was counted on a Cobra II Autogamma counter (Packard Instrument Company, Downers Grove, Ill.). Percent specific lysis was calculated from the mean of triplicates as 100 × [(experimental release − spontaneous release)/(maximum release − spontaneous release)]. Maximum and spontaneous releases were determined in wells containing no effectors in the presence or absence of 2% Triton X-100, respectively. In experiments where CTL activity of CD8+ and CD4+ T cells was tested, effector cells were depleted by incubation on ice for 30 min with predetermined dilutions of culture supernatants from hybridomas 3.155 (anti-CD8) (ATCC TIB 211) (46) and RL172 (anti-CD4) (8), followed by 30 min at 37°C in the presence of 1:6-diluted rabbit complement (Pel-Freez, Brown Deer, Wis.). Spontaneous release did not exceed 20% of the maximum release. The standard error ranged between 0.02 to 6.1% of the mean.
RESULTS
Expression of TSA-1 in transiently transfected cells.
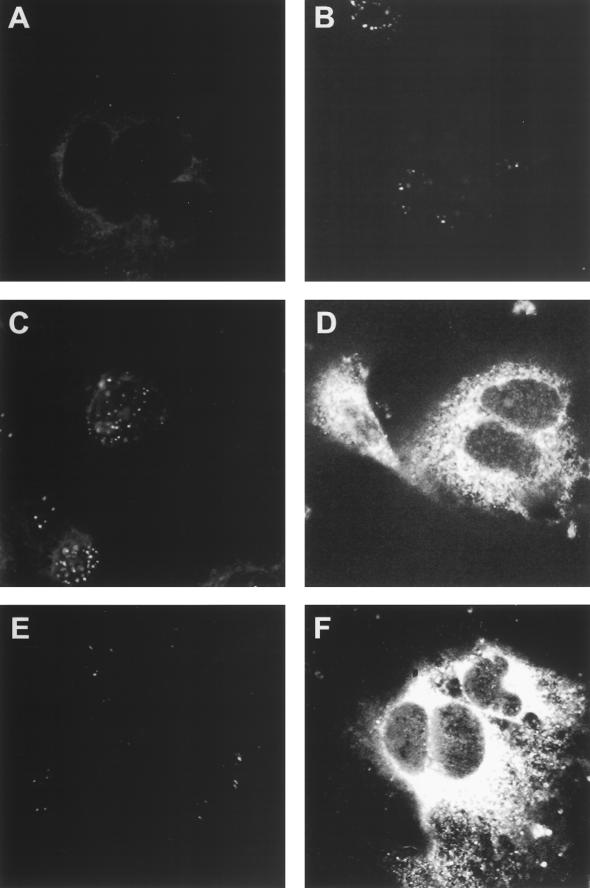
To study the effectiveness of genetic immunization against T. cruzi, the TSA-1 gene was subcloned into the VR1012 mammalian expression vector (19), containing the cytomegalovirus promoter and the bovine growth hormone polyadenylation sequences. The constructs VR1012 TSA1.7 and VR1012 TSA2.1 were generated to drive the expression of two N-terminally truncated TSA-1 gene products lacking and bearing, respectively, the five nonapeptide repeats located near the C-terminal end of the TSA-1 protein. Both plasmid constructs expressed the inserted TSA-1 gene fragment upon transient transfection of COS-7 cells. The cytoplasmic expression of TSA-1 in VR1012 TSA1.7- and VR1012 TSA2.1-transfected cells was intense as detected by immunofluorescent staining with a polyclonal anti-T. cruzi serum (Fig. 1D and F). In contrast, similarly transfected cells stained with normal mouse serum showed no evidence of immunofluorescence (Fig. 1C and E). No expression was detected in cells transfected with the unmodified VR1012 vector and stained with either serum (Fig. 1A and B).
FIG. 1.
Expression of TSA-1 in transiently transfected cells. COS-7 cells were transfected with 10 μg of unmodified VR1012 plasmid (A and B), TSA-1-encoding VR1012 TSA1.7 (C and D), or VR1012 TSA2.1 (E and F) by using Lipofection. After 72 h, cells were fixed in ice-cold methanol and stained by immunofluorescence with a polyclonal anti-T. cruzi serum obtained from acutely infected mice (B, D, and F) or with a control normal mouse serum (A, C, and E) followed by a fluorescein isothiocyanate-conjugated secondary antibody. Photomicrographs (magnification, ×100) were taken by confocal microscopy. Note the cytoplasmic localization of the transgene-expressed TSA-1 products (D and F).
Immunization with TSA-1 plasmid DNA elicits a parasite-specific antibody response.
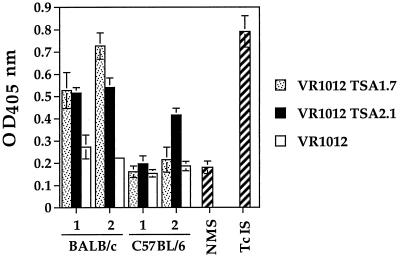
A strong humoral immune response has been widely implicated as a major effector mechanism that participates in the immune control of T. cruzi (28, 29, 71), and immunization of mice with a recombinant N-proximal portion of TSA-1 induces an antibody response which correlates with survival after a lethal challenge infection (66). To ascertain whether a T. cruzi-specific antibody response could be elicited by the expression of the TSA-1 protein fragments following intramuscular DNA immunization, BALB/c and B6 mice were injected twice with 100 μg of VR1012 TSA1.7, VR1012 TSA2.1, or control plasmid VR1012. The presence of parasite-specific antibodies in pooled sera prepared from each group of mice was assessed by ELISA (Fig. 2). Three weeks following the first dose, sera from BALB/c mice immunized with either VR1012 TSA1.7 or VR1012 TSA2.1 showed comparable antibody responses against the sonicated parasite material used as capture antigen. Two weeks after the second dose, while a boosting of the parasite-specific antibody level was detected in the sera from the VR1012 TSA1.7-immunized group, the level of antibodies in the sera from the VR1012 TSA2.1-immunized animals remained essentially unchanged. When a similar analysis was conducted for the pooled sera from similarly immunized B6 mice, the antibody levels after the first dose did not exceed the level found in normal mouse serum. However, after the second dose, only the VR1012 TSA2.1-immunized group showed a parasite-specific antibody response. In all cases, the antibody levels detected in the sera from groups of mice immunized with unmodified VR1012 vector were no different than the level measured in normal mouse serum.
FIG. 2.
T. cruzi-specific serum antibody response in TSA-1 DNA-vaccinated mice. BALB/c and B6 mice were injected with 50 μg of VR1012 TSA1.7, VR1012 TSA2.1, or unmodified VR1012 plasmid in each tibialis anterior muscle. Mice were boosted after 4 weeks with the same dose of plasmid. The presence of parasite-specific antibodies was assessed by ELISA with a 1:100 dilution of sera pooled from individual tail blood samples (four or five mice per group) and collected 3 and 2 weeks after the first (bars 1) and second (bars 2) doses. Negative and positive controls were sera from normal mice (NMS) and from mice acutely infected with T. cruzi (TcIS). OD450, optical density at 450 nm.
Induction of a long-lasting TSA-1-specific CTL response in TSA-1 plasmid DNA-immunized B6 mice.
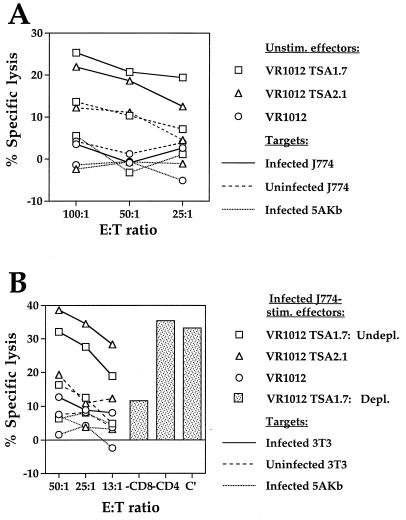
TSA-1515–522 is a target of H-2Kb-restricted protective CTL responses induced in B6 mice infected with T. cruzi (61). We therefore wanted to determine whether immunization of this strain of mice with the TSA-1-encoding DNA vectors could induce a TSA-1515–522-specific CTL response. Two weeks after the second intramuscular injection of either VR1012 TSA1.7 or VR1012 TSA2.1, immune SCs were stimulated with TSA-1515–522, and 6 days later, the lytic activity of effectors was tested against peptide-sensitized target cells. CTL activity was antigen specific, MHC class I restricted, and dependent on CD8+ T lymphocytes (Fig. 3A). The H-2b effector cells lysed matched RMA-S cells (H-2b) sensitized with TSA-1515–522 but were unable to lyse the same cells pulsed with control peptide OVA257–264 or MHC-mismatched P815 cells (H-2d) pulsed with TSA-1515–522. Detected lytic activity was abrogated by CD8+-T-cell depletion but not by depletion of CD4+ effectors. In no case did TSA-1515–522-stimulated SCs from mice immunized with unmodified VR1012 vector display CTL activity against peptide-sensitized target cells. Similar TSA-1515–522-specific CTL activity was detected in the peptide-stimulated SC cultures established 7 months after mice had received the second 100-μg dose of the TSA-1-encoding DNA vectors (Fig. 3B). The magnitude of such recall CTL responses was comparable to the CTL activity detected for TSA-1515–522-stimulated effectors from T. cruzi-infected mice. Hence, immunization of B6 mice with both TSA-1-encoding DNA constructs generates a long-lasting TSA-1515–522-specific CTL response which closely resembles the recall response induced in T. cruzi-infected animals.
FIG. 3.
Induction of long-lasting TSA-1515–522-specific, CD8+-T-cell-dependent, MHC class I-restricted CTL in TSA-1 DNA-immunized B6 mice. Four weeks following the first 100-μg intramuscular dose of plasmid DNA, B6 (H-2b) mice were injected via the same route with an identical dose of the priming vaccine, consisting of either control VR1012 plasmid or the TSA-1-expressing constructs VR1012 TSA1.7 and VR1012 TSA2.1. (A) Immune SCs were obtained 2 weeks after the second immunization and stimulated in vitro with peptide TSA-1515–522 (1 μM). After 6 days, recall CTL activity of undepleted (Undepl.) responder cultures was assessed in a 5-h 51Cr release assay against RMA-S (H-2b) and P815 (H-2d) target cells sensitized with TSA-1515–522 peptide (0.05 μM) at the indicated E/T ratios. RMA-S cells pulsed with OVA257–264 peptide (0.05 μM) were used as negative-control target cells. CTL activity of effector cells depleted (Depl.) of CD4+ or CD8+ T cells was measured at a 50:1 E/T ratio against TSA-1515–522-sensitized (0.05 μM) target cells. (B) Immune SCs from DNA-vaccinated or T. cruzi-infected mice were obtained 7 and 6 months after the second immunization or parasite challenge, respectively, and processed as described for panel A.
The CTL response induced in BALB/c mice by TSA-1 plasmid DNA immunization is parasite specific, MHC class I restricted, and CD8+ T cell dependent.
Despite the fact that the target antigens recognized by CTL from T. cruzi-infected BALB/c mice (H-2d) have not been identified, SCs from these animals display genetically restricted CTL activity against T. cruzi-infected target cells (36). Thus, we used this system to determine whether parasite-specific CTL could be induced in BALB/c mice following immunization with the TSA-1-encoding plasmid DNA constructs. First, spleens were collected 2 weeks after the second dose of DNA, and on the same day the CTL activity of SCs against infected and uninfected target cells was measured (Fig. 4A). Infection of J774 cells (H-2d) with T. cruzi efficiently targeted these macrophages for lysis by the H-2d effector cells harvested from either VR1012 TSA1.7- or VR1012 TSA2.1-immunized mice. In contrast, minimal or no lysis against uninfected J774 cells and against mismatched T. cruzi-infected 5A.Kb.α3 fibroblasts (H-2k; H-2Kb) was detected. None of the target cells tested was recognized by effector cells obtained from control VR1012-immunized animals. Next, CTL activity of immune SCs that had been stimulated for 6 days with T. cruzi-infected J774 macrophages against uninfected and T. cruzi-infected fibroblasts was assessed (Fig. 4B). Again, the specificity and MHC class I-restricted nature of the recall CTL response was demonstrated by the ability of effector cells derived from VR1012 TSA1.7- and VR1012 TSA2.1-immunized mice to lyse infected but not uninfected 3T3 cells (H-2d) and by their inability to recognize infected 5A.Kb.α3 cells (H-2k; H-2Kb). When the phenotype of the VR1012 TSA1.7-derived effectors was tested, it was found that they were CD8+ CD4−, because the lytic activity of these cells was significantly reduced by the depletion of CD8+ T cells and was minimally affected by the depletion of CD4+ T cells. Similarly stimulated VR1012 immune SCs failed to lyse all the target cells tested. Together, these data indicated that immunization of BALB/c mice with TSA-1-encoding DNA plasmids efficiently primed parasite-specific CD8+ CTL precursors and that these in vivo-expanded cells were in sufficient numbers to allow the detection of their genetically restricted lytic activity without in vitro restimulation.
FIG. 4.
Induction of parasite-specific, MHC class I-restricted, CD8+-T-cell-dependent CTL response in TSA-1 DNA-immunized BALB/c mice. Two weeks after the second 100-μg intramuscular dose of either VR1012 TSA1.7, VR1012 TSA2.1, or unmodified VR1012 plasmid, immune SCs from BALB/c (H-2d) mice were prepared. (A) Unstimulated SCs were tested for CTL recognition of T. cruzi-infected or uninfected J774 macrophages (H-2d) and T. cruzi-infected 5A.Kb.α3 fibroblasts (H-2k; H-2Kb) in a 5-h 51Cr release assay at the indicated E/T ratios. (B) Following a 6-day stimulation period with irradiated T. cruzi-infected J774 macrophages, effector cells were assayed at the indicated E/T ratios for CTL activity on 51Cr-labeled T. cruzi-infected or uninfected 3T3 fibroblasts (H-2d) and T. cruzi-infected 5A.Kb.α3 cells. Effector cells depleted (Depl.) of CD4+ or CD8+ T cells were tested for CTL activity at a 50:1 E/T ratio against T. cruzi-infected 3T3 cells. Levels of infection in stimulator cells and target cells ranged from 65 to 75%.
A TSA-1 plasmid DNA-based vaccine significantly protects mice from T. cruzi-induced mortality.
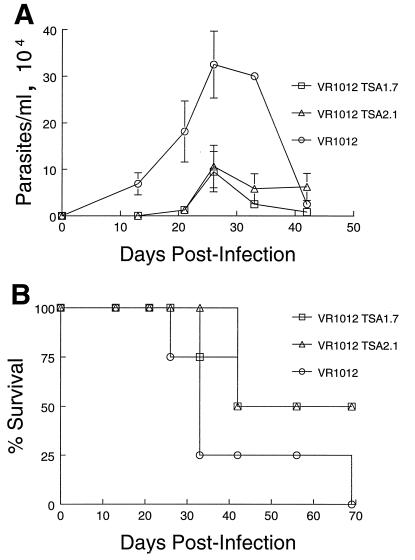
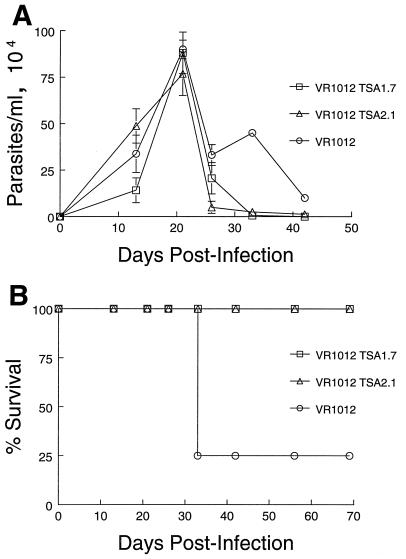
Having established that B6 and BALB/c mice generated T. cruzi-specific immune responses upon immunization with either of the TSA-1-expressing constructs, we next determined whether DNA vaccination could provide these animals with any degree of protection against challenge with T. cruzi. Two weeks after the second immunizing dose, groups of B6 and BALB/c mice were challenged with 105 or 103 T. cruzi BFT, respectively. The difference in the challenging dose was to compensate for the observed differences in susceptibility of the two strains of mice. Both strains of mice showed a significant degree of protection against T. cruzi-induced mortality. As illustrated in one of three conducted experiments, B6 mice vaccinated with either of the TSA-1-encoding vectors showed a 7-day delay in the onset of parasitemia and a consistently reduced level of parasites compared to control animals immunized with the unmodified VR1012 vector (Fig. 5A). Moreover, all control animals died before 45 days postinfection, whereas 50% of mice in each of the test groups survived the infection (Fig. 5B). In the case of BALB/c mice, however, the steady increase in parasitemia noted in TSA-1 DNA-vaccinated animals was strikingly similar to the kinetics of infection observed for mice immunized with the unmodified plasmid DNA (Fig. 6A). Despite similar levels of circulating parasites in test and control animals, none of the mice vaccinated with either of the TSA-1-encoding vectors succumbed to T. cruzi infection, whereas 75% of control mice developed fatal infections within 27 days postinfection (Fig. 6B). Overall, protection against an otherwise lethal inoculum with trypomastigotes was observed in 73 and 55% of VR1012 TSA1.7- and VR1012 TSA2.1-vaccinated B6 mice, respectively, and in 91 and 86% of similarly vaccinated BALB/c mice, respectively (Table 1). In contrast, control VR1012-vaccinated mice remained highly susceptible to T. cruzi-induced lethality, as only 9% overall survival was observed for both strains (Table 1).
FIG. 5.
Parasitemia and protection from T. cruzi-induced lethality in TSA-1 plasmid DNA-vaccinated B6 mice. Mice were injected intramuscularly with 100 μg of VR1012 TSA1.7, VR1012 TSA2.1, or control VR1012 plasmid at 0 and 4 weeks, followed 2 weeks later by intraperitoneal infection with 105 T. cruzi (Brazil strain) BFT. (A) Blood parasite levels in individual mice were monitored by using hemacytometer counts in 10 μl of tail vein blood diluted in an ammonium chloride lysing solution. Values represent mean ± standard errors of the means for surviving mice. (B) Mortality of vaccinated B6 mice infected with T. cruzi.
FIG. 6.
Parasitemia and protection from T. cruzi-induced lethality in TSA-1 plasmid DNA-vaccinated BALB/c mice. Mice were intramuscularly primed and boosted 4 weeks later with 100 μg of VR1012 TSA1.7, VR1012 TSA2.1, or control VR1012 plasmid. After 2 weeks, immunized animals were infected by intraperitoneal injection with 103 T. cruzi (Brazil strain) BFT. (A) Blood parasite levels were monitored as described for Fig. 5A. (B) Mortality of vaccinated BALB/c mice infected with T. cruzi.
TABLE 1.
Protection against lethal T. cruzi challenge conferred by DNA vaccinationa
| Plasmid DNA | B6 mice
|
BALB/c mice
|
||
|---|---|---|---|---|
| No. of survivors/no. challenged | % Survival | No. of survivors/no. challenged | % Survival | |
| VR1012 | 0/3 | 0/3 | ||
| 0/4 | 1/4 | |||
| 1/4 | 0/4 | |||
| Total | 1/11 | 9 | 1/11 | 9 |
| VR1012 TSA1.7 | 3/3 | 3/3 | ||
| 2/4 | 4/4 | |||
| 3/4 | 3/4 | |||
| Total | 8/11 | 73 | 10/11 | 91 |
| VR1012 TSA2.1 | 2/3 | 2/3 | ||
| 2/4 | 4/4 | |||
| 2/4 | NDb | |||
| Total | 6/11 | 55 | 6/7 | 86 |
Mice were primed intramuscularly with 100 μg of VR1012 TSA1.7, VR1012 TSA2.1, or control VR1012 plasmid and boosted 4 weeks later with a similar dose of the respective construct. After 2 weeks, immunized animals were infected by intraperitoneal injection with 103 T. cruzi (Brazil strain) BFT. Percent survival was assessed at day 100 postinfection.
ND, not determined.
DISCUSSION
Several observations on T. cruzi-infected hosts regarding the mechanisms involved in disease development and protective immunity provide strong support for the development of vaccines as a means to prevent or lessen the severity of Chagas’ disease (20–22, 41, 53, 56). Thus far, the exploration of vaccines against T. cruzi has been widely avoided due to the fear that such intervention methods would exacerbate rather than prevent a disease that many still consider to have an autoimmune etiology (26). However, a growing body of evidence indicates that it is the persistence of T. cruzi in the diseased tissue and not the parasite-induced immune responses to self molecules which correlates best with the induction and maintenance of the inflammatory disease process (5, 7, 25, 31, 56). This link between parasite load and severity of disease is further supported by the critical role that CD8+ T cells play in parasite control and survival after infection. CD8+ T cells constitute the major component in inflammatory foci of T. cruzi-infected tissues (22, 40, 47, 51), and in their absence (52, 54, 55), infected mice have increased mortality rates and tissue parasite loads with a decreased or absent inflammatory response. The recent demonstration of CD8+ CTL in T. cruzi-infected mice and humans with a specificity for defined trypomastigote and amastigote surface molecules (32, 61, 62) and of the immunoprotective phenotype that these cells express (61) prompted us to initiate the development of immunization strategies to further characterize the vaccine potential of parasite components known to be targets of protective anti-T. cruzi immune responses.
DNA-based immunization has been shown in animal models to easily, safely, and effectively elicit and modulate the spectrum of immune responses necessary for the prevention of infectious diseases (17, 34, 50, 59, 69, 70) and for the treatment of neoplastic (10, 24, 49), allergic (23, 39), and autoimmune (60) disorders. Thus, we chose this vaccination method to induce T. cruzi-specific antibody and class I-restricted CD8+ CTL responses in two inbred mouse strains and to assess its protective efficacy against parasite challenge. Our recent demonstration of TSA-1 as a target of protective CTL (61) made this parasite molecule a prime model antigen to evaluate this immunization method, inasmuch as (i) the N-proximal portion of TSA-1 had already been shown to induce antibody responses which correlate with survival after lethal T. cruzi infection (66) and (ii) TSA-1 is a member of the large 85-kDa family of trypomastigote surface proteins which are recognized by human sera and rodent-derived protective antibodies (2, 37).
Plasmid DNA vaccines VR1012 TSA1.7 and VR1012 TSA2.1 were constructed to drive the expression of products TSA-178–652 and TSA-178–790, which are truncated at the N terminus by 77 residues and at the C terminus by 183 and 45 amino acids, respectively. The main reasons for such a design were twofold: first, because removal of the N-terminal endoplasmic reticulum translocation signal sequence would ensure the cytoplasmic retention of de novo-synthesized TSA-1 protein, its subsequent cytosolic degradation, and an efficient priming of CTL responses; second, because conventional TSA-1 protein-based immunization of BALB/c mice has shown that the C-proximal portion encompassing residues 618 to 835 contains epitopes which interfere with the generation of antibodies to the protective determinants within residues 78 to 619 of the N-proximal portion (66).
Both VR1012 TSA-1 constructs directed the in vitro expression of cytoplasmically retained products with immunoreactivity to sera from T. cruzi-infected mice, and in BALB/c mice, both TSA-1-encoding vectors, with and without the repeat sequence, elicited parasite-specific antibody responses. Such responses were detected after the priming dose, and a modest boosting was achieved after the second dose with the VR1012 TSA2.1 vector. By contrast, in B6 mice, parasite-specific antibodies were detected only after the second dose of the VR1012 TSA2.1 vector alone. Similar strain-dependent variability in the induction of antibody responses following plasmid DNA immunization has been reported for the Plasmodium yoelii circumsporozoite and hepatocyte-erythrocyte proteins (13). This discrepancy, however, might be a reflection of the well-established genetic control of immune responses to T. cruzi (58, 67). We are currently seeking to improve the antibody responses to levels comparable to or higher than those generated by T. cruzi infection through the immunization with TSA-1-encoding DNA vaccines that codeliver cytokine genes that had been reported to enhance both humoral and cellular immune responses (9, 16, 24, 27, 68).
The fact that immunization with TSA-1-expressing plasmid DNA vaccines efficiently elicited MHC class I-restricted CTL responses in B6 (H-2b) and BALB/c (H-2d) mice is notable, inasmuch as prior to these studies, T. cruzi-specific CD8+ CTL had been primed only by parasite infection (32, 36, 61) and TSA-1 had been identified only as a CTL target molecule of B6 mice (61). The demonstration with B6 mice that TSA-1 DNA vaccination and T. cruzi infection were able to prime CD8+ CTL populations with specificity for the same protective H-2Kb-restricted TSA-1515–522 epitope indicated that similar immunogenic peptides are generated when a cell is transiently transfected in vivo or when it is expressed by an infected cell. In agreement with other studies where DNA immunization has been found to elicit long-lasting CTL responses (6, 57), TSA-1515–522-specific CTL were still detected 7 months after administration of the last dose of the TSA-1-encoding DNA. The longevity of the response may be explained by the persistence of the plasmid vaccine in vivo (64) or by recent reports which indicate that CTL memory does not require antigen persistence or CD4 T-cell help (1, 11). Regardless of the mechanisms involved, the ability of genetic immunization to maintain a long-lasting response to protective T. cruzi CTL epitopes may have significant potential for the development of DNA vaccines capable of preventing or treating an established T. cruzi infection.
While the presence of class I-restricted CTL responsive to T. cruzi-infected cells has been demonstrated in BALB/c mice (36), their target antigens have not been identified. Hence, in the absence of known TSA-1-derived H-2d-restricted CTL peptide epitopes, two alternative strategies were used to determine that TSA-1-expressing DNA vaccines had successfully primed parasite antigen-specific CTL responses. In the first strategy, where the CTL assay was performed on immune SCs without in vitro stimulation, significant genetically restricted CTL reactivity against T. cruzi-infected target cells was detected. These results suggest the priming of a substantial number of TSA-1-specific CTL precursors of which a large population remain in a state of activation that allows for their direct detection 2 weeks after the last dose of the DNA vaccine. Similar findings on the detection of CTL activity with unstimulated SCs from mice immunized with DNA vaccines have been reported for the Vif and Nef proteins of human immunodeficiency virus type 1 (27) and for the simian virus 40 T antigen (49). In the second strategy, the stimulating and targeting activities of T. cruzi-infected cells were used to confirm the specificity and MHC class I-restricted lytic activity displayed by in vitro-expanded CTL precursors. These findings and the fact that the lytic activity was CD8+ T cell dependent indicate that the observed response was T cell and not NK cell mediated and attest to the value of this method of immunization for priming potent MHC class I-restricted CTL responses in vivo.
Perhaps the most significant finding of these studies was that vaccination with TSA-1-expressing plasmid DNA afforded B6 and BALB/c mice significant levels of protection against lethal T. cruzi challenge infection. Overall survival rates of B6 mice vaccinated with VR1012 TSA1.7 or VR1012 TSA2.1 were 73 and 55%, respectively. The same constructs furnished BALB/c mice with nearly complete protection, as 91 and 86% of vaccinated animals, respectively, survived T. cruzi infection. These results are in sharp contrast to the 9% survival observed for animals immunized with the unmodified VR1012 plasmid for both strains of mice. It should be noted, though, that immunization with the TSA-1-encoding vectors did not prevent recipient mice from getting infected, and DNA-vaccinated mice from both strains developed parasitemias, albeit at different levels. In B6 mice, the number of circulating parasites in test animals was lower than that observed for recipients of the control DNA vaccine, whereas in BALB/c mice, parasitemias were frequently similar in both groups of animals.
The results presented here lay the foundation for DNA immunization as a strategy for the design of anti-T. cruzi vaccines. Using TSA-1 as the model antigen, we demonstrated that this type of antigen delivery was efficient in the induction of parasite-specific antibody and CTL responses as well as in providing significant protection in two inbred strains of mice against T. cruzi-induced lethality. Work is now in progress to determine whether the simultaneous delivery of plasmids encoding additional parasite antigens (33, 44) and immunomodulatory cytokines (9, 16, 24, 27, 68) can improve protection and induce efficacious immune responses in genetically diverse strains of mice. Such information may provide strong support for the development of DNA-based vaccines that not only might protect humans at risk of infection with T. cruzi but also may alleviate or prevent the pathogenic responses characteristic of chronic Chagas’ disease by reducing or perhaps eliminating tissue parasites from infected patients.
ACKNOWLEDGMENTS
We are grateful to Mark Heiges and Tami Rosario for excellent technical assistance. We also thank Jerry Manning and David Fouts for providing the TSA-1 genomic DNA clone and Peter Hobart for providing the VR1012 vector.
This work was supported by National Institutes of Health grant AI33106. R.L.T. is a Burroughs Wellcome Fund Scholar in Molecular Parasitology.
REFERENCES
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Alves M, Abuin G, Kuwajima V, Colli W. Partial inhibition of trypomastigote entry into cultured mammalian cells by monoclonal antibodies against a surface glycoprotein of Trypanosoma cruzi. Mol Biochem Parasitol. 1986;21:75–82. doi: 10.1016/0166-6851(86)90081-2. [DOI] [PubMed] [Google Scholar]
- 3.Andrews N, Einstein M, Nussenzweig V. Presence of antibodies to the major surface glycoprotein of Trypanosoma cruzi amastigotes in sera from Chagasic patients. Am J Trop Med Hyg. 1989;40:46–49. doi: 10.4269/ajtmh.1989.40.46. [DOI] [PubMed] [Google Scholar]
- 4.Barros H, Verbisck N, DaSilva S, Araguth M, Mortara R. Distribution of epitopes of Trypanosoma cruzi amastigotes during the intracellular life cycle within mammalian cells. J Eukaryot Microbiol. 1997;44:332–344. doi: 10.1111/j.1550-7408.1997.tb05675.x. [DOI] [PubMed] [Google Scholar]
- 5.Bellotti G, Bocchi E A, Villela de Moraes M A, Higuchi M L, Barbero-Marcial M, Sosa E, Esteves-Filho A, Kalil R, Weiss R, Jatene A, Pileggi F. In vivo detection of Trypanosoma cruzi antigens in hearts of patients with chronic Chagas’ heart disease. Am Heart J. 1996;131:301–307. doi: 10.1016/s0002-8703(96)90358-0. [DOI] [PubMed] [Google Scholar]
- 6.Böhm W, Kuhröber A, Paier T, Mertens T, Reimann J, Schirmbeck R. DNA vector constructs that prime hepatitis B surface antigen-specific cytotoxic T lymphocyte and antibody responses in mice after intramuscular injection. J Immunol Methods. 1996;193:29–40. doi: 10.1016/0022-1759(96)00035-x. [DOI] [PubMed] [Google Scholar]
- 7.Brandariz S, Schijman A, Vigliano C, Arteman P, Viotti R, Beldjord C, Levin M J. Detection of parasite DNA in Chagas’ heart disease. Lancet. 1995;346:1370–1371. doi: 10.1016/s0140-6736(95)92388-8. [DOI] [PubMed] [Google Scholar]
- 8.Ceredig R, Lowenthal J, Nabholz M, MacDonald H. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985;314:98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- 9.Chow Y, Chiang B, Lee Y, Chi W, Lin W, Chen Y, Tao M. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol. 1998;160:1320–1329. [PubMed] [Google Scholar]
- 10.Conry R, Lo Buglio A, Curiel D. Polynucleotide-mediated immunization therapy of cancer. Semin Oncol. 1996;23:135–147. [PubMed] [Google Scholar]
- 11.DiRosa F, Matzinger P. Long-lasting CD8+ T cell memory in the absence of CD4+ T cells or B cells. J Exp Med. 1996;183:2153–2163. doi: 10.1084/jem.183.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly J, Ulmer J, Shiver J, Liu M. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 13.Doolan D, Sedegah M, Hedstrom R, Hobart P, Charoenvit Y, Hoffman S. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ T cell-, interferon γ-, and nitric oxide-dependent immunity. J Exp Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dragon E, Brothers V, Wrightsman R, Manning J. A Mr 90 000 surface polypeptide of Trypanosoma cruzi as a candidate for a Chagas’ disease diagnostic antigen. Mol Biochem Parasitol. 1985;16:213–229. doi: 10.1016/0166-6851(85)90065-9. [DOI] [PubMed] [Google Scholar]
- 15.Fouts D, Ruef B, Ridley P, Wrightsman R, Peterson D, Manning J. Nucleotide sequence and transcription of a trypomastigote surface antigen gene of Trypanosoma cruzi. Mol Biochem Parasitol. 1991;46:189–200. doi: 10.1016/0166-6851(91)90043-6. [DOI] [PubMed] [Google Scholar]
- 16.Geissler M, Gesien A, Wands J. Enhancement of cellular and humoral immune responses to hepatitis C virus core protein using DNA-based vaccines augmented with cytokine-expressing plasmids. J Immunol. 1997;158:1231–1237. [PubMed] [Google Scholar]
- 17.Gurunathan S, Sacks D, Brown D, Reiner S, Charest H, Glaichenhaus N, Seder R. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J Exp Med. 1997;186:1137–1147. doi: 10.1084/jem.186.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson W, Chapman W, Waits V. Immunization of mice with irradiated Trypanosoma cruzi grown in cell culture: relations of number of parasites, immunizing injections and route of immunization to resistance. Int J Parasitol. 1976;6:341–347. doi: 10.1016/0020-7519(76)90057-6. [DOI] [PubMed] [Google Scholar]
- 19.Hartikka J, Sawdey M, Cornefert-Jensen F, Margalith M, Barnhart K, Nolasco M, Vahlsing H, Meek J, Marquet M, Hobart P, Norman J, Manthorpe M. An improved plasmid DNA expression vector for direct injection into skeletal muscle. Hum Gene Ther. 1996;7:1205–1217. doi: 10.1089/hum.1996.7.10-1205. [DOI] [PubMed] [Google Scholar]
- 20.Higuchi M, De Brito T, Reis M, Barbosa A, Bellotti G, Pereira-Barreto A, Pileggi F. Correlation between Trypanosoma cruzi parasitism and myocardial inflammatory infiltrate in human chronic chagasic myocarditis: light microscopy and immunohistochemical findings. Cardiovasc Pathol. 1993;2:101–106. doi: 10.1016/1054-8807(93)90021-S. [DOI] [PubMed] [Google Scholar]
- 21.Higuchi M, Reis M, Aiello V, Benvenutti L, Gutierrez P, Bellotti G, Pileggi F. Association of an increase in CD8+ T cells with the presence of Trypanosoma cruzi antigens in chronic, human, chagasic myocarditis. Am J Trop Med Hyg. 1997;56:485–489. doi: 10.4269/ajtmh.1997.56.485. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi M d L, Gutierrez P, Aiello V, Palomino S, Bocchi E, Kalil J, Bellotti G, Pileggi F. Immunohistochemical characterization of infiltrating cells in human chronic chagasic myocarditis: comparison with myocardial rejection process. Virchows Arch A Pathol Anat Histopathol. 1993;423:157–160. doi: 10.1007/BF01614765. [DOI] [PubMed] [Google Scholar]
- 23.Hsu C, Chua K, Tao M, Lai Y, Wu D, Huang S, Hsieh K. Immunoprophylaxis of allergen-induced immunoglobulin E synthesis and airway hyperresponsiveness in vivo by genetic immunization. Nat Med. 1996;2:540–544. doi: 10.1038/nm0596-540. [DOI] [PubMed] [Google Scholar]
- 24.Irvine K, Rao J, Rosenberg S, Restifo N. Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J Immunol. 1996;156:238–245. [PMC free article] [PubMed] [Google Scholar]
- 25.Jones E, Colley D, Tostes S, Lopes E, Vnencak-Jones C, McCurley T. Amplification of a Trypanosoma cruzi DNA sequence from inflammatory lesions in human chagasic cardiomyopathy. Am J Trop Med Hyg. 1993;48:348–357. doi: 10.4269/ajtmh.1993.48.348. [DOI] [PubMed] [Google Scholar]
- 26.Kalil J, Cunha-Neto E. Autoimmunity in Chagas disease cardiomyopathy: fulfilling the criteria at last? Parasitol Today. 1996;12:396–399. doi: 10.1016/0169-4758(96)10058-2. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Ayyavoo V, Bargarazzi M, Chattergoon M, Dang K, Wang B, Boyer J, Wiener D. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J Immunol. 1997;158:816–826. [PubMed] [Google Scholar]
- 28.Krettli A, Brener Z. Protective effects of specific antibodies in Trypanosoma cruzi infections. J Immunol. 1976;116:755–760. [PubMed] [Google Scholar]
- 29.Krettli A, Brener Z. Resistance against Trypanosoma cruzi associated to anti-living trypomastigote antibodies. J Immunol. 1982;128:2009–2012. [PubMed] [Google Scholar]
- 30.Kuhn R, Murnane J. Trypanosoma cruzi: immune destruction of parasitized mouse fibroblasts in vitro. Exp Parasitol. 1977;41:66–73. doi: 10.1016/0014-4894(77)90130-8. [DOI] [PubMed] [Google Scholar]
- 31.Lane J, Olivares-Villagomez D, Vnencak-Jones C, McCurley T, Carter C. Detection of Trypanosoma cruzi with the polymerase chain reaction and in situ hybridization in infected murine cardiac tissue. Am J Trop Med Hyg. 1997;56:588–595. doi: 10.4269/ajtmh.1997.56.588. [DOI] [PubMed] [Google Scholar]
- 32.Low H, Santos M, Wizel B, Tarleton R. Amastigote surface proteins of Trypanosoma cruzi are targets for CD8+ cytotoxic T lymphocytes. J Immunol. 1998;160:1817–1823. [PubMed] [Google Scholar]
- 33.Low H, Tarleton R. Molecular cloning of the gene encoding the 83 kDa amastigote surface protein and its identification as a member of the Trypanosoma cruzi sialidase superfamily. Mol Biochem Parasitol. 1997;88:137–149. doi: 10.1016/s0166-6851(97)00088-1. [DOI] [PubMed] [Google Scholar]
- 34.Lowrie D, Silva C, Colston M, Ragno S, Tascon R. Protection against tuberculosis by a plasmid DNA vaccine. Vaccine. 1997;15:834–838. doi: 10.1016/s0264-410x(97)00073-x. [DOI] [PubMed] [Google Scholar]
- 35.Moncayo A. Chagas’ disease: epidemiology and prospects for interruption of transmission in the Americas. World Health Stat Q. 1992;45:276–279. [PubMed] [Google Scholar]
- 36.Nickell S, Stryker G, Arevalo C. Isolation from Trypanosoma cruzi-infected mice of CD8+, MHC-restricted cytotoxic T cells that lyse parasite-infected target cells. J Immunol. 1993;150:1446–1457. [PubMed] [Google Scholar]
- 37.Ouaissi M, Taibi A, Cornette J, Velge P, Marty B, Loyens M, Esteva M, Rizvi F, Capron A. Characterization of major surface and excretory-secretory immunogens of Trypanosoma cruzi trypomastigotes and identification of potential protective antigen. Parasitology. 1990;100:115–124. doi: 10.1017/s0031182000060182. [DOI] [PubMed] [Google Scholar]
- 38.Peterson D, Wrightsman R, Manning J. Cloning of a major surface-antigen gene of Trypanosoma cruzi and identification of a nonapeptide repeat. Nature. 1986;322:566–568. doi: 10.1038/322566a0. [DOI] [PubMed] [Google Scholar]
- 39.Raz E, Tighe H, Sato M, Corr M, Dudler J, Roman M, Swain S, Spiegelberg H, Carson D. Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci USA. 1996;93:5141–5145. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reis D D, Jones E M, Tostes S J, Lopes E R, Gazzinelli G, Colley D G, McCurley T L. Characterization of inflammatory infiltrates in chronic chagasic myocardial lesions: presence of tumor necrosis factor-alpha+ cells and dominance of granzyme A+, CD8+ lymphocytes. Am J Trop Med Hyg. 1993;48:637–644. doi: 10.4269/ajtmh.1993.48.637. [DOI] [PubMed] [Google Scholar]
- 41.Reis M, Higuchi M, Benvenutti L, Aiello V, Gutierrez P, Bellotti G, Pileggi F. An in situ quantitative immunohistochemical study of cytokines and IL-2R+ in chronic human chagasic myocarditis: correlation with the presence of myocardial Trypanosoma cruzi antigens. Clin Immunol Immunopathol. 1997;83:165–172. doi: 10.1006/clin.1997.4335. [DOI] [PubMed] [Google Scholar]
- 42.Rotzschke O, Falk K, Stevanovic S, Jung G, Walden P, Rammensee H. Exact prediction of a natural T cell epitope. Eur J Immunol. 1991;21:2891–2894. doi: 10.1002/eji.1830211136. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 44.Santos M, Garg N, Tarleton R. The identification and molecular characterization of Trypanosoma cruzi amastigote surface protein-1, a member of the trans-sialidase gene super-family. Mol Biochem Parasitol. 1997;86:1–11. [PubMed] [Google Scholar]
- 45.Santos-Buch C A, Acosta A M. Pathology of Chagas’ disease. In: Tizard I, editor. Immunology and pathology of trypanosomiasis. Boca Raton, Fla: CRC Press; 1985. pp. 145–183. [Google Scholar]
- 46.Sarmiento M, Glasebrook A, Fitch F. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 47.Sato M, Yamashiro-Kanashiro E, Tanji M, Kaneno R, Higuchi M, Duarte A. CD8+ T cells and natural cytotoxic activity among spleen, blood, and heart lymphocytes during the acute phase of Trypanosoma cruzi infection in rats. Infect Immun. 1992;60:1024–1030. doi: 10.1128/iai.60.3.1024-1030.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schenkman S, Eichinger D, Pereira M, Nussenzweig V. Structural and functional properties of Trypanosoma trans-sialidase. Annu Rev Microbiol. 1994;48:499–523. doi: 10.1146/annurev.mi.48.100194.002435. [DOI] [PubMed] [Google Scholar]
- 49.Schirmbeck R, Böhm W, Reimann J. DNA vaccination primes MHC class I-restricted simian virus 40 tumor antigen-specific CTL in H-2d mice that reject syngeneic tumors. J Immunol. 1996;157:3550–3558. [PubMed] [Google Scholar]
- 50.Sedegah M, Hedstrom R, Hobart P, Hoffman S. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun J, Tarleton R. Predominance of CD8+ T lymphocytes in the inflammatory lesions of mice with acute Trypanosoma cruzi infection. Am J Trop Med Hyg. 1993;48:161–169. doi: 10.4269/ajtmh.1993.48.161. [DOI] [PubMed] [Google Scholar]
- 52.Tarleton R. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J Immunol. 1990;144:717–724. [PubMed] [Google Scholar]
- 53.Tarleton R. Immunity to Trypanosoma cruzi. In: Kaufmann S, editor. Host response to intracellular pathogens. R.G. Austin, Tex: Landes Company; 1996. pp. 227–247. [Google Scholar]
- 54.Tarleton R, Grusby M, Postan M, Glimcher L. Trypanosoma cruzi infection in MHC-deficient mice: further evidence for the role of both class I- and class II-restricted T cells in immune resistance and disease. Int Immunol. 1996;8:13–22. doi: 10.1093/intimm/8.1.13. [DOI] [PubMed] [Google Scholar]
- 55.Tarleton R, Koller B, Latour A, Postan M. Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature. 1992;356:338–340. doi: 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- 56.Tarleton R, Zhang L, Downs M. “Autoimmune rejection” of neonatal heart transplants in experimental Chagas disease is a parasite-specific response to infected host tissue. Proc Natl Acad Sci USA. 1997;94:3932–3937. doi: 10.1073/pnas.94.8.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomson S, Sherritt M, Medveczky J, Elliott S, Moss D, Fernando G, Brown L, Suhrbier A. Delivery of multiple CD8+ cytotoxic T cell epitopes by DNA vaccination. J Immunol. 1998;160:1717–1723. [PubMed] [Google Scholar]
- 58.Trischmann T, Bloom B. Genetics of murine resistance to Trypanosoma cruzi. Infect Immun. 1982;35:546–551. doi: 10.1128/iai.35.2.546-551.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ulmer J, Donnelly J, Parker S, Rhodes G, Felgner P, Dwarki V, Gromkowski S, Deck R, DeWitt C, Friedman A, Hawe L, Leander K, Martinez D, Perry H, Shiver J, Montgomery D, Liu M. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 60.Waisman A, Ruiz P, Hirschberg D, Gelman A, Oksenberg J, Brocke S, Mor F, Cohen I, Steinman L. Suppressive vaccination with DNA encoding a variable region gene of the T-cell receptor prevents autoimmune encephalomyelitis and activates Th2 immunity. Nat Med. 1996;2:899–905. doi: 10.1038/nm0896-899. [DOI] [PubMed] [Google Scholar]
- 61.Wizel B, Nunes N, Tarleton R. Identification of Trypanosoma cruzi trans-sialidase family members as targets of protective CD8+ TC1 responses. J Immunol. 1997;159:6120–6130. [PubMed] [Google Scholar]
- 62.Wizel B, Palmieri M, Mendoza C, Arana B, Sidney J, Sette A, Tarleton R. Human infection with Trypanosoma cruzi induces parasite antigen-specific cytotoxic T lymphocyte responses. J Clin Investig. 1998;102:1062–1071. doi: 10.1172/JCI3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wizel B, Rogers W, Houghten R, Lanar D, Tine J, Hoffman S. Induction of murine cytotoxic T lymphocytes against Plasmodium falciparum sporozoite surface protein 2. Eur J Immunol. 1994;24:1487–1495. doi: 10.1002/eji.1830240705. [DOI] [PubMed] [Google Scholar]
- 64.Wolff J, Ludtke J, Acsadi G, Williams P, Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genet. 1992;1:363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 65.World Health Organization. Twelfth Programme Report of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, Geneva, Switzerland. 1995. Tropical disease research. Progress 1975–94. Highlights 1993–94; pp. 125–134. [Google Scholar]
- 66.Wrightsman R, Dawson B, Fouts D, Manning J. Identification of immunodominant epitopes in Trypanosoma cruzi trypomastigote surface antigen-1 protein that mask protective epitopes. J Immunol. 1994;153:3148–3154. [PubMed] [Google Scholar]
- 67.Wrightsman R, Krassner S, Watson J. Genetic control of responses to Trypanosoma cruzi in mice: multiple genes influencing parasitemia and survival. Infect Immun. 1982;36:637–645. doi: 10.1128/iai.36.2.637-644.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiang Z, Ertl H. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 69.Xiang Z, Spitalnik S, Tran M, Wunner W, Cheng J, Ertl H. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology. 1994;199:132–140. doi: 10.1006/viro.1994.1105. [DOI] [PubMed] [Google Scholar]
- 70.Yokoyama M, Zhang J, Whitton J. DNA immunization confers protection against lethal lymphocytic choriomeningitis virus infection. J Virol. 1995;69:2684–2688. doi: 10.1128/jvi.69.4.2684-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshida N. Trypanosoma cruzi: recognition of trypomastigote surface antigens by lytic antisera from mice resistant to acute infection. Exp Parasitol. 1986;61:184–191. doi: 10.1016/0014-4894(86)90151-7. [DOI] [PubMed] [Google Scholar]