Abstract

Currently, there are no FDA-approved medications for the treatment of psychostimulant use disorders (PSUD). We have previously discovered “atypical” dopamine transporter (DAT) inhibitors that do not display psychostimulant-like behaviors and may be useful as medications to treat PSUD. Lead candidates (e.g., JJC8-091, 1) have shown promising in vivo profiles in rodents; however, reducing hERG (human ether-à-go-go-related gene) activity, a predictor of cardiotoxicity, has remained a challenge. Herein, a series of 30 (([1,1′-biphenyl]-2-yl)methyl)sulfinylalkyl alicyclic amines was synthesized and evaluated for DAT and serotonin transporter (SERT) binding affinities. A subset of analogues was tested for hERG activity, and the IC50 values were compared to those predicted by our hERG QSAR models, which showed robust predictive power. Multiparameter optimization scores (MPO > 3) indicated central nervous system (CNS) penetrability. Finally, comparison of affinities in human DAT and its Y156F and Y335A mutants suggested that several compounds prefer an inward facing conformation indicating an atypical DAT inhibitor profile.
Keywords: atypical dopamine transporter inhibitors, dopamine transporter, DAT, serotonin transporter, psychostimulants, cocaine, human ether-à-go-go-related gene, hERG, multiparameter optimization score, MPO
Deaths related to use of psychostimulants, such as cocaine and methamphetamine, have surged by more than 13-fold between 1999 and 2021, with a steep increase of nearly 300% recorded between 2013 and 2019.1−3 Indeed, the current opioid overdose crisis includes fatalities of people who have used cocaine or methamphetamine that has been contaminated with fentanyl and has caused the death of these unsuspecting individuals.4 A combination of FDA approved medication with behavioral therapy, termed medication-assisted treatment, has proven more effective in treating substance use disorders (SUD) than employing monotherapies.5−8 However, psycho-stimulant use disorders (PSUD) still lack FDA approved pharmacotherapies. Thus, effective medications for the treatment of PSUDs are urgently needed.
The rewarding effects imparted by psychostimulants stem primarily from their inhibition of dopamine (DA) reuptake via DAT9−14 resulting in a surge of synaptic DA and increased neurotransmission that can lead to euphoria and potential misuse.9−14 Molecular docking simulations15,16 and X-ray crystallography17 suggest that cocaine prefers binding DAT in an outward-facing conformation, whereas atypical DAT inhibitors, such as modafinil, JHW007, and JJC8-091 (1; Figure 1), bind to the same site as DA and cocaine, but in a more occluded inward-facing conformation, which has been associated with their lack of cocaine-like behaviors including predicted addictive liability.18−25
Figure 1.

Chemical structures of modafinil and analogues.
Modafinil (Figure 1) is a weak DAT inhibitor that has shown some positive effects in treating PSUD in humans but is not FDA approved as a medication for that purpose.26−28 A wide range of atypical DAT inhibitors modeled after the bisphenyl scaffold of modafinil have been synthesized and evaluated.23,29−35 For example, preclinically, compound 1 (Figure 1) exhibited a noncocaine-like behavioral profile, and its pretreatment reduced cocaine-induced reinstatement to drug seeking behaviors in rats.22 In addition, compound 1 was effective in models of reducing both short and long access methamphetamine self-administration.36 More recently, the sulfenyl-cis-2,6-dimethylpiperazine modafinil analogue, RDS03-94 (2; Figure 1), was found to have ∼10-fold higher DAT affinity than 1, in rat brain tissue, but with modest cocaine-like locomotor stimulant effects, in mice.31 Moreover, 2 showed a reduced hERG (human ether-à-go-go-related gene)/DAT ratio compared to 1, improving the predicted cardiac safety profile at a therapeutically relevant dose.31 hERG affinity has become an important metric in drug development, as blockade of hERG may prolong the QT interval, which could lead to the lethal cardiac arrhythmia torsade de pointes.37−39 Nevertheless, 2 showed relatively poor metabolic stability in mouse liver microsomes,31 which has led to exploration of new scaffolds and computational modeling studies. A new bisphenyl analogue, S,S-CE-158, has recently been highlighted as a high affinity DAT inhibitor that may have promise for improving cognitive impairment through increasing DA levels in the nucleus accumbens core.40 It will be very interesting to see if this compound also is effective in inhibiting behavioral actions of psychostimulant drugs such as cocaine or methamphetamine.
To further explore the structure–activity relationships (SAR) in this class of compounds, diverse modifications on the piperazine linker and the 2-propanol terminus have been investigated,31−34 but the bisphenyl scaffold itself has not been probed. However, a series of biphenyl analogues of modafinil, such as compound 3 (Figure 1), were examined for their effect on sleep in rats.41,42 The peripheral aryl group was functionalized with various substituents and was placed ortho-, meta-, and para- to the sulfeinylacetamide moiety.41 The ortho-aryl substituted compounds, especially compound 3, were found to be the most promising at promoting wakefulness within the study, illuminating an interesting template to explore chemically.41
Moreover, our machine learning-based quantitative SAR (QSAR) models of hERG activity predicted that the biphenyl series may have reduced hERG activity compared to the 4′F-bisphenyl analogues.16 These studies inspired us to synthesize a series of new compounds combining the biphenyl moiety of 3 and sulfe(i)nyl-piperazine 2-propanol scaffolds of 1 and 2 to further our SAR of atypical DAT inhibitors with potentially reduced hERG activity, with a focus on this diaryl pharmacophore.
All final compounds were evaluated for DAT and SERT binding in rat brain (Table 1). A subset of analogues was tested for hERG channel activity compared to 1 and previously reported analogues 2, 23–35. Additionally, we compared the hERG affinities predicted by our QSAR models and their corresponding experimental measurements. Furthermore, compounds 9k, 10b, 10i, 10k, 10l, and 21a were also tested and compared to cocaine and 1 for binding at WT DAT, and the Y156F and Y335A mutants, which were heterologously expressed in cell lines. The ratios between the binding affinities in WT DAT compared to those in either the Y156F or Y335A DAT mutants have previously been used to predict if a compound demonstrates a classical (outward facing) or more atypical (inward facing) DAT binding profile.20,21
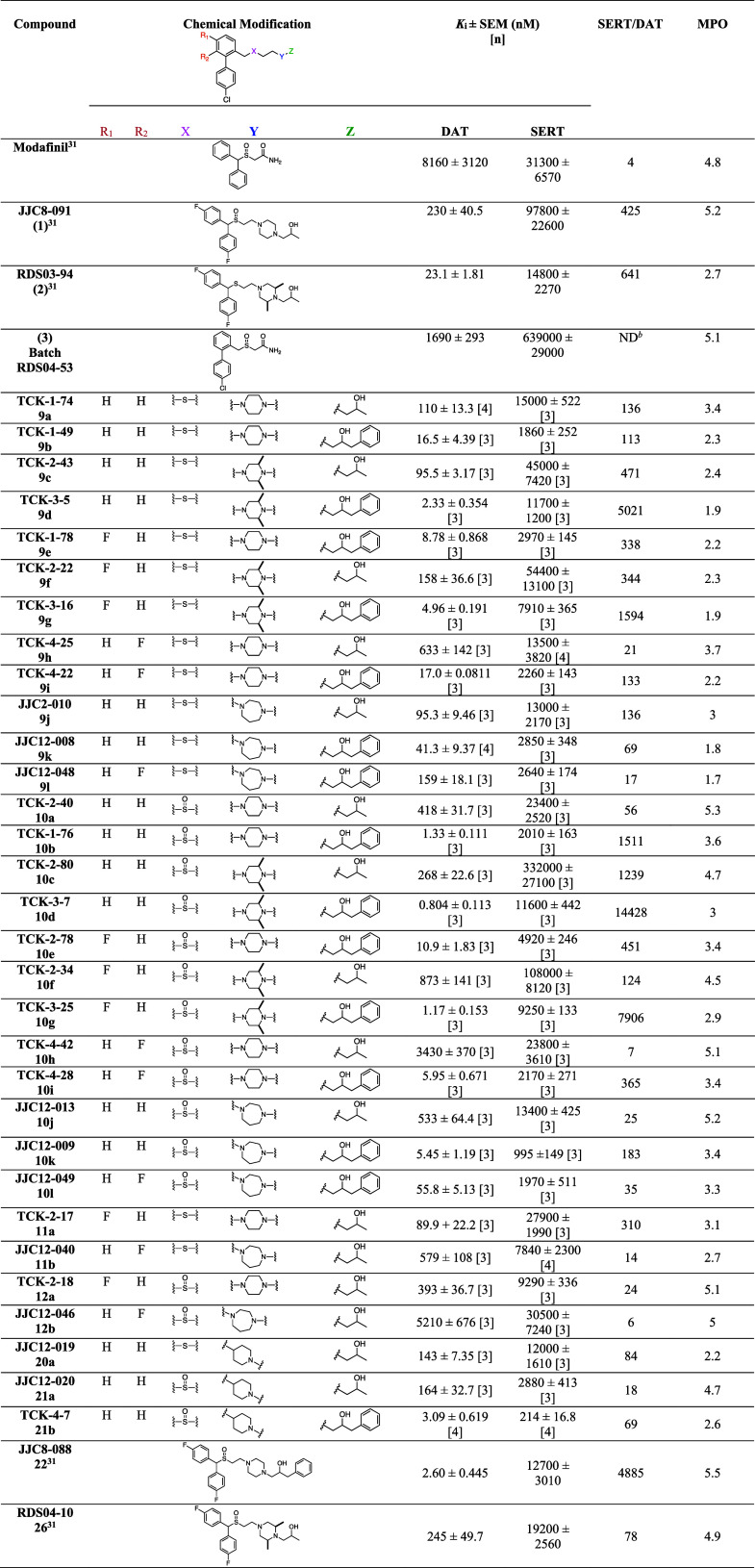
Table 1. Binding Data for Sulfenyl- and Sulfinylalkylamine Analoguesa.
Each Ki value represents data from at least three independent experiments, each performed in triplicate. Ki values were analyzed by PRISM. Binding assay procedures are described in detail in Experimental Methods. [n] = number of experiments.
ND; not determined.
Results and Discussion
Chemical Synthesis
Synthesis of novel piperazine and diazepane sulfenylalkylamines (9a–9l and 11a–11b) and sulfinylalkylamines (10a–l and 12a–b) was achieved as depicted in Schemes 1 and 2. Compounds 5a–c were obtained by coupling 4a–c with mercaptoethanol under basic condition in 92–97% yield. The alcohols were converted to intermediates 6a–c with 4-chlorobenzeneboronic acid under Suzuki coupling conditions in 62–77% yield. Compounds 7a–c were obtained by bromination of 6a–c, via the Appel reaction in 55–99% yield. Alkylation of 7a–c with the appropriate piperazine or 7b–c with 1,4-diazepane furnished key intermediates 8a–g or 11a–b between 82 and ∼100% yield. As previously reported,23 epoxide ring opening using the appropriate oxiranes gave 9a–l. Subsequent sulfenyl oxidation into the sulfinyl with H2O2 in methanol/acetic acid under room temperature gave final products 10a–l in 35–93% yield (Scheme 1). Alkylation of intermediate 7b and 7c with 1-(piperazin-1-yl)propan-2-ol or 1-(1,4-diazepan-1-yl)propan-2-ol afforded compounds 11a and 11b in 70 and 37% yield, respectively. Lastly, oxidation of the sulfenyl compounds was performed as described for compounds 10a–l, to afford 12a–b in 71–90% yield (Scheme 2). Of note, all final compounds are racemic or diastereomeric mixtures and were not separated.
Scheme 1. Synthesis of Compounds 9a–9l and 10a–l.
Reagents and conditions: (a) mercaptoethanol, K2CO3, ACN, 55 °C, overnight; (b) 4-chlorobenzeneboronic acid, Pd(PPh3)4, Na2CO3 solution (2.7 M in H2O), toluene/EtOH = 1/1, 90 °C, overnight; (c) PPh3, CBr4, ACN, room temperature, 2 h; (d) piperazine or 1,4-diazepane, K2CO3, ACN, 60 °C, overnight; (e) appropriate oxirane, isopropanol, 90 °C, overnight; (f) H2O2, AcOH/MeOH, overnight.
Scheme 2. Synthesis of Compounds 11a–b and 12a–b.
Reagents and conditions: (a) 1-(piperazin-1-yl)propan-2-ol or 1-(1,4-diazepan-1-yl)propan-2-ol, K2CO3, ACN, 60 °C, overnight; (b) H2O2, AcOH/MeOH, overnight.
Syntheses of the piperidine sulfenylalkylamine (20a and 20b) and sulfinylalkylamine analogues (21a and 21b) were achieved as depicted in Scheme 3. Bromination of alcohol 13 to give 15 was achieved under Appel conditions in 69% yield. Thiourea was used to convert compound 14 to thiol 16 in 89% yield. Conjugation of 15 with 16 to give 17 under alkylation conditions in acetonitrile (ACN) was followed by Suzuki coupling with 4-chlorobenzeneboronic acid to obtain the Boc-protected biphenyl intermediate 18 in 62% yield. Deprotection with TFA afforded 19 in quantitative yield. As described above, the piperidine sulfenylalkylamine (20a and 20b) were prepared with the appropriate oxirane in 48–62% yield followed by oxidation with H2O2 in methanol/acetic acid to give sulfinylalkylamine analogues (21a and 21b) in 34–69% yield.
Scheme 3. Synthesis of Compounds 20a–b and 21a–b.
Reagents and conditions: (a) PPh3, CBr4, DCM, room temperature, overnight; (b) thiourea, H2O/ethanol = 1/0.7, reflux, 45 min; (c) K2CO3, ACN, 60 °C, overnight; (d) 4-chlorobenzeneboronic acid, Pd(PPh3)4, Na2CO3 solution (2.7 M in H2O), toluene/ethanol = 1/1, 90 °C, overnight; (e) TFA, CH2Cl2, room temperature, overnight; (f) appropriate oxirane, isopropanol, 90 °C, overnight; (g) H2O2, AcOH/MeOH, overnight.
SAR at DAT and SERT
All final compounds were evaluated for binding at DAT and SERT in rat brain membranes and compared to 1 and 2. Previously reported compounds JJC8-088 (22) and RDS04-010 (23) were also included for comparison. The DAT and SERT binding affinities (Ki values) and SERT/DAT ratios are presented in Table 1.
In our previously reported 4′F-bisphenyl series, two major SAR features have been consistent: (1) sulfides typically have higher affinities at DAT than their analogous sulfoxides and (2) a terminal phenyl ring further increases binding affinities. Nevertheless, in both cases, these features reduce metabolic stability, which is detrimental to interpreting in vivo studies and precludes further development.
In the first set of compounds evaluated, the (bis(4-fluorophenyl)methyl) substituent was replaced with a 4′-chloro-2-methyl-1,1′-biphenyl group. Compared to 1, 10a showed a similar DAT affinity (Ki = 418 nM). Compound 9a, also devoid of the 2,6-dimethyl substituents of 2 on the piperazine ring, showed a 4.8-fold reduction in DAT affinity compared to 2. However, based on the comparable DAT affinity (Ki = 95.5 nM) of 9c, the 2,6-dimethyl substituents did not play a major role in the affinities of these compounds. Likewise, 10c showed comparable DAT affinity (Ki = 268 nM) to 1. Compound 10b showed comparably high affinity for DAT (Ki = 1.33 nM) to 22 as did 10d (Ki = 0.804 nM), but here SAR diverged from the 4′F-bisphenyl analogues in that the sulfide, 9d, showed a ∼ 3-fold lower affinity at DAT. Adding a F at R1, served to modestly decrease DAT affinities for all the sulfoxides, compared to their unsubstituted counterparts, e.g., 10e vs 10b. However, the sulfides were not adversely affected, e.g., 9d vs 9g. When the F was moved to R2, DAT affinities were uniformly decreased, e.g., 9h vs 9a.
Another modification of the piperazine ring in 1 or the 2,6-dimethyl piperazine in compound 2 to improve metabolic stability was to replace these piperazines with either a homopiperazine or a piperidine.34 This modification to compound 2 decreased DAT affinity by ∼4-fold, for 9j (Ki = 95.3 nM) and, as observed in the 4′F-bisphenyl series, the sulfoxide 10j showed lower affinity at DAT (Ki = 533 nM) compared to 1. Interestingly, the reverse was true with the compounds with terminal phenyl rings, where 10k had a similar DAT affinity compared to 22 (Ki = 5.45 v. 2.60 nM, respectively). Unlike the previous pair, the sulfide, 9k, had ∼8-fold lower affinity than the sulfoxide, 10k, further demonstrating that the importance of these two substituents in how these molecules bind DAT.22 Overall, the F-substituents in the R1 and R2 positions served to decrease affinities at DAT in the homopiperazine series. Finally, the replacement of the piperazine function with piperidine was relatively well-tolerated at DAT, although 20a showed ∼6-fold lower affinity than 2 at DAT. The sulfoxide and sulfide analogues, 20a and 21a, showed essentially the same DAT affinities (Ki = 143 and 164 nM, respectively).
All of these analogues were selective for DAT over SERT. However, selectivities were dramatically different ranging from 6-fold for 12b to >14,000-fold for 10d.
In addition to searching for atypical DAT inhibitors with high to moderate DAT binding affinities, we also attempted to optimize central nervous system (CNS) permeability by aiming for a multiparameter optimization (MPO)43,44 score of >3. MPO scores in this series ranged from 1.8 to 5.3, with approximately half of the compounds meeting our criteria of >3. Physicochemical parameters used for the MPO calculations are tabulated for those compounds also tested for hERG channel activity (Table S1). Of note, all compounds with MPO scores of 4 or higher were sulfoxides.
Molecular Pharmacology and Mutagenesis Studies
We next evaluated the binding ratios of compounds 9k, 10b, 10i, 10k, 10l, and 21a compared to cocaine and compound 1 in hDAT wildtype (WT) and the Y156F mutant to preliminarily assess the nature of DAT binding in vitro, as has been described previously with earlier generation analogues of benztropine and modafinil.20−23 In hDAT WT, substrate binding has been predicted to generate a H-bond between the OH-group of Tyr156 and Asp79. The H-bond contributes to the conformational changes that eventually expose the binding site toward the intracellular environment. Molecular docking simulations have shown that cocaine prefers to bind hDAT in an outward facing conformation that does not interfere with this H-bond formation. Consequently, cocaine binds with similar affinities to the WT and hDAT Y156F where the OH group of tyrosine (Y) residue has been removed by replacing it with a phenylalanine (F), yielding the hDAT Y156F mutant.15,23 However, binding of the atypical DAT inhibitors prefer a more occluded DAT conformation when binding, which depends on the Tyr156–Asp79 H-bond formation.15,20,45 Hence, their binding affinities are typically decreased in hDAT Y156F resulting in a >2-fold decrease in Ki between hDAT WT and hDAT Y156F (see Table 2, affinity ratio).19,20
Table 2. Ki Values for Inhibition of [3H]WIN35,428 Binding by Indicated Compounds to hDAT WT and hDAT Y156Fa.
| compound | WT Ki (nM) | n | Y156F Ki (nM) | n | affinity ratio |
|---|---|---|---|---|---|
| cocaine | 287 [244; 336] | 3 | 326 [247; 431] | 3 | 1.1 |
| 1 | 1020 [927; 1130] | 3 | 7560 [3980; 14400] | 3 | 7.4 |
| 9k | 55.4 [49.4; 62.2] | 3 | 402 [360; 450] | 3 | 7.3 |
| 10b | 6.74 [6.44; 7.05] | 3 | 248 [195; 317] | 5 | 37 |
| 10i | 50.6 [49.3; 51.9] | 3 | 618 [469; 882] | 4 | 12 |
| 10k | 37.5 [35.4; 39.7] | 3 | 346 [309; 387] | 3 | 9.2 |
| 10l | 244 [219; 272] | 3 | 1090 [7610; 1560] | 4 | 4.5 |
| 21a | 86.9 [84.8; 89] | 3 | 1130 [1040; 1230] | 3 | 13 |
The Ki values are determined from their respective IC50 values in inhibiting [3H]WIN35,428 binding using the Cheng–Prusoff equation. Experiments were performed on intact COS7 cells transiently expressing hDAT WT or hDAT Y156F mutant. All measurements were performed in technical triplicate with the indicated n number of biological replicates. Values are shown as mean [SEM interval] and are calculated from pIC50 and the SE interval from pIC50 ± SE. See Experimental Methods section for details.
Inhibition of [3H]WIN35,428 binding on COS7 cells transiently expressing hDAT WT or hDAT Y156F was determined for a subset of biphenyl analogues. As shown in Table 2, all these compounds showed a Y156F/WT hDAT ratio of >2, suggesting a different binding preference than cocaine that is more effected by the disruption of the Y156–D79 H-bond formation.
In the next set of experiments, these same analogues were tested for inhibition of [3H]DA uptake in hDAT WT and hDAT Y335A (Table 3). In this case, the Y335A mutation induces a more inward facing conformation of hDAT, which dramatically reduces the binding affinity of cocaine.21,46 However, if most of these analogues are predicted to prefer a more inward occluded conformation, this mutation would be expected to have less of an adverse effect on binding relative to cocaine. Indeed, for most of the analogues tested, the Y335A/WT ratios were <70. However, compounds 10b (TCK1-76) and 10k (JJC12-009) showed similar binding affinity ratios to cocaine of 72 and 69, respectively.
Table 3. Ki Values for Inhibition of [3H]DA Uptake by Indicated Compounds to hDAT WT and the Y335A Mutanta.
| compound | WT Ki (nM) | n | Y335A Ki (nM) | n | affinity ratio |
|---|---|---|---|---|---|
| cocaine | 219 [191; 250] | 5 | 13800 [12700; 15100] | 6 | 70 |
| 9b | 39.8 [30.6; 51.8] | 6 | 1010 [895; 1140] | 4 | 25 |
| 9k | 78.8 [73.2; 84.8] | 3 | 1670 [1540; 1810] | 3 | 21 |
| 10b | 7.93 [6.99; 8.99] | 3 | 573 [469; 700] | 5 | 72 |
| 10i | 50.5 [42.1; 60.5] | 4 | 1580 [1450; 1730] | 3 | 31 |
| 10k | 42.7 [40.2; 45.4] | 3 | 2930 [2660; 3230] | 3 | 69 |
| 10l | 225 [179; 283] | 3 | 6130 [5740; 6540] | 3 | 27 |
| 21a | 238 [217; 262] | 3 | 8660 [7840; 9560] | 3 | 36 |
The Ki values are determined from their respective IC50 values in inhibiting [3H]DA uptake using the Cheng–Prusoff equation. Experiments were performed on intact COS7 cells transiently expressing hDAT WT or hDAT Y335A. All measurements were performed in technical triplicate with the indicated n number of biological replicates. Values are shown as mean [SEM interval] and are calculated from pIC50 and the SE interval from pIC50 ± SE. See Experimental Methods section for details.
hERG Predicted and Experimental Data
As described in the introduction, hERG channel activity is considered to be a predictor of cardiotoxicity and thus decreasing hERG activity in this series of compounds was another goal for this study. We evaluated 14 compounds in this biphenyl series and compared their experimentally determined and computationally predicted hERG channel activity data to 16 previously described 4′F-bisphenyl analogues. Our aim was to determine if the biphenyl analogues would improve the hERG/DAT affinity ratio, with the goal of >30.
Based on the experimentally determined hERG activity, as shown in Table 4, the only 4′F-bisphenyl analogues to show a hERG/DAT affinity ratio of >30 were the sulfoxides 22 and 27. The most notably high hERG/DAT affinity ratios of >500 were all biphenyl analogues that were also sulfoxides and bore terminal phenyl rings. Of note, these ratios were largely driven by very high DAT affinities in the low nanomolar range. More modest hERG/DAT affinity ratios of >30 were observed for the biphenyl analogues 9c, 10i, and 10k.
Table 4. Predicted and Experimental hERG Activity Dataa.

The experiment response of hERG currents to various compounds at various concentrations were measured through whole-cell voltage clamp experiments. The results of each compound were fitted to Hill equation to obtain the IC50 and Hill coefficient values. IC50 values and Hill coefficients were determined from at least three independent experiments performed in triplicate and are reported as mean ± SEM (μM). The predictions were made using the hERG QSAR models trained with the ChEMBL 31 data set.
We previously gathered all available hERG data from the ChEMBL database and selected IC50 data to train our machine-learning QSAR models for hERG after careful filtering and curation. Utilizing both random forest (RF) and eXtreme gradient boosting (XGBoost) algorithms, we constructed and optimized our QSAR models. The results showed that XGBoost-trained models surpassed those trained with RF, and models using clamp data slightly outperformed those using binding data in external validation. Compared to our previous QSAR models trained with the ChEMBL 25 data set,16 in this study, the benchmarking results of our updated models trained with the ChEMBL 31 data set demonstrated enhanced predictive power with the coefficient of determination (R2) increasing from 0.70 to 0.73. The linear and monotonic correlations, represented by the Pearson and Spearman Rank correlation coefficients (RPearson and RSpearman, respectively), also improved: RPearson from 0.84 to 0.88 and RSpearman from 0.74 to 0.84 (Figure 2A,B). As described in the Experimental Methods section, the ChEMBL 31 data set has 525 more data points than the ChEMBL 25 data set, which clearly enriched the training data set, resulting in better performance.
Figure 2.
Correlations between predicted and experimental hERG affinities. For the 18 compounds that we have made predictions previously, we compared the results from the QSAR models built from either the ChEMBL 25 (A) or the ChEMBL 31 (B) data set. The calculations of both Pearson and Spearman Rank correlation coefficients (R(p) and R(s), respectively) show that the ChEMBL 31 data set-based models outperform those based on ChEMBL 25 data set. Panel (C) shows the correlations of all 30 compounds from Table 4 using QSAR models built from the ChEMBL 31 data set. The 18 compounds used as the validation data set previously are shown in green, and the 12 new compounds added in this study are shown in yellow. Note that the green markers in (B,C) are in the same locations. For each compound, the mean (center point) and standard deviation (lines) of the predicted pIC50 values are plotted. 4′F-bisphenyl compounds are shown as “•”, while biphenyl compounds are shown as ‘x’.
Therefore, the models trained with the ChEMBL 31 data set were used to make predictions for 12 additional compounds (marked yellow in Figure 2C; the predicted IC50 values are reported in Table 4). The resulting RPearson and RSpearman indicate a strong, positive linear and monotonic relationship between experimental and predicted pIC50 (0.82 and 0.73, respectively). This strong monotonic relationship indicates that the hERG affinities can be predicted with reliability. Our QSAR models have practical utility in guiding future SAR exploration and screening potential candidates for synthesis, either independently or in conjunction with experimental assays.
Conclusions
In summary, based on previous machine learning-based QSAR model-derived hERG channel activity predictions,16 we designed and synthesized 30 (([1,1′-biphenyl]-2-yl)methyl)sulfinylalkyl alicyclic amines as novel and high affinity atypical DAT inhibitors with reduced hERG activity. We evaluated these compounds for binding at DAT and SERT and compared them to several previously described 4′F-bisphenyl analogues. We determined that replacement of the 4′F-bisphenyl moiety with the (4′-(chloro-[1,1′-biphenyl]-2-yl)methyl)-substituent was generally well tolerated at DAT and all compounds remained selective over SERT.
Interestingly, as in the 4′F-bisphenyl series, the compounds with terminal phenyl rings showed highest affinities for DAT, but in contrast to the 4′F-bisphenyl series, the sulfoxides in the (4′-(chloro-[1,1′-biphenyl]-2-yl)methyl)-series typically showed higher affinities for DAT than their sulfide analogues. This finding is important as the sulfoxides have previously been shown to be more metabolically stable than the sulfides and further, the sulfoxide plays an important role in the inward facing conformation of DAT that these compounds prefer and has been related to nonpsychostimulant behavioral profiles.48,49 In addition, the sulfoxides generally had MPO scores > 3, suggesting these compounds would be more brain penetrant than the sulfides.
Importantly, although addiction is a human behavior for which animal models are still used to determine therapeutic potential, computational predictive tools like the ones described herein, as well as in vitro studies using transfected cell lines, to predict behavior can be used to implement the principle of three R’s (i.e., replacement, reduction, and refinement), as first described by Russell and Burch.50 This remains a guiding tenet for the use of animals in research.51,52 Importantly, using computational and in vitro predictive studies, we can now strategize new directions in which to take our drug design that have predicted MPO scores of >3 and those with low predicted hERG activity or high hERG/DAT ratios before synthesizing and testing the next series of compounds in vivo.
What we conclude from this study is that the (4′-(chloro-[1,1′-biphenyl]-2-yl)methyl)-analogues, and especially those with a sulfoxide and terminal phenyl ring might provide an avenue for safer medications (lower hERG activity/higher hERG/DAT ratios). However, based on our previous SAR studies34 as well as preliminary stability studies in rat liver microsomes (Table S3), the compounds with a piperazine linker and terminal phenyl ring will need further modification to achieve better metabolic stability. These results underscore the effectiveness of using cell-based models and predictive tools such as MPO scores and especially, our hERG QSAR models in guiding the rational design of novel DAT inhibitors.
Finally, the Y156F and Y335A hDAT mutation studies supported an atypical DAT profile for most of the analogues tested. Additional molecular simulations are ongoing to investigate this further and ultimately aid in identifying new lead compounds for further development.
Experimental Methods
Synthesis
All reagents and solvents used for chemical synthesis and buffer preparation were purchased from commercial sources unless otherwise stated and used without additional purification. Spectroscopic data and yields are reported for the compounds as free bases. All flash chromatography was performed using prepacked silica gel cartridges (Teledyne ISCO, RediSep Rf Gold 20–40 μm and RediSep Rf Flash columns 40 to 60 μm) in a combiFlash instrument (combiFlash RF or combiFlash EZ prep, Teledyne ISCO). 1H and 13C spectra were acquired using a Varian Mercury Plus 400 spectrometer. 1H chemical shifts are reported as parts per million (δ ppm) relative to tetramethylsilane (0.00 ppm). All the coupling constants are measured in Hz. Chemical shifts for 13C NMR spectra are reported as parts per million (δ ppm) relative to deuterated solvents. Chemical shifts, multiplicities and coupling constants (J) have been reported and calculated using Vnmrj Agilent-NMR 400MR or MNova 9.0. Combustion analyses were performed by Atlantic Microlab, Inc. (Norcross, GA) or Robertson Microlit Laboratories (Ledgewood, NJ) and agree within ±0.4% of calculated values.
Electrospray Ionization Mass Spectrometry
Neat solutions of samples were dissolved and diluted in ACN. Samples were analyzed by direct injection (10 μL) using a Vanquish UHPLC system (ThermoFisher, Waltham, MA) with tandem Orbitrap Exploris 120 mass spectrometer (ThermoFisher). The flow rate was 200 μL/min with an isocratic mobile phase of 80% ACN for the 4 min run. Analysis was performed using a heated electron spray ionization (HESI) source in positive ion mode. In MS mode, the mass resolution was set at 120,000, while in MS/MS mode, the mass resolution was set at 15,000. HPLC analysis was performed using an Agilent system coupled with DAD (Diode Array Detector). Melting point determination was conducted using an OptiMelt automated melting point system and are uncorrected. Based on NMR, combustion data and HPLC, all final compounds are >95% pure.
2-((2-Iodobenzyl)thio)ethan-1-ol (5a)
Commercially available 1-(bromomethyl)-2-iodobenzene (246 mg, 0.83 mmol) was dissolved in ACN (10 mL). K2CO3 (120 mg, 0.91 mmol) and 2-mercaptoethanol (0.07 mL, 0.99 mmol) were added. The mixture was heated to 55 °C and stirred for 18 h. The insoluble salts were removed via vacuum filtration and the crude mixture was purified through column chromatography (EtOAc in hexanes 10–100%) to yield product (237 mg, 0.81 mmol, 97%) as clear oil. 1H NMR (400 MHz, CDCl3) δ: 7.84 (d, J = 8.0 Hz, 1H), 7.36 (d, J = 8.0 Hz, 1H), 7.30 (t, J = 7.4 Hz, 1H), 6.94 (t, J = 7.4 Hz, 1H), 3.85 (s, 2H), 3.74 (s, 2H), 2.71 (t, J = 5.4 Hz, 2H), 2.18 (br, 1H). 13C (100 MHz, CDCl3) δ: 30.1, 36.2, 55.6, 95.8, 123.7, 124.2, 125.3, 135.2, 135.7.
2-((4-Fluoro-2-iodobenzyl)thio)ethan-1-ol (5b)
Compound 5b was prepared as 5a using commercially available 1-(bromomethyl)-4-fluoro-2-iodobenzene (11.07 g, 34.07 mmol) to yield product (9.78 g, 31.34 mmol, 92%) as clear oil. 1H NMR (400 MHz, CDCl3) δ: 7.57 (dd, J = 8.0, 2.6 Hz, 1H), 7.33 (dd, J = 8.5, 5.8 Hz, 1H), 7.04 (ddd, J = 8.5, 8.0, 2.7 Hz, 1H), 3.83 (s, 2H), 3.75 (q, J = 5.9 Hz, 2H), 2.70 (t, J = 5.9 Hz, 2H), 2.11 (t, J = 6.1 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 162.2, 159.7, 151.0, 136.4, 136.4, 130.5, 130.4, 126.8, 126.6, 115.6, 115.4, 99.6, 99.5, 77.3, 77.0, 76.7, 60.4, 40.1, 35.2, 34.8.
2-((3-Fluoro-2-iodobenzyl)thio)ethan-1-ol (5c)
Compound 5c was prepared as 5a using commercially available 1-(bromomethyl)-3-fluoro-2-iodobenzene (1.00 g, 3.18 mmol) to yield product (954 mg, 3.06 mmol, 96%) as clear oil. 1H NMR (400 MHz, CDCl3) δ: 7.29–7.24 (m, 1H), 7.16 (dd, J = 1.2, 7.6 Hz, 1H), 6.95 (td, J = 1.6, 8 Hz, 1H), 3.90 (s, 2H), 3.78–3.72 (m, 2H), 2.74–2.69 (br, 2H), 2.08 (t, J = 5.6 Hz, 1H). 13C (100 MHz, CDCl3) δ: 163.2, 160.1, 142.6, 129.6, 129.6, 125.4, 125.4, 114.3, 114.1, 60.4, 40.5, 40.5, 34.9.
2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)thio)ethan-1-ol (6a)
In a glass tube, 5a (900 mg, 3.06 mmol) and 4-chlorobenzeneboronic acid (730 mg, 4.59 mmol) were suspended in degassed toluene (4 mL) and ethanol (4 mL), followed by addition of Na2CO3 solution, (2.7 M in H2O, 3.06 mL). Pd(PPh3)4 (354 mg, 0.31 mmol) was added last, the tube was sealed, and the reaction mixture was allowed to stir at 90 °C for 18 h. The crude reaction mixture was diluted with ethyl acetate (10 mL) washed with brine (2 times, 10 mL), dried over MgSO4 and the solvent removed in vacuo. The organic mixture was purified via column chromatography (EtOAc in hexanes, 0–100%) to yield the product as yellow oil (530 mg, 62%). 1H NMR (400 MHz, CDCl3) δ: 7.47–7.20 (m, 8H), 3.68 (s, 2H), 3.55 (t, J = 5.2 Hz, 2H), 2.60 (t, J = 6 Hz, 2H), 1.99 (br, 1H). 13C NMR (100 MHz, CDCl3) δ: 140.9, 139.3, 135.2, 133.4, 130.6, 130.2, 130.2, 128.4, 128.0, 127.3, 60.1, 35.3, 33.4.
2-(((4′-Chloro-5-fluoro-[1,1′-biphenyl]-2-yl)methyl)thio)ethan-1-ol (6b)
Compound 6b was prepared as 6a using 5b (1.33 g, 4.26 mmol) to yield the product as yellow oil (834 mg, 66%). 1H NMR (400 MHz, CDCl3) δ: 7.43–7.32 (m, 5H), 7.04 (t, J = 8 Hz, 1H), 6.93 (d, J = 9.6 Hz, 1H), 3.63 (s, 2H), 3.56 (q, J = 6 Hz, 2H), 2.60 (t, J = 6 Hz, 2H), 1.86 (t, J = 5.4 Hz, 1H).
2-(((4′-Chloro-6-fluoro-[1,1′-biphenyl]-2-yl)methyl)thio)ethan-1-ol (6c)
Compound 6c was prepared as 6a using 5c (954 mg, 3.06 mmol) to yield the product as yellow oil (702 mg, 2.37 mmol, 77%). 1H NMR (400 MHz, CDCl3) δ: 7.46–7.03 (m, 7H), 3.57–3.53 (m, 4H), 2.59 (t, J = 6.4 Hz, 2H), 1.84 (t, J = 5.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 161.0, 158.6, 138.4, 134.8, 134.1, 132.0, 131.4, 129.2, 129.1, 128.6, 125.5, 125.4, 114.5, 114.3, 60.2, 35.2, 33.1, 33.1.
(2-Bromoethyl)((4′-chloro-[1,1′-biphenyl]-2-yl)methyl)sulfane (7a)
Compound 6a (850 mg, 3.02 mmol) was dissolved in 20 mL DCM under argon and cooled to 0 °C; CBr4 (2.00 g, 6.04 mmol) was added followed by PPh3 (1.98 g, 7.55 mmol). The reaction was allowed to warm to room temperature and stirred for 2 h. The solvent was removed in vacuo and the crude mixture was resuspended in ethyl acetate. The resulting suspension was sonicated for 10 min, after which the mixture was filtered. The filtrate was evaporated, and the crude mixture was further purified via column chromatography (EtOAc in hexanes, 0–10%) to yield 7a as clear oil (573 mg, 1.68 mmol, 55%). 1H NMR (400 MHz, CDCl3) δ: 7.46–7.20 (m, 8H) 3.71 (s, 2H), 3.22 (t, J = 7.6 Hz, 2H), 2.78 (t, J = 8 Hz, 2H).
(2-Bromoethyl)((4′-chloro-5-fluoro-[1,1′-biphenyl]-2-yl)methyl)sulfane (7b)
Compound 7b was prepared as 7a using 6b (8.81 g, 29.7 mmol) to yield 7b as clear oil (8.42 g, 78%). 1H NMR (400 MHz, CDCl3) δ: 7.44–7.32 (m, 5H), 7.08–6.92 (m, 2H), 3.66 (s, 2H), 3.24 (t, J = 9.6 Hz, 2H), 2.77 (t, J = 9.0 Hz, 2H).
(2-Bromoethyl)((4′-chloro-6-fluoro-[1,1′-biphenyl]-2-yl)methyl)sulfane (7c)
Compound 7c was prepared as 7a using 6c (702 mg, 2.37 mmol) to yield 7c as clear oil (851 mg, 2.37 mmol, 99%). 1H NMR (400 MHz, CDCl3) δ: 7.45–7.04 (m, 7H), 3.59 (s, 2H), 3.22 (t, J = 7.6 Hz, 2H), 2.77 (t, J = 9.2 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ: 161.0, 158.6, 138.2, 138.2, 134.2, 131.8, 131.4, 129.3, 129.2, 128.6, 125.5, 125.4, 114.7, 114.5, 60.4, 33.8, 33.4, 33.4, 30.1, 30.0, 14.2.
1-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)piperazine (8a)
Compound 7a (2.45 g, 7.17 mmol) was suspended in ACN (100 mL). Piperazine (3.71 g, 43.0 mmol) was added to the mixture followed by K2CO3 (1.98 mg, 14.35 mmol). The mixture was heated to 60 °C and stirred for 18 h. The insoluble salts were filtered off, and the resulting crude mixture was concentrated and purified via column chromatography (MeOH in DCM, 5–20%) to yield yellow oil (2.35 g, 6.77 mmol, 94%). 1H NMR (400 MHz, DMSO-d6) δ: 7.51–7.19 (m, 8H), 3.69 (s, 2H), 3.02 (s, 4H), 2.55–2.39 (m, 8H).
1-(2-(((4′-Chloro-5-fluoro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)piperazine (8b)
Compound 8b was prepared as 8a using 7a (1.47 g, 4.30 mmol) to yield yellow oil (1.45 g, 3.97 mmol, 97%). 1H NMR (400 MHz, acetone-d6) δ: 7.58–7.47 (m, 5H), 7.13 (t, J = 8.4 Hz, 1H), 7.02 (d, J = 9.6 Hz, 1H), 3.79 (s, 2H), 3.61 (s, 4H), 3.38 (s, 4H), 3.07 (t, J = 7.2 Hz, 2H), 2.81 (t, J = 7.6 Hz, 2H).
1-(2-(((4′-Chloro-6-fluoro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)piperazine (8c)
Compound 8c was prepared as 8a using 7c (851 mg, 2.37 mmol) to yield yellow oil (642 mg, 1.76 mmol, 74%). 1H NMR (400 MHz, CDCl3) δ: 7.42–6.99 (m, 7H), 3.55 (s, 2H), 2.86–2.82 (m, 4H), 2.52–2.33 (m, 8H). 13C NMR (100 MHz, CDCl3) δ: 160.9, 158.4, 138.8, 133.8, 132.0, 131.5, 129.0, 128.9, 128.4, 125.4, 125.4, 114.2, 114.0, 60.2, 58.6, 54.3, 45.9, 33.8, 29.2, 14.1.
(3S,5R)-1-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)-3,5-dimethylpiperazine (8d)
Compound 8d was prepared as 8a using 7a (957 mg, 2.80 mmol) and Cis-2,6-dimethylpiperazine (383 mg, 3.36 mmol) to yield yellow oil (990 mg, 2.80 mmol, 94%). 1H NMR (400 MHz, CDCl3) δ: 7.47–7.20 (m, 8H), 3.69 (s, 2H), 2.90–2.41 (m, 8H), 1.60 (t, J = 10.8 Hz, 2H), 1.05 (d, J = 6.0 Hz, 6H).
(3S,5R)-1-(2-(((4′-Chloro-5-fluoro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)-3,5-dimethylpiperazine (8e)
Compound 8e was prepared as 8a using 7b (1.56 g, 4.35 mmol) and cis-2,6-dimethylpiperazine (383 mg, 3.36 mmol) to yield yellow oil (1.54 g, 4.35 mmol, 90%). 1H NMR (400 MHz, CDCl3) δ: 7.44–7.33 (m, 5H), 7.02 (t, J = 8.0 Hz, 1H), 6.92 (d, J = 9.6 Hz, 1H), 3.63 (s, 2H), 2.90–2.83 (m, 2H), 2.68 (d, J = 10.4 Hz, 2H), 2.52 (t, J = 7.2 Hz, 2H), 2.40 (t, J = 7.2 Hz, 2H), 1.56 (t, J = 10.4 Hz, 2H), 1.03 (d, J = 5.6 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ: 162.6, 160.2, 142.7, 142.6, 138.3, 133.7, 131.9, 131.8, 131.4, 130.5, 128.5, 116.9, 116.7, 114.8, 114.6, 109.8, 109.8, 60.5, 58.1, 50.5, 33.6, 29.3, 19.9.
1-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)-1,4-diazepane (8f)
Compound 8f was prepared as 8a using 7a (1.7 g, 5.0 mmol) and 1,4-diazepane (2.0 g, 4 eq, 20.0 mmol) to yield as yellow oil (1.8 g, 5.0 mmol, 100%). 1H NMR (400 MHz, CDCl3) δ: 7.53–7.19 (m, 8H), 3.67 (s, 2H), 2.92–2.84 (m, 4H), 2.65–2.49 (m, 8H), 1.72–1.70 (m, 2H).
1-(2-(((4′-Chloro-6-fluoro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)-1,4-diazepane (8g)
Compound 8g was prepared as 8a using 7c (1.28 g, 3.56 mmol) and 1,4-diazepane (1.43 g, 14.2 mmol) to yield product (1.1 g, 2.9 mmol, 82%) as yellow oil. 1H NMR (400 MHz, CDCl3) δ: 7.43–7.41 (m, 2H), 7.30–7.25 (m, 4H), 7.05–7.02 (m, 1H), 3.55 (s, 2H), 2.98–2.86 (m, 4H), 2.62–2.47 (m, 8H), 1.83–1.72 (m, 2H).
1-(4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)piperazin-1-yl)propan-2-ol (9a)
Compound 8a (96 mg, 0.28 mmol) was suspended in isopropyl alcohol (1 mL) in a glass tube and cooled to 0 °C. DIPEA (9 μL, 0.06 mmol) and propylene oxide (0.20 mL, 0.28 mmol) were added, and the tube was sealed. The mixture was heated to 90 °C and stirred for 18 h. The solvent was evaporated in vacuo, and the crude mixture was purified via column chromatography (10% NH4OH in MeOH/DCM, 0–25%) to give yellow oil (78 mg, 0.19 mmol, 70%). 1H NMR (400 MHz, CDCl3) δ: 7.48–7.22 (m, 8H), 3.86 (br, 1H), 3.76 (s, 2H) 2.67–2.36 (m, 14H), 1.06 (d, J = 5.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ: 140.8, 139.4, 135.6, 133.2, 130.7, 130.2 128.4, 128.3, 127.8, 127.1, 65.6, 62.2, 58.0, 53.2, 53.1, 34.3, 29.4, 20.0. The free base was converted to the fumarate salt and recrystallized from methanol to give a white solid. mp 195–196 °C. Anal. (C22H29ClN2OS·2C4H4O4) C, H, N.
1-(4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)piperazin-1-yl)-3-phenylpropan-2-ol (9b)
Compound 8a (50 mg, 0.14 mmol) in anhydrous isopropyl alcohol (2 mL) was cooled to 0 °C in a glass tube. 2-Benzyloxirane (0.02 mL, 0.14 mmol) was added; the tube was sealed and heated to 90 °C for 18 h. The solvent was removed in vacuo, and the crude content was purified via column chromatography (DMA 0–25%) to give 9b as yellow oil (65 mg, 0.14 mmol, 94%). 1H NMR (400 MHz, CDCl3) δ: 7.44–7.17 (m, 13H), 3.93–3.87 (m, 1H), 3.65 (s, 2H), 2.82–2.32 (m, 16H). 13C NMR (100 MHz, CDCl3) δ: 140.8, 139.4, 138.2, 135.6, 133.2, 131.3, 130.9, 130.7, 130.2, 129.3, 128.6, 128.5, 128.4, 128.3, 127.9, 127.8, 127.2, 126.3, 67.2, 63.4, 58.0, 53.0, 41.4, 34.3, 29.7, 29.4. The free base was converted to the fumarate salt and recrystallized from methanol to give a white solid. mp 202–203 °C. Anal. (C28H33ClN2OS·2C4H4O4) C, H, N.
1-((2S,6R)-4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)-2,6-dimethylpiperazin-1-yl)propan-2-ol (9c)
Compound 9c was prepared as 9a using 8d (744 mg, 1.98 mmol) and propylene oxide (2.1 mL, 29.76 mmol) to give yellow oil (660 mg, 1.52 mmol, 77%). 1H NMR (400 MHz, CDCl3) δ: 7.46–7.19 (m, 8H), 3.67–3.65 (m, 3H), 2.63–2.32 (m, 10H), 1.81–1.77 (m, 2H), 1.10 (d, J = 6.4 Hz, 3H), 1.04–1.02 (m, 6H). 13C NMR (100 MHz, CDCl3) δ: 140.7, 139.3, 135.4, 133.1, 130.6, 130.1, 128.2, 127.8, 127.1, 127.0, 65.3, 60.8, 58.4, 58.3, 57.7, 56.1, 34.2, 29.2, 20.2, 19.3, 19.1. The free base was converted to the oxalate salt and recrystallized from methanol to give white foam. Anal. (C24H33ClN2OS·2C2H2O4·H2O) C, H, N.
1-((2S,6R)-4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)-2,6-dimethylpiperazin-1-yl)-3-phenylpropan-2-ol (9d)
Compound 9d was prepared as 9b using 8d (458 mg, 1.22 mmol) and 2-benzyloxirane (1.00 mL, 7.63 mmol) to give 9d as yellow oil (452 mg, 73%). 1H NMR (400 MHz, CDCl3) δ: 7.46–7.18 (m, 13H), 3.82–3.76 (m, 1H), 3.67 (s, 2H), 2.83–2.31 (m, 12H), 1.79–1.72 (m, 2H), 0.99 (dd, J = 2.4, 6.4 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ: 140.8, 139.4, 138.4, 135.6, 133.2, 130.7, 130.18, 130.16, 129.3, 128.3, 128.3, 127.8, 127.1, 126.2, 70.0, 58.2, 57.8, 56.2, 41.9, 34.3, 29.3, 19.3, 19.0. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. mp 81–83 °C. Anal. (C30H37ClN2OS·2C2H2O4·0.5H2O) C, H, N.
1-(4-(2-(((4′-Chloro-5-fluoro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)piperazin-1-yl)-3-phenylpropan-2-ol (9e)
Compound 9e was prepared as 9b using 8b (200 mg, 0.55 mmol) and 2-benzyloxirane (72 μL, 0.55 mmol) to give product as yellow oil (230 mg, 0.46 mmol, 84%). 1H NMR (400 MHz, acetone-d6) δ: 7.56–7.00 (m, 12H), 4.50–4.12 (m, 1H), 3.74 (s, 2H), 2.84–2.51 (m, 16H). 13C NMR (100 MHz, acetone-d6) δ: 167.8, 165.3, 147.9, 147.8, 144.5, 143.9, 138.4, 137.55, 137.51, 137.47, 136.1, 136.0, 135.9, 134.6, 133.6, 133.4, 133.1, 131.0, 121.7, 121.5, 119.8, 119.5, 73.0, 69.0, 63.3, 58.5, 58.2, 46.6, 38.3, 34.5. The free base was converted to the fumarate salt and recrystallized from methanol to give a white solid. mp 170–176 °C. Anal. (C28H32ClFN2OS·2C4H4O4) C, H, N.
1-((2S,6R)-4-(2-(((4′-Chloro-5-fluoro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)-2,6-dimethylpiperazin-1-yl)propan-2-ol (9f)
Compound 9f was prepared as 9a using 8e (944 mg, 2.40 mmol) and isopropyl alcohol (3 mL) to give yellow oil (350 mg, 32%). 1H NMR (400 MHz, CDCl3) δ: 7.43–7.26 (m, 5H), 7.02 (t, J = 8.0 Hz, 1H), 6.91 (d, J = 9.6 Hz, 1H), 3.68–3.62 (m, 3H), 2.66–2.32 (m, 10H), 1.80–1.76 (m, 2H), 1.10 (d, J = 6.0 Hz, 3H), 1.02 (s, 6H). 13C NMR (100 MHz, CDCl3) δ: 163.7, 161.2, 143.6, 143.5, 139.2, 134.6, 132.7, 132.6, 132.2, 131.3, 131.2, 129.3, 129.2, 117.7, 117.4, 115.6, 115.4, 65.8, 61.2, 58.9, 58.7, 58.1, 56.5, 33.9, 29.6, 20.5, 19.5, 19.3. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. mp 112–114 °C. Anal. (C24H32ClFN2OS·2C2H2O4·0.5H2O) C, H, N.
1-((2S,6R)-4-(2-(((4′-Chloro-5-fluoro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)-2,6-dimethylpiperazin-1-yl)-3-phenylpropan-2-ol (9g)
Compound 9g was prepared as 9b using 8e (587 mg, 1.49 mmol) and 2-benzyloxirane (1.23 mL, 9.34 mmol) to give product as yellow oil (658 mg, 84%). 1H NMR (400 MHz, CDCl3) δ: 7.44–7.19 (m, 10H), 7.01 (td, J = 2.8, 8.4 Hz, 1H), 6.91 (dd, J = 2.8, 9.4, 1H), 3.82–3.75 (m, 1H), 3.62 (s, 2H), 2.82–2.31 (m, 12H), 1.79–1.72 (m, 2H), 0.99 (dd, J = 2.4, 6.2 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ: 162.6, 160.2, 142.7, 142.6, 138.4, 138.3, 133.7, 131.9, 131.8, 131.42, 131.37,130.7, 130.5, 129.3, 128.5, 128.3, 128.1, 126.2, 116.9, 116.7, 114.8, 114.6, 70.0, 60.91, 60.88, 60.4, 58.2, 57.8, 56.1, 41.9, 33.6, 29.4, 19.3, 19.1, 19.0. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. mp 142–146 °C. Anal. (C30H36ClFN2OS·2C2H2O4) C, H, N.
1-(4-(2-(((4′-Chloro-6-fluoro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)piperazin-1-yl)propan-2-ol (9h)
Compound 9h was prepared as 9a using 8c (279 mg, 0.765 mmol) and propylene oxide (0.54 mL, 7.65 mmol) to give yellow oil (26 mg, 0.06 mmol, 8%). 1H NMR (400 MHz, CDCl3) δ: 7.41–7.01 (m, 7H), 3.85–3.77 (m, 1H), 3.56 (s, 2H), 2.64–2.18 (m, 14H), 1.12 (d, J = 6.4 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ: 161.0, 158.6, 138.7, 133.9, 132.0, 131.5, 129.1, 129.0, 128.5, 128.4, 128.2, 125.4, 125.4, 114.4, 114.2, 65.5, 62.2, 58.0, 53.1, 33.95, 33.92, 29.5, 20.0. The free base was converted to the fumarate salt and recrystallized from methanol to give white foam. Anal. (C22H28ClFN2OS·2C4H4O4) C, H, N.
1-(4-(2-(((4′-Chloro-6-fluoro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)piperazin-1-yl)-3-phenylpropan-2-ol (9i)
Compound 9i was prepared as 9b using 8c (223 mg, 0.61 mmol) and 2-benzyloxirane (287 μL, 2.14 mmol) to give product as yellow oil (272 mg, 0.54 mmol, 89%). 1H NMR (400 MHz, CDCl3) δ: 7.43–7.01 (m, 12H), 3.94–3.88 (m, 1H), 3.56 (s, 2H), 2.84–2.32 (m, 16H). 13C NMR (100 MHz, CDCl3) δ: 161.0, 158.6, 138.8, 138.7, 138.3, 133.9, 132.1, 131.5, 129.3, 129.1, 129.0, 128.5, 128.41, 128.36, 128.2, 126.3, 125.5, 125.4, 114.4, 114.2, 67.2, 63.4, 58.0, 53.1, 41.4, 34.0, 33.9, 29.5. The free base was converted to the fumarate salt and recrystallized from methanol to give a white solid. mp 184–186 °C. Anal. (C28H32ClFN2OS·2C4H4O4·0.5H2O) C, H, N.
1-(4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)-1,4-diazepan-1-yl)propan-2-ol (9j)
Compound 9j was prepared as 9a using 8f (420 mg, 1.16 mmol) and propylene oxide (135 mg, 0.16 mL, 2 eq, 2.33 mmol) to give product (140 mg, 334 μmol, 29%) as yellow oil. 1H NMR (400 MHz, CDCl3) δ: 7.47–7.19 (m, 8H), 3.74–3.70 (m, 3H), 2.82–2.48 (m, 13H), 2.23–2.17 (m, 1H), 1.76–1.75 (m, 2H), 1.12–1.11 (d, J = 6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 140.5, 139.2, 135.4, 133.0, 130.5, 130.4, 130.02, 129.99, 129.9, 128.1, 128.0, 127.6, 126.8, 65.4, 62.7, 57.6, 55.3, 55.1, 54.2, 53.5, 53.1, 34.0, 29.9, 27.5, 19.5. The free base was converted to oxalate salt and recrystallized in methanol to get a white solid. mp 155–157 °C. Anal. (C23H31ClN2OS·2C2H2O4·0.25H2O) C, H, N.
1-(4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)-1,4-diazepan-1-yl)-3-phenylpropan-2-ol (9k)
Compound 9k was prepared as 9b using 8f (360 mg, 997 μmol) and 2-benzyloxirane (161 mg, 157 μL, 1.2 eq, 1.20 mmol) to give product (340 mg, 687 μmol, 69%) as yellow oil. 1H NMR (400 MHz, CDCl3) δ: 7.45–7.19 (m, 13H), 3.84–3.80 (m, 1H), 3.66 (s, 2H), 2.84–2.46 (m, 15H), 2.33–2.27 (m, 1H), 1.77–1.70 (m, 2H); 13C NMR (100 MHz, CDCl3) δ: 140.8, 136.6, 136.2, 128.8, 128.6, 128.5, 128.4, 127.4, 127.1, 121.4, 121.2, 121.0, 64.6, 64.4, 53.3, 53.1, 51.2, 44.3, 44.2, 43.0, 41.7, 41.2, 36.0, 35.7, 24.5. The free base was converted to fumaric salt and recrystallized in methanol to get a white solid. mp 168–170 °C. Anal. (C29H35ClN2O2S·2C4H4O4) C, H, N.
1-(4-(2-(((4′-Chloro-6-fluoro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)-1,4-diazepan-1-yl)-3-phenylpropan-2-ol (9l)
Compound 9l was prepared as 9b using 8g (1.00 g, 2.64 mmol) and 2-benzyloxirane (425 mg, 417 μL, 1.2 eq, 3.17 mmol) to give product (900 mg, 1.75 mmol, 67%) as yellow oil. 1H NMR (400 MHz, CDCl3) δ: 7.42–7.41 (m, 2H), 7.29–7.23 (m, 9H), 7.05–7.03 (m, 1H), 3.81 (m, 1H), 3.54 (s, 2H), 2.79–2.45 (m, 15H), 2.33–2.27 (m, 1H), 1.74–1.73 (m, 2H); 13C NMR (100 MHz, CDCl3) δ: 161.0, 158.6, 138.9, 138.5, 133.9, 132.1, 131.5, 129.3, 129.0, 128.9, 128.6, 128.5, 128.4, 128.2, 126.6, 126.4, 126.2, 125.4, 114.3, 114.1, 68.1, 67.9, 63.7, 62.9, 62.8, 57.7, 57.5, 55.8, 55.4, 54.5, 54.4, 53.8, 42.6, 41.3, 41.2, 33.9, 30.3, 29.3, 27.8. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. mp 90–92 °C. Anal. (C29H34ClFN2OS·2C2H2O4) C, H, N.
1-(4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)piperazin-1-yl)propan-2-ol (10a)
To compound 9a (400 mg, 0.99 mmol) in MeOH (10 mL) was added acetic acid (1 mL) and H2O2 (0.60 mL). The reaction was stirred for 18 h. The reaction was neutralized with sat. NaHCO3 solution (10 mL), extracted with ethyl acetate (20 mL × 3), and the organic extracts were washed with brine (20 mL × 2) and dried over MgSO4. The crude product was purified via column chromatography (10% NH4OH in MeOH/DCM, 0–25%) to give yellow oil (388 mg, 0.92 mmol, 93%). 1H NMR (400 MHz, acetone-d6) δ: 7.24–7.01 (m, 8H), 4.11 (br, 1H), 3.79 (s, 2H), 3.14–3.32 (m, 14H), 0.76 (d, J = 6.0 Hz, 3H). 13C NMR (100 MHz, acetone-d6) δ: 142.0, 139.6, 134.1, 133.1, 131.7, 131.6, 130.4, 130.0, 128.5, 128.1, 104.6, 101.2, 66.2, 62.5, 62.0, 55.8, 49.9, 49.4, 47.3, 46.8, 20.7. The free base was converted to the fumarate salt and recrystallized from methanol to give a white solid. mp 165–167 °C. Anal. (C22H29ClN2O2S·2C4H4O4) C, H, N.
1-(4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)piperazin-1-yl)-3-phenylpropan-2-ol (10b)
Compound 10b was prepared as 10a using 9b (336 mg, 0.70 μmol) to obtain product (122 mg, 245 μmol, 35%) as yellow oil. 1H NMR (400 MHz, acetone-d6) δ: 7.53–7.19 (m, 13H), 4.07 (d, J = 5.2 Hz, 2H), 4.03–3.96 (m, 1H), 2.94–2.40 (m, 16H). 13C NMR (100 MHz, acetone-d6) δ: 141.8, 139.5, 139.0, 132.9, 131.6, 131.4, 130.2, 130.0, 129.5, 128.3, 128.0,127.9, 125.9, 67.5, 63.3, 55.6, 54.1, 53.0, 52.0, 50.3, 49.4, 41.5, 29.5, 28.5, 28.4. The free base was converted to the fumarate salt and recrystallized from methanol to give a white solid. mp 170–175 °C. Anal. (C28H33ClN2O2S·2C4H4O4) C, H, N.
1-((2S,6R)-4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)-2,6-dimethylpiperazin-1-yl)propan-2-ol (10c)
Compound 10c was prepared as 10a using 9c (1.06 g, 2.45 mmol) to give yellow oil (878 mg, 80%). 1H NMR (400 MHz, CDCl3) δ: 7.48–7.27 (m, 8H), 4.02 (s, 2H), 3.70–3.66 (m, 1H), 2.66–2.42 (m, 10H), 1.89–1.78 (m, 2H), 1.10 (d, J = 5.2 Hz, 3H), 1.04 (s, 6H). 13C NMR (100 MHz, CDCl3) δ: 141.9, 139.1, 133.9, 131.5, 131.2, 130.7, 128.9, 128.6, 128.5, 128.5, 65.7, 61.1, 60.5, 58.4, 56.5, 56.2, 50.6, 49.4, 20.6, 19.5, 19.4, 19.3, 19.2. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. mp 61–63 °C. Anal. (C24H33ClN2O2S·2C2H2O4·0.4H2O) C, H, N.
1-((2S,6R)-4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)-2,6-dimethylpiperazin-1-yl)-3-phenylpropan-2-ol (10d)
Compound 10d was prepared as 10a using 9d (30 mg, 589 μmol) to give yellow oil (273 mg, 589 μmol, 88%). 1H NMR (400 MHz, CDCl3) δ: 7.48–7.19 (m, 13H), 3.99 (s, 2H), 3.81–3.74 (m, 1H), 2.82–2.40 (m, 12H), 1.86–1.71 (m, 2H), 0.98 (dd, J = 2.4, 6 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ: 141.7, 138.9, 138.3, 131.2, 130.9, 130.5, 129.3, 128.6, 128.4, 128.3, 128.3, 128.2, 126.3, 70.0, 61.0, 60.4, 60.3, 58.1, 58.0, 56.3, 56.0, 50.3, 49.3, 41.8, 41.8, 19.2, 19.1, 19.0, 18.9. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. mp 105–107 °C. Anal. (C30H37ClN2O2S·2C2H2O4·0.5H2O) C, H, N.
1-(4-(2-(((4′-Chloro-5-fluoro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)piperazin-1-yl)-3-phenylpropan-2-ol (10e)
Compound 10e was prepared as 10a using 9e (2.06 g, 4.13 mmol) to give yellow oil (1.97 g, 3.82 mmol, 93%). 1H NMR (400 MHz, CDCl3) δ: 7.28–6.78 (m, 12H), 3.78–3.68 (m, 3H), 2.65–2.12 (m, 16H). 13C NMR (100 MHz, CDCl3) δ: 164.0, 163.5, 161.6, 161.0, 143.8, 143.8, 138.4, 138.0, 134.2, 133.25, 133.17, 131.9, 131.8, 130.8, 129.4, 128.8, 128.4, 126.4, 126.1, 124.6, 117.5, 117.3, 116.1, 115.9, 115.4, 115.2, 67.4, 63.5, 57.3, 55.4, 53.2, 53.1, 50.8, 50.7, 49.6, 48.6, 41.5. The free base was converted to the fumarate salt and recrystallized from methanol to give a white solid. mp 143–145 °C. Anal. (C28H32ClFN2O2S·2C4H4O4·0.5H2O) C, H, N.
1-((2S,6R)-4-(2-(((4′-Chloro-5-fluoro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)-2,6-dimethylpiperazin-1-yl)propan-2-ol (10f)
Compound 10f was prepared as 10a using 9f (141 mg, 0.31 mmol) to give yellow oil (97 mg, 313 μmol, 66%). 1H NMR (400 MHz, CDCl3) δ: 7.47–7.39 (m, 3H), 7.26 (d, J = 6.8 Hz, 2H), 7.09 (t, J = 8.4 Hz, 1H), 6.99 (d, J = 9.2 Hz, 1H), 3.94 (s, 2H), 3.72 (m, 1H), 2.66–2.46 (m, 10H), 2,03–1.87 (m, 2H), 1.12–1.06 (m, 9H). 13C NMR (100 MHz, CDCl3) δ: 163.4, 161.0, 143.74, 143.66, 137.9, 134.2, 133.1, 133.1, 130.7, 128.8, 124.3, 117.4, 117.2, 115.3, 115.1, 109.8, 65.1, 60.1, 59.5, 58.5, 56.4, 55.2, 50.3, 49.2, 20.4, 18.8. The free base was converted to the oxalate salt and recrystallized from methanol to give white foam. Anal. (C24H32ClFN2O2S·2C2H2O4·0.5H2O) C, H, N.
1-((2S,6R)-4-(2-(((4′-Chloro-5-fluoro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)-2,6-dimethylpiperazin-1-yl)-3-phenylpropan-2-ol (10g)
Compound 10g was prepared as 10a using 9g (307 mg, 582 μmol) to obtain product (212 mg, 390 μmol, 67.0%) as yellow oil. 1H NMR (400 MHz, CDCl3) δ: 7.47–6.97 (m, 12H), 3.92 (dd, J = 12.8, 19.4 Hz, 2H), 3.81–3.75 (m, 1H), 2.82–2.41 (m, 12H), 1.86–1.72 (m, 2H), 0.98 (dd, J = 3.2, 6 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ: 163.4, 161.0, 143.7, 143.6, 138.3, 137.9, 134.2, 133.1, 133.0, 130.7, 129.3, 128.8, 128.4, 126.3, 124.4, 124.4, 117.4, 117.2, 115.3, 115.1, 70.0, 61.0, 60.4, 58.1, 56.2, 56.0, 55.3, 50.3, 49.4, 41.8, 41.8, 19.2, 19.1, 19.0, 18.9, 14.2. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. mp 108–110 °C. Anal. (C30H36ClFN2O2S·2C2H2O4·1.5H2O) C, H, N.
1-(4-(2-(((4′-Chloro-6-fluoro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)piperazin-1-yl)propan-2-ol (10h)
Compound 10h was prepared as 10a using 9h (800 mg, 1.89 mmol) to give product yellow oil (600 mg, 1.37 mmol, 72%). δ: 7.44–7.11 (m, 7H), 3.89 (s, 2H), 3.82–3.78 (m, 1H), 2.68–2.60 (m, 6H), 2.40–2.18 (m, 8H), 1.12 (d, J = 6.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 165.6, 161.2, 158.8, 135.3, 134.4, 131.7, 131.6, 131.5, 131.4, 129.5, 129.3, 129.2, 129.1, 128.8, 126.8, 126.7, 115.7, 115.5, 65.5, 62.2, 56.1, 53.0, 50.6, 49.8, 19.9. mp 163–165 °C. Anal. (C22H28ClFN2O2S·2C4H4O4·0.5H2O) C, H, N.
1-(4-(2-(((4′-Chloro-6-fluoro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)piperazin-1-yl)-3-phenylpropan-2-ol (10i)
Compound 10i was prepared as 10a using 9i (179.6 mg, 0.36 mmol) to give yellow oil (66 mg, 0.13 mmol, 36%). 1H NMR (400 MHz, CDCl3) δ: 7.42–7.10 (m, 12H), 3.93–3.84 (m, 3H), 2.83–2.27 (m, 16H). 13C NMR (100 MHz, CDCl3) δ: 161.2, 158.8, 138.2, 134.4, 131.7, 131.6, 131.5, 131.5, 129.6, 129.5, 129.3, 129.3, 129.1, 128.8, 128.4, 126.8, 126.7, 126.3, 115.7, 115.5, 67.2, 63.4, 56.0, 53.0, 50.6, 49.7, 49.7, 41.3. The free base was converted to the fumarate salt and recrystallized from methanol to give a white solid. mp 172–174 °C. Anal. (C28H32ClFN2O2S·2C4H4O4) C, H, N.
1-(4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)-1,4-diazepan-1-yl)propan-2-ol (10j)
Compound 10j was prepared as 10a using 9j (220 mg, 525 μmol) to give product (130 mg, 299 μmol, 57%) as clear oil. 1H NMR (400 MHz, CDCl3) δ: 7.49–7.27 (m, 8H), 4.01–3.97 (m, 2H), 3.74–3.70 (m, 1H), 2.87–2.50 (m, 13H), 2.23–2.16 (m, 1H), 1.77–1.72 (m, 2H), 1.12–1.11 (d, J = 6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 141.4, 138.6, 133.4, 130.9, 130.7, 130.2, 128.3, 128.1, 128.0, 127.9, 65.3, 62.6, 55.9, 55.2, 55.1, 54.1, 54.0, 53.4, 50.3, 50.2, 50.1, 27.4, 19.5. The free base was converted to oxalate salt and recrystallized in methanol to get a white solid. mp 147–149 °C. Anal. (C23H31ClN2O2S·2C2H2O4·0.75H2O) C, H, N.
1-(4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)-1,4-diazepan-1-yl)-3-phenylpropan-2-ol (10k)
Compound 10k was prepared as 10a using 9k (1.48 g, 3.64 mmol) to give product (160 mg, 313 μmol, 55%) as clear oil. 1H NMR (400 MHz, CDCl3) δ: 7.46–7.02 (m, 13H), 4.03–3.95(m, 2H), 3.82–3.81 (m, 1H), 2.86–2.52 (m, 15H), 2.33–2.28 (m, 1H), 1.73–1.70 (m, 2H); 13C NMR (100 MHz, CDCl3) δ: 141.4, 138.6, 138.1, 133.4, 130.9, 130.7, 130.2, 129.1, 128.5, 128.4, 128.3, 128.2, 128.12, 128.05, 126.0, 67.7, 63.2, 55.9, 55.2, 55.1, 54.1, 54.0, 53.4, 50.3, 50.1, 41.0, 27.4. The free base was converted to oxalate salt and recrystallized in methanol to get a white solid. mp 156–158 °C. Anal. (C29H35ClN2O2S·2C2H2O4) C, H, N.
1-(4-(2-(((4′-Chloro-6-fluoro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)-1,4-diazepan-1-yl)-3-phenylpropan-2-ol (10l)
Compound 10l was prepared as 10a using 9l (550 mg, 1.07 mmol) to give product (330 mg, 624 μmol, 58%) as clear oil. 1H NMR (400 MHz, CDCl3) δ: 7.41–7.13 (m, 12H), 3.86–3.81 (m, 3H), 2.82–2.55 (m, 15H), 2.33–2.27 (m, 1H), 1.72–1.71 (m, 2H); 13C NMR (100 MHz, CDCl3) δ: 161.2, 158.8, 138.4, 134.4, 131.72, 131.66, 131.6, 129.5, 129.4, 129.3, 129.1, 128.8, 128.3, 126.7, 126.3, 115.7, 115.5, 67.9, 63.6, 55.9, 55.5, 54.4, 54.3, 53.7, 50.9, 50.5, 41.3, 27.8. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. mp 90–92 °C. Anal. (C29H34ClFN2O2S·2C2H2O4·0.25H2O) C, H, N.
1-(4-(2-(((4′-Chloro-5-fluoro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)piperazin-1-yl)propan-2-ol (11a)
Compound 11a was prepared as 8a using 7b (890 mg, 2.47 mmol) and commercially available 1-(piperazin-1-yl)propan-2-ol (428 mg, 2.97 mmol) to yield yellow oil (720 mg, 1.70 mmol, 70%). 1H NMR (400 MHz, CDCl3) δ: 7.43–7.33 (m, 5H), 7.02 (t, J = 8.0 Hz, 1H), 6.92 (d, J = 9.2 Hz, 1H), 3.85–3.76 (m, 1H), 3.63 (s, 2H), 2.65–2.18 (m, 14H), 1.12 (d, J = 6.0 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ: 162.6, 160.2, 142.7, 142.6, 138.3, 133.7, 131.8, 131.8, 131.4, 130.7, 130.5, 130.4, 128.5, 128.4, 116.9, 116.7, 114.8, 114.6, 65.6, 62.2, 58.0, 53.1, 33.7, 29.5, 20.0. The free base was converted to the fumarate salt and recrystallized from methanol to give a white solid. mp 188–190 °C. Anal. (C22H28ClFN2OS·2C4H4O4) C, H, N.
1-(4-(2-(((4′-Chloro-6-fluoro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)-1,4-diazepan-1-yl)propan-2-ol (11b)
Compound 11b was prepared as 8a using 7c (1.12 g, 3.11 mmol) and commercial available 1-(1,4-diazepan-1-yl)propan-2-ol (493 mg, 3.11 mmol) to give product (500 mg, 1.14 mmol, 37%) as yellow oil. 1H NMR (400 MHz, CDCl3) δ: 7.42–7.41 (m, 2H), 7.30–7.22 (m, 4H), 7.04–7.00 (m, 1H), 3.72 (m, 1H), 3.54 (s, 2H), 2.81–2.48 (m, 13H), 2.22–2.16 (m, 1H), 1.76–1.74 (m, 2H), 1.11–1.09 (d, J = 5.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 161.0, 158.5, 138.9, 133.9, 132.1, 131.5, 129.0, 128.9, 128.5, 128.4, 128.2, 125.4, 114.3, 114.1, 65.7, 62.9, 57.7, 55.5, 55.4, 54.5, 53.8, 33.9, 30.3, 27.8, 19.8. The free base was converted to the oxalate salt and recrystallized from methanol to give an orange solid. mp 165–167 °C. Anal. (C23H30ClFN2OS·2C2H2O4) C, H, N.
1-(4-(2-(((4′-Chloro-5-fluoro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)piperazin-1-yl)propan-2-ol (12a)
Compound 12a was prepared as 10a using 11a (552 mg, 1.30 mmol) to give yellow oil (405 mg, 71%). 1H NMR (400 MHz, CDCl3) δ: 7.49–7.26 (m, 5H), 7.09 (t, J = 8.0 Hz, 1H), 6.99 (d, J = 9.2 Hz, 1H), 3.95 (s, 2H), 3.81 (m, 1H), 2.75–2.19 (m, 14H), 1.12 (d, J = 5.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ: 163.4, 160.9, 143.7, 143.6, 137.8, 134.1, 133.1, 133.0, 130.7, 128.9, 128.8, 124.4, 117.4, 117.2, 115.3, 115.1, 109.8, 65.5, 62.2, 55.4, 53.0, 50.7, 49.5, 20.0. The free base was converted to the fumarate salt and recrystallized from methanol to give a white solid. mp 165–167 °C. Anal. (C22H28ClFN2O2S·2C4H4O4·0.4H2O) C, H, N.
1-(4-(2-(((4′-Chloro-6-fluoro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)-1,4-diazepan-1-yl)propan-2-ol (12b)
Compound 12b was prepared as 10a using 11b (300 mg, 686 μmol) to give product (280 mg, 618 μmol, 90%) as clear oil. 1H NMR (400 MHz, CDCl3) δ: 7.44–7.24 (m, 6H), 7.15–7.11 (m, 1H), 3.87 (s, 2H), 3.70 (m, 1H), 2.83–2.54 (m, 13H), 2.21–2.16 (m, 1H), 1.73–1.74 (m, 2H), 1.11–1.09 (d, J = 5.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 161.2, 158.8, 134.4, 131.7, 131.7, 131.6, 129.5, 129.4, 129.3, 129.1, 128.8, 126.7, 115.7, 115.5, 65.6, 62.9, 55.9, 55.5, 55.4, 54.4, 54.3, 53.7, 50.9, 50.5, 27.8, 19.8. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. mp 90–92 °C. Anal. (C23H30ClFN2O2S·2C2H2O4) C, H, N.
tert-Butyl 4-(2-bromoethyl)piperidine-1-carboxylate (15)
To commercially available tert-butyl 4-(2-hydroxyethyl)piperidine-1-carboxylate (1.00 g, 4.36 mmol) in anh. dichloromethane (20 mL) was added CBr4 (3.14 g, 9.46 mmol) followed by triphenylphosphine (1.34 g, 5.10 mmol) portion wise. The mixture was stirred at room temperature overnight. The resulting reaction mixture was diluted with hexanes (50 mL) and washed with water (40 mL), then brine (40 mL × 2) and dried over MgSO4. The crude mixture was purified via column chromatography (0–30%, hexanes/EtOAc) to yield product (875 mg, 2.99 mmol, 69%) as clear oil. 1H NMR (400 MHz, CDCl3) δ: 4.07 (br, 2H), 3.42 (t, J = 7.2 Hz, 2H), 2.68 (t, J = 12.8 Hz, 2H), 1.79 (q, J = 6.4 Hz, 2H), 1.69–1.59 (m, 3H), 1.43 (s, 9H), 1.14–1.04 (m, 2H). 13C NMR (100 MHz, CDCl3) δ: 154.8, 79.3, 43.7, 39.1, 34.4, 31.4, 31.0, 28.4.
(2-Iodophenyl)methanethiol (16)
To a solution of thiourea (500 mg, 6.57 mmol) in warm water (5 mL) (allowed thiourea to dissolve) were added ethanol (3.5 mL) and 1-(bromomethyl)-2-iodobenzene (1.95 g, 6.57 mmol). The mixture was stirred at reflux for 45 min. The solvent was evaporated under vacuum to give S-(2-iodobenzyl)isothiouronium chloride, which was used without further purification. The salt was added to a solution of NaOH (0.6 g) in water (6 mL). The mixture was stirred at reflux for 2 h, cooled to room temperature using an ice bath, acidified with 6 M HCl solution, and then extracted with Et2O (3 × 100 mL). The combined organic extracts were dried and concentrated. The crude mixture was purified via column chromatography (0–50%, hexanes/EtOAc) to give product (1.46 g, 5.83 mmol, 89%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 7.82 (dd, J = 1.2, 8 Hz, 1H), 7.38 (dd, J = 1.6, 7.6 Hz, 1H), 7.31 (td, J = 1.2, 7.4 Hz, 1H), 6.93 (td, J = 1.6, 7.4 Hz, 1H), 6.82 (d, J = 8 Hz, 2H).
tert-Butyl 4-(2-((2-iodobenzyl)thio)ethyl)piperidine-1-carboxylate (17)
To 16 (532 mg, 2.13 mmol) and 15 (653 mg, 2.24 mmol) in ACN (10 mL) was added K2CO3 (588 mg, 4.25 mmol). The reaction was stirred vigorously at 60 °C for 18 h. Inorganic solids were removed via filtration, and the solvent was removed in vacuo. Crude content was purified via column chromatography (0–50%, hexanes/EtOAc) to give product (771 mg, 1.67 mmol, 79%) as clear oil. 1H NMR (400 MHz, CDCl3) δ: 7.84 (dd, J = 1.2, 8 Hz, 1H), 7.36–7.28 (m, 2H), 6.93 (td, J = 1.6, 7.4 Hz, 1H), 3.81 (s, 2H), 2.65 (t, J = 11.6 Hz, 2H), 2.51 (t, J = 7.6, 2H), 1.62–1.45 (m, 16H), 1.11–1.01 (m, 2H). 13C NMR (100 MHz, CDCl3) δ: 154.8, 140.9, 139.8, 129.9, 128.7, 128.3, 100.6, 79.2, 41.5, 35.9, 35.0, 31.8, 28.9, 28.4.
tert-Butyl 4-(2-(((4′-chloro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)piperidine-1-carboxylate (18)
In a glass tube, 17 (900 mg, 3.06 mmol) and 4-chlorobenzeneboronic acid (730 mg, 4.59 mmol) were suspended in degassed toluene (4 mL) and ethanol (4 mL), followed by addition of Na2CO3 solution, (2.7 M in H2O, 3.06 mL). Pd(PPh3)4 (354 mg, 0.31 mmol) was added last, and the tube was sealed, and the reaction mixture was allowed to stir at 90 °C for 18 h. The crude reaction mixture was diluted with ethyl acetate (10 mL), washed with brine (10 mL × 2), dried over MgSO4, and the solvent removed in vacuo. The organic mixture was purified via column chromatography (EtOAc in hexanes, 0–100%) to yield the product as yellow oil (530 mg, 62%). 1H NMR (400 MHz, CDCl3) δ: 7.45–7.19 (m, 8H), 3.64 (s, 2H), 2.64 (t, J = 10.8 Hz, 2H), 2.40 (t, J = 6.8 Hz, 2H), 1.61–1.35 (m, 16H), 1.06–0.97 (m, 2H); 13C NMR (100 MHz, CDCl3) δ: 154.8, 140.8, 139.4, 133.2, 130.7, 130.2, 128.3, 127.8, 127.1, 79.2, 35.8, 35.0, 33.9, 31.8, 29.5, 28.5.
4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)piperidine (19)
Compound 18 (569 mg, 1.28 mmol) in DCM (10 mL) and TFA (1 mL) was stirred at room temperature for 18 h. The reaction was neutralized with sat. NaHCO3 solution (5 mL), extracted with ethyl acetate (5 mL × 3), the organic extracts were washed with brine (5 mL × 2), and dried over MgSO4. The crude product purified via column chromatography (10% NH4OH in MeOH/DCM, 0–25%) to obtain product (440 mg, quant.) as yellow oil. 1H NMR (400 MHz, CDCl3) δ: 7.44–7.19 (m, 8H), 3.64 (s, 2H), 3.35 (d, J = 12.4 Hz, 2H), 2.79 (q, J = 10.8 Hz, 2H), 2.38 (t, J = 6.8 Hz, 2H), 1.73 (d, J = 12.4 Hz, 2H), 1.54–1.39 (m, 6H); 13C NMR (100 MHz, CDCl3) δ: 140.8, 139.4, 135.4, 133.3, 130.7, 130.2, 130.1, 128.8, 128.5, 128.3, 127.9, 127.2, 43.9, 34.8, 33.9, 32.7, 28.9, 28.3.
1-(4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)piperidin-1-yl)propan-2-ol (20a)
Compound 20a was prepared as 9a using 19 (1.1 g, 3.2 mmol) and propylene oxide (1.8 g, 2.2 mL, 32 mmol) to give product (800 mg, 1.98 mmol, 62%) as yellow oil. 1H NMR (400 MHz, CDCl3) δ: 7.55–7.00 (m, 8H), 3.90–3.76 (m, 1H), 3.63 (s, 2H), 2.96 (d, J = 12 Hz, 1H), 2.74 (d, J = 12 Hz, 1H), 2.40 (t, J = 7.6 Hz, 2H), 2.27–2.14 (m, 3H), 1.84 (t, J = 12 Hz, 1H), 1.57 (d, J = 10.4 Hz, 2H), 1.37–1.12 (m, 8H); 13C NMR (100 MHz, CDCl3) δ: 140.7, 139.5, 135.7, 133.2, 130.7, 130.2, 130.1, 128.5, 128.3, 127.8, 127.1, 65.9, 62.3, 55.6, 52.1, 35.8, 34.7, 33.9, 32.3, 32.0, 29.7, 20.0. Anal. (C23H30ClNOS) C, H, N.
1-(4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)thio)ethyl)piperidin-1-yl)-3-phenylpropan-2-ol (20b)
Compound 20b was prepared as 9b using 19 (443 mg, 1.28 mmol) and 2-benzyloxirane (601 μL, 4.48 mmol) to give product as yellow oil (293 mg, 0.61 mmol, 48%). 1H NMR (400 MHz, acetone-d6) δ: 7.43–7.18 (m, 13H), 3.99–3.93 (m, 3H), 3.72–3.51 (m, 3H), 2.92–2.67 (m, 4H), 2.38 (t, J = 6.8 Hz, 2H), 1.62 (d, J = 10.8 Hz, 2H), 1.38–1.25 (m, 6H).
1-(4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)piperidin-1-yl)propan-2-ol (21a)
Compound 21a was prepared as 10a using 20a (350 mg, 866 μmol) to give product (250 mg, 595 μmol, 69%) as clear oil. 1H NMR (400 MHz, CDCl3) δ: 7.51–7.27 (m, 8H), 4.00 (s, 2H), 3.80–3.76 (m, 1H), 2.97 (d, J = 11.2 Hz, 1H), 2.75 (d, J = 12 Hz, 1H), 2.54–2.40 (m, 2H), 2.27–2.21 (m, 3H), 1.85 (t, J = 12 Hz, 1H), 1.62–1.52 (m, 4H), 1.21–1.08 (m, 6H); 13C NMR (100 MHz, CDCl3) δ: 141.6, 138.8, 133.7, 131.2, 131.1, 130.9, 130.6, 130.5, 128.6, 128.5, 128.4, 128.1, 65.9, 62.4, 62.3, 56.0, 55.4, 52.0, 49.0, 35.0, 32.3, 32.2, 32.0, 31.8, 28.6, 20.0. Anal. (C23H30ClNO2S) C, H, N.
1-(4-(2-(((4′-Chloro-[1,1′-biphenyl]-2-yl)methyl)sulfinyl)ethyl)piperidin-1-yl)-3-phenylpropan-2-ol (21b)
Compound 21b was prepared as 10a using 20b (293 mg, 0.61 mmol) to give product as yellow oil (102 mg, 0.206 mmol, 34%) 1H NMR (400 MHz, CDCl3) δ: 7.44–7.19 (m, 13H), 4.09–4.03 (m, 1H), 3.97 (d, J = 12.8 Hz, 2H), 3.11 (dd, J = 10.8, 31.8 Hz, 2H), 2.87 (dd, J = 6.4, 13.6 Hz, 1H), 2.65 (dd, J = 6, 13.6 Hz, 1H), 2.60–2.32 (m, 5H), 2.10 (t, J = 11.2 Hz, 1H), 1.98 (s, 1H), 1.65–1.34 (m, 6H). 13C NMR (100 MHz, CDCl3) δ: 141.6, 138.8, 137.8, 133.7, 131.2, 130.9, 130.5, 129.3, 128.7, 128.5, 128.3, 127.9, 126.5, 67.0, 63.3, 56.0, 55.0, 52.2, 48.5, 41.7, 34.1, 30.6, 30.6, 30.4, 30.4, 28.2. HRMS M + H+, 496.20610 (calc. 496.20770). Anal. (C29H34ClNO2S) C, H, N.
Radioligand Binding Assays in Rat Brain Tissue
Radioligand binding assays were performed similar to what was reported previously.31,53
DAT Binding Assay
Frozen striatum tissue dissected from male Sprague–Dawley rat brains (supplied on ice by BioIVT, Hicksville, NY) were homogenized in 20 volumes (w/v) of ice-cold modified sucrose phosphate buffer (0.32 M sucrose, 7.74 mM Na2HPO4, and 2.26 mM NaH2PO4, pH adjusted to 7.4) using a Brinkman Polytron (Setting 6 for 20 s) and centrifuged at 45,995g for 10 min at 4 °C. The resulting pellet was resuspended in buffer, recentrifuged, and suspended in ice cold buffer again to a concentration of 20 mg/mL, (original wet weight, OWW). Experiments were conducted in 96-well polypropylene plates containing 50 μL of various concentrations of the test compound, diluted using 30% DMSO vehicle, 300 μL of sucrose phosphate buffer, 50 μL of [3H]WIN35,428 (final concentration 1.5 nM; Kd = 14.6 nM; PerkinElmer Life Sciences, Waltham, MA), and 100 μL of tissue (2.0 mg/well OWW). All compound dilutions were tested in triplicate, and the competition reactions started with the addition of tissue; the plates were incubated for 120 min at 0–4 °C. Nonspecific binding was determined using 10 μM final concentration of indatraline.
SERT Binding Assay
Frozen brainstem tissue dissected from male Sprague–Dawley rat (supplied on ice by BioIVT, Hicksville, NY) were homogenized in 20 volumes (w/v) of 50 mM Tris buffer (120 mM NaCl and 5 mM KCl, adjusted to pH 7.4) at 25 °C using a Brinkman Polytron (at setting 6 for 20 s) and centrifuged at 34,957g for 10 min at 4 °C. The resulting pellet was resuspended in buffer, recentrifuged, and suspended in buffer again to a concentration of 20 mg/mL, OWW. Experiments were conducted in 96-well polypropylene plates containing 50 μL of various concentrations of the test compounds, diluted using 30% DMSO vehicle, 300 μL of Tris buffer, 50 μL of [3H]citalopram (final concentration 1.5 nM; Kd = 6.91 nM; PerkinElmer Life Sciences, Waltham, MA), and 100 μL of tissue (2.0 mg/well OWW). All compound dilutions were tested in triplicate, and the competition reactions started with the addition of tissue, and the plates were incubated for 60 min at room temperature. Nonspecific binding was determined using 10 μM final concentration of fluoxetine. For all binding assays, incubations were terminated by rapid filtration through PerkinElmer Uni-Filter-96 GF/B (DAT), GF/B or GF/C SAN (SERT) presoaked in either 0.3% (SERT) or 0.05% (DAT) polyethylenimine, using a Brandel 96-Well Plates Harvester manifold (Brandel Instruments, Gaithersburg, MD). The filters were washed a total of three times with 3 mL (3 × 1 mL/well) of ice-cold binding buffer. 65 μL PerkinElmer MicroScint20 Scintillation Cocktail was added to each filter well. All the plates/filters were counted using a PerkinElmer MicroBeta Microplate Counter. For each experiment, aliquots of the prepared radioligand solutions were measured to calculate the exact amount of radioactivity added, taking into account the experimentally determined top-counter efficiency for each radioligand. Ki values have been extrapolated by constraining the bottom of the dose–response curves (=0% residual specific binding) in the nonlinear regression analysis. Ki values were calculated using GraphPad Prism 8 version 8.4.0 for Macintosh (GraphPad Software, San Diego, CA) utilizing One site- Fit Ki model. Kd values for the radioligands were determined via separate homologous competitive binding or radioligand binding saturation experiments. Ki values were determined from at least three independent experiments performed in triplicate and are reported as mean ± SEM and rounded to three significant digits.
Molecular Pharmacology
Site-Directed Mutagenesis
The Y156F or Y335A mutation was introduced with QuickChange (adapted from Stratagene, La Jolla, CA) on hDAT WT cDNA cloned into the pcDNA3 expression vector. Clones carrying these mutations were detected by DNA sequencing (Eurofins Genomics, DE), and plasmids were amplified by transformation [XL1 blue cells (Stratagene)] and harvested using the maxi prep kit (Qiagen) according to the manufacturer’s manual.
Cell Culture and Transfection
COS7 cells were grown in Dulbecco’s modified Eagle’s medium 041 01885 supplemented with 10% fetal calf serum, 2 mM l-glutamine, and 0.01 mg/mL gentamicin at 37 °C in 10% CO2. hDAT WT, Y335A and Y156F were transiently transfected into COS7 cells with Lipo2000 (Invitrogen) according to manufacturer’s manual using a cDNA/Lipo2000 ratio of 3:6.
[3H]WIN35,428 Binding Experiments
Binding assays were carried out essentially as described.54 Transfected COS7 cells were plated in 24-well dishes (105 cells/well) coated with poly ornithine (Sigma). 48 h after transfection, cells were washed with 500 μL uptake buffer (UB) (25 mM HEPES, 130 mM NaCl, 5.4 mM KCl, 1.2 mM CaCl2, 1.2 mM MgSO4, 1 mM l-ascorbic acid and 5 mM d-glucose, pH 7.4), and the nonlabeled compound was added to the cells in a total volume of 500 μL UB. The assay was initiated by the addition of 10 nM [3H]WIN35,428 (82 Ci/mmol) (Novandi Chemistry, SE). The reactions were incubated at 5 °C until equilibrium was obtained (>90 min). Then cells were washed twice with 500 μL of ice cold UB, lysed in 250 μL of 1% SDS, and left for 1 h at 37 °C. All experiments were carried out with 10 concentrations of unlabeled ligand within a concentration range from 1 nM to 1 mM, performed in triplicates. All concentrations were compared to the control where no unlabeled ligand was added. Nonspecific binding was determined with the addition of 50 μM nomifensine.
All samples were transferred to 24-well counting plates (PerkinElmer, Waltham, MA); 500 μL (24 well) of Opti-phase Hi Safe 3 scintillation fluid (PerkinElmer) was added followed by counting of the plates in a Wallac Tri-Lux β-scintillation counter (PerkinElmer).
[3H]DA Uptake Experiments
Uptake assays were carried out essentially as described.54 COS7 cells transfected with either wild-type hDAT or mutant hDAT Y335A were cultured in 24-well dishes coated with poly ornithine (Sigma) at a density of 105 cells per well or in 12-well dishes at a density of 1.5 × 105 cells per well. After 48 h of incubation, the cells were rinsed with 400 μL of uptake buffer (UB) (25 mM HEPES, 130 mM NaCl, 5.4 mM KCl, 1.2 mM CaCl2, 1.2 mM MgSO4, 1 mM l-ascorbic acid, and 5 mM d-glucose at pH 7.4). Subsequently, 50 μL of nonlabeled compound was introduced to the cells along with 400 μL of UB. The assay was incubated at 22 °C with gentle agitation for 30 min to reach binding equilibrium. Uptake was initiated by the addition of 50 μL 13.3 nM [3H]DA (specific activity of 30–45 Ci/mmol, PerkinElmer). After 5 min of uptake for hDAT WT or 10 min for hDAT Y335A, the reaction was stopped by washing the cells twice with 500 μL of ice-cold UB. The cells were then lysed in 250 μL of a 1% SDS solution and incubated at 37 °C for 1 h.
All experiments were conducted using 10 concentrations of an unlabeled ligand, spanning from 1 nM to 1 mM, and were performed in triplicates. All concentrations were compared to the control where no unlabeled ligand was added. Nonspecific binding was determined with the addition of 50 μM nomifensine. Subsequently, all samples were transferred to 24-well counting plates (PerkinElmer), and 500 μL of Opti-phase Hi Safe 3 scintillation fluid (PerkinElmer) was added to each well. The plates were then counted using a Wallac Tri-Lux β-scintillation counter (PerkinElmer).
hERG Channel Activity Method
The WT-hERG constructs were transiently transfected into human embryonic kidney (HEK) 293 cells by the calcium phosphate method and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin (GIBCO, CA).55 Transfected HEK cells were grown on cover glass chips. After 24 to 72 h incubation, the glass chips were moved in a 2 cm3 chamber with constant extracellular medium superfusion. Various concentrations of compounds were superfused at a rate of ∼1.5 mL/min during experiments. The conventional whole-cell voltage-clamp methods were used to measure hERG currents.55 The pipet solution contained (in mM) KCl 10, K-aspartate 110, MgCl2 5, Na2 ATP 5, EGTA 10, HEPES 5, CaCl2 1, corrected to pH 7.2 with KOH. The extracellular solution contained (in mM) NaCl 140, KCl 5.4, CaCl2 1, MgCl2 1, HEPES 5, glucose 5.5, pH 7.4, with NaOH. The holding potential was −80 mV. The hERG currents were activated by 1 s depolarization to +50 mV and then hyparpolarized to −100 mV to induce tail currents. The amplitudes of tail currents were measured in response to testing compounds. All compounds were dissolved in DMSO (20–50 mM) first and then diluted into the extracellular solution before the experiments. N = 3 to 6 at the concentrations around IC50 of the compounds. All the experiments were performed at room temperature. AXOPATCH 200B amplifier and Clampex software (Axon Instruments) was used. The data were sampled at 2 kHz and analyzed with Clampfit (Axon, USA). IC50 and Hill’s coefficient of each compound were calculated by fitting all the data to Hill’s equation with SigmaPlot 14.
QSAR Modeling to Predict hERG Activity
Machine learning-based QSAR models were built to correlate the chemical structures of the 4'F-bisphenyl and biphenyl DAT inhibitors with binding affinities at hERG. Our previous study16 showed that the machine-learning-based QSAR models trained with the hERG clamp IC50 data set and eXtreme gradient boosting (XGBoost) algorithm have the best predictive power. Therefore, we chose to use this combination of data set and algorithm to generate QSAR models for this study.
To extract the correct and relevant information, we queried and retrieved these data from ChEMBL database56 using the same filtering pipeline used in our previous study.16 The same list of keywords for hERG data was used to comprehensively retrieve relevant entries. However, two significant changes were made for this study. First, we upgraded the ChEMBL version from 25 to 31. Second, we only kept the largest pChEMBL value among the compounds with the same chemical structure (Tanimoto similarity >0.999) that appeared in the database and removed the others. After these filter changes and adding back 55 compounds with high-quality measurements of hERG affinity,57 there were 1930 IC50 data points in the clamp data set, a drastic increase of 525 data points from ChEMBL 25 (1405). This data set was used to search the hyper-parameter space as described previously.16 Ten production models were then generated using the resulting hyper-parameters and 100% of the data.
These QSAR models were used to predict the 4'F-bisphenyl and biphenyl DAT inhibitors. For each compound, the mean and standard deviation of the predicted values from the ten models are reported in Table 4.
Acknowledgments
We thank Lone Rosenquist for excellent technical assistance. We thank James Paule for obtaining RLM data at Johns Hopkins Drug Discovery, Johns Hopkins University. Support for this research was provided by the National Institute on Drug Abuse-Intramural Research Program [Z1A DA000389 (AHN) and Z1A DA000606 (LS)] and the Medications Development Program. CJL is supported by the Lundbeck Foundation (R344-2020-1020) and Danish Council for Independent Research (3101-00381B).
Glossary
Abbreviations
- ACN
acetonitrile
- ANOVA
analysis of variance
- Boc
tert-butyloxycarbonyl
- CDI
carbonyldiimidazole
- CFT
β-carbomethoxy-3-β-(4-fluorophenyl)tropane
- CNS
central nervous system
- DA
dopamine
- DAT
dopamine transporter
- DCM
dichloromethane
- EI
electron ionization
- FDA
food and drug administration
- hDAT
human dopamine transporter
- hERG
human ether-à-go-go-related gene
- HESI
heated electrospray ionization
- IC50
half maximal inhibitory concentration
- Kd
dissociation constant
- Ki
inhibitor constant
- MeOH
methanol
- MPO
multiparameter optimization
- MS
mass spectrometry
- NMR
nuclear magnetic resonance
- NT
not tested
- OWW
original wet weight
- ppm
parts-per-million
- PSUD
psychostimulant use disorders
- QSAR
quantitative structure–activity relationships
- RT
room temperature
- SEM
standard error of the mean
- SAR
structure–activity relationships
- SERT
serotonin transporter
- TEA
triethylamine
- TFA
trifluoroacetic acid
- WT
wild type
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.3c00322.
Microanalysis data on all final compounds and SMILES data (PDF)
Author Contributions
A.H.N. and T.C.K. designed the project; T.C.K., G. A. C.-H., and A.H.N. wrote the manuscript with input of all authors; T.C.K., C.J.L, H.J.D., L.S., and A.H.N. designed and/or supervised the experiments and data analyses; T.C.K., S.J.W., J.G., G.A. C.-H., A.V.O., K.W.S., and K.H.L performed experiments. T.C.K and J.C. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- NIDA . Overdose Death Rates. 2022. https://nida.nih.gov/drug-topics/trends-statistics/overdose-death-ratess (accessed, May 09, 2023).
- NIDA . Methamphetamine-involved Overdose Deaths Nearly Tripled between 2015 to 2019, NIH Study Finds. 2021. https://nida.nih.gov/news-events/news-releases/2021/09/methamphetamine-involved-overdose-deaths-nearly-tripled-between-2015-to-2019-nih-study-finds (accessed, May 23, 2022).
- Foundation N . Stimulant Deaths on the Rise, Compounded by Rise in Synthetic Opioids. 2021. https://nihcm.org/publications/stimulant-deaths-on-the-rise-compounded-by-rise-in-synthetic-opioids#:~:text=In%202019%2C%20stimulants%20were%20involved,also%20opioids%20or%20other%20drugs)https://nihcm.org/publications/stimulant-deaths-on-the-rise-compounded-by-rise-in-synthetic-opioids#:~:text=In%202019%2C%20stimulants%20were%20involved,also%20opioids%20or%20other%20drugs) (accessed, May 23, 2022).
- Friedman J.; Shover C. L. Charting the fourth wave: Geographic, temporal, race/ethnicity and demographic trends in polysubstance fentanyl overdose deaths in the United States, 2010–2021. Addiction 2023, 118 (12), 2477–2485. 10.1111/add.16318. [DOI] [PubMed] [Google Scholar]
- Mattick R. P.; Breen C.; Kimber J.; Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst. Rev. 2009, 2009 (3), CD002209. 10.1002/14651858.CD002209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick R. P.; Breen C.; Kimber J.; Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2014, 2014 (2), CD002207. 10.1002/14651858.CD002207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. P.; Gryczynski J.; O’Grady K. E.; Sharfstein J. M.; Warren G.; Olsen Y.; Mitchell S. G.; Jaffe J. H. Opioid agonist treatments and heroin overdose deaths in Baltimore, Maryland, 1995–2009. Am. J. Public Health 2013, 103 (5), 917–922. 10.2105/AJPH.2012.301049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C. J.; Cao J.; Newman A. H.; Xi Z. X. Progress in agonist therapy for substance use disorders: Lessons learned from methadone and buprenorphine. Neuropharmacology 2019, 158, 107609. 10.1016/j.neuropharm.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster R. L.; Kuhar M. J.; Schuster C. R.. Pharmacological Aspects of Drug Dependence: Toward an Integrated Neurobehavioral Approach; Springer, 1996. [Google Scholar]
- Schmitt K. C.; Zhen J.; Kharkar P.; Mishra M.; Chen N.; Dutta A. K.; Reith M. E. A. Interaction of cocaine-, benztropine-, and GBR12909-like compounds with wildtype and mutant human dopamine transporters: molecular features that differentially determine antagonist binding properties. J. Neurochem. 2008, 107 (4), 928–940. 10.1111/j.1471-4159.2008.05667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt K. C.; Reith M. E. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann. N.Y. Acad. Sci. 2010, 1187, 316–340. 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. E.; Volz T. J.; Riddle E. L.; Gibb J. W.; Hanson G. R. New insights into the mechanism of action of amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 681–698. 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Wood S.; Sage J. R.; Shuman T.; Anagnostaras S. G. Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol. Rev. 2014, 66 (1), 193–221. 10.1124/pr.112.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras B.; Kuhar M. J.. The Effects of Drug Abuse on the Human Nervous System; Elsevier, 2014. [Google Scholar]
- Beuming T.; Kniazeff J.; Bergmann M. L.; Shi L.; Gracia L.; Raniszewska K.; Newman A. H.; Javitch J. A.; Weinstein H.; Gether U.; Loland C. J. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat. Neurosci. 2008, 11 (7), 780–789. 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. H.; Fant A. D.; Guo J.; Guan A.; Jung J.; Kudaibergenova M.; Miranda W. E.; Ku T.; Cao J.; Wacker S.; Duff H. J.; Newman A. H.; Noskov S. Y.; Shi L. Toward Reducing hERG Affinities for DAT Inhibitors with a Combined Machine Learning and Molecular Modeling Approach. J. Chem. Inf. Model. 2021, 61 (9), 4266–4279. 10.1021/acs.jcim.1c00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. H.; Penmatsa A.; Gouaux E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 2015, 521 (7552), 322–327. 10.1038/nature14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai R. I.; Newman A. H.; Katz J. L. Antagonism of the stimulant effects of cocaine by the dopamine transporter ligand, JHW007. Behav. Pharmacol. 2005, 16, S78. 10.1097/00008877-200509001-00244. [DOI] [Google Scholar]
- Tanda G.; Newman A. H.; Katz J. L. Discovery of drugs to treat cocaine dependence: behavioral and neurochemical effects of atypical dopamine transport inhibitors. Adv. Pharmacol. 2009, 57, 253–289. 10.1016/S1054-3589(08)57007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loland C. J.; Mereu M.; Okunola O. M.; Cao J.; Prisinzano T. E.; Mazier S.; Kopajtic T.; Shi L.; Katz J. L.; Tanda G.; Newman A. H. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol. Psychiatry 2012, 72 (5), 405–413. 10.1016/j.biopsych.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loland C. J.; Desai R. I.; Zou M. F.; Cao J.; Grundt P.; Gerstbrein K.; Sitte H. H.; Newman A. H.; Katz J. L.; Gether U. Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol. Pharmacol. 2008, 73 (3), 813–823. 10.1124/mol.107.039800. [DOI] [PubMed] [Google Scholar]
- Newman A. H.; Cao J.; Keighron J. D.; Jordan C. J.; Bi G. H.; Liang Y.; Abramyan A. M.; Avelar A. J.; Tschumi C. W.; Beckstead M. J.; Shi L.; Tanda G.; Xi Z. X. Translating the atypical dopamine uptake inhibitor hypothesis toward therapeutics for treatment of psychostimulant use disorders. Neuropsychopharmacology 2019, 44 (8), 1435–1444. 10.1038/s41386-019-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J.; Slack R. D.; Bakare O. M.; Burzynski C.; Rais R.; Slusher B. S.; Kopajtic T.; Bonifazi A.; Ellenberger M. P.; Yano H.; He Y.; Bi G. H.; Xi Z. X.; Loland C. J.; Newman A. H. Novel and High Affinity 2-[(Diphenylmethyl)sulfinyl]acetamide (Modafinil) Analogues as Atypical Dopamine Transporter Inhibitors. J. Med. Chem. 2016, 59 (23), 10676–10691. 10.1021/acs.jmedchem.6b01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramyan A. M.; Stolzenberg S.; Li Z.; Loland C. J.; Noe F.; Shi L. The Isomeric Preference of an Atypical Dopamine Transporter Inhibitor Contributes to Its Selection of the Transporter Conformation. ACS Chem. Neurosci. 2017, 8 (8), 1735–1746. 10.1021/acschemneuro.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avelar A. J.; Cao J.; Newman A. H.; Beckstead M. J. Atypical dopamine transporter inhibitors R-modafinil and JHW 007 differentially affect D2 autoreceptor neurotransmission and the firing rate of midbrain dopamine neurons. Neuropharmacology 2017, 123, 410–419. 10.1016/j.neuropharm.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu M.; Bonci A.; Newman A. H.; Tanda G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology 2013, 229 (3), 415–434. 10.1007/s00213-013-3232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis C. A.; Kampman K. M.; Lynch K. G.; Plebani J. G.; Pettinati H. M.; Sparkman T.; O’Brien C. P. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J. Subst Abuse Treat 2012, 43 (3), 303–312. 10.1016/j.jsat.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A. L.; Reid M. S.; Li S. H.; Holmes T.; Shemanski L.; Slee A.; Smith E. V.; Kahn R.; Chiang N.; Vocci F.; Ciraulo D.; Dackis C.; Roache J. D.; Salloum I. M.; Somoza E.; Urschel H. C.; Elkashef A. M. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009, 104 (1–2), 133–139. 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaba P.; Ilic M.; Aher N. Y.; Dragacevic V.; Wieder M.; Zehl M.; Wackerlig J.; Beyl S.; Sartori S. B.; Ebner K.; Roller A.; Lukic N.; Beryozkina T.; Gonzalez E. R. P.; Neill P.; Khan J. A.; Bakulev V.; Leban J. J.; Hering S.; Pifl C.; Singewald N.; Lubec J.; Urban E.; Sitte H. H.; Langer T.; Lubec G. Structure-Activity Relationships of Novel Thiazole-Based Modafinil Analogues Acting at Monoamine Transporters. J. Med. Chem. 2020, 63 (1), 391–417. 10.1021/acs.jmedchem.9b01938. [DOI] [PubMed] [Google Scholar]
- Kalaba P.; Aher N. Y.; Ilic M.; Dragacevic V.; Wieder M.; Miklosi A. G.; Zehl M.; Wackerlig J.; Roller A.; Beryozkina T.; Radoman B.; Saroja S. R.; Lindner W.; Gonzalez E. P.; Bakulev V.; Leban J. J.; Sitte H. H.; Urban E.; Langer T.; Lubec G. Heterocyclic Analogues of Modafinil as Novel, Atypical Dopamine Transporter Inhibitors. J. Med. Chem. 2017, 60 (22), 9330–9348. 10.1021/acs.jmedchem.7b01313. [DOI] [PubMed] [Google Scholar]
- Slack R. D.; Ku T. C.; Cao J.; Giancola J. B.; Bonifazi A.; Loland C. J.; Gadiano A.; Lam J.; Rais R.; Slusher B. S.; Coggiano M.; Tanda G.; Newman A. H. Structure-Activity Relationships for a Series of (Bis(4-fluorophenyl)methyl)sulfinyl Alkyl Alicyclic Amines at the Dopamine Transporter: Functionalizing the Terminal Nitrogen Affects Affinity, Selectivity, and Metabolic Stability. J. Med. Chem. 2020, 63 (5), 2343–2357. 10.1021/acs.jmedchem.9b01188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okunola-Bakare O. M.; Cao J.; Kopajtic T.; Katz J. L.; Loland C. J.; Shi L.; Newman A. H. Elucidation of structural elements for selectivity across monoamine transporters: novel 2-[(diphenylmethyl)sulfinyl]acetamide (modafinil) analogues. J. Med. Chem. 2014, 57 (3), 1000–1013. 10.1021/jm401754x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J.; Prisinzano T. E.; Okunola O. M.; Kopajtic T.; Shook M.; Katz J. L.; Newman A. H. SARs at the Monoamine Transporters for a Novel Series of Modafinil Analogues. ACS Med. Chem. Lett. 2011, 2 (1), 48–52. 10.1021/ml1002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okorom A. V.; Camacho-Hernandez G. A.; Salomon K.; Lee K. H.; Ku T. C.; Cao J.; Won S. J.; Friedman J.; Lam J.; Paule J.; Rais R.; Klein B.; Xi Z.-X.; Shi L.; Loland C. J.; Newman A. H. Modifications to 1-(4-(2-Bis(4-fluorophenyl)methyl)sulfinyl)alkyl Alicyclic Amines That Improve Metabolic Stability and Retain an Atypical DAT Inhibitor Profile. J. Med. Chem. 2023, 10.1021/acs.jmedchem.3c02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaba P.; Pacher K.; Neill P. J.; Dragacevic V.; Zehl M.; Wackerlig J.; Kirchhofer M.; Sartori S. B.; Gstach H.; Kouhnavardi S.; Fabisikova A.; Pillwein M.; Monje-Quiroga F.; Ebner K.; Prado-Roller A.; Singewald N.; Urban E.; Langer T.; Pifl C.; Lubec J.; Leban J. J.; Lubec G. Chirality Matters: Fine-Tuning of Novel Monoamine Reuptake Inhibitors Selectivity through Manipulation of Stereochemistry. Biomolecules 2023, 13 (9), 1415. 10.3390/biom13091415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall B. J.; Ho C. P.; Cao J.; Vendruscolo J. C. M.; Schmeichel B. E.; Slack R. D.; Tanda G.; Gadiano A. J.; Rais R.; Slusher B. S.; Koob G. F.; Newman A. H.; Vendruscolo L. F. Atypical dopamine transporter inhibitors attenuate compulsive-like methamphetamine self-administration in rats. Neuropharmacology 2018, 131, 96–103. 10.1016/j.neuropharm.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin M. J.; Kuchel P. W.; Campbell T. J.; Vandenberg J. I. Drug binding to the inactivated state is necessary but not sufficient for high-affinity binding to human ether-a-go-go-related gene channels. Mol. Pharmacol. 2008, 74 (5), 1443–1452. 10.1124/mol.108.049056. [DOI] [PubMed] [Google Scholar]
- Gintant G.; Sager P. T.; Stockbridge N. Evolution of strategies to improve preclinical cardiac safety testing. Nat. Rev. Drug Discovery 2016, 15 (7), 457–471. 10.1038/nrd.2015.34. [DOI] [PubMed] [Google Scholar]
- Kalyaanamoorthy S.; Barakat K. H. Binding modes of hERG blockers: an unsolved mystery in the drug design arena. Expert Opin. Drug Discovery 2018, 13 (3), 207–210. 10.1080/17460441.2018.1418319. [DOI] [PubMed] [Google Scholar]
- Lubec J.; Kalaba P.; Hussein A. M.; Feyissa D. D.; Kotob M. H.; Mahmmoud R. R.; Wieder O.; Garon A.; Sagheddu C.; Ilic M.; Dragacevic V.; Cybulska-Klosowicz A.; Zehl M.; Wackerlig J.; Sartori S. B.; Ebner K.; Kouhnavardi S.; Roller A.; Gajic N.; Pistis M.; Singewald N.; Leban J. J.; Korz V.; Malikovic J.; Plasenzotti R.; Sitte H. H.; Monje F. J.; Langer T.; Urban E.; Pifl C.; Lubec G. Reinstatement of synaptic plasticity in the aging brain through specific dopamine transporter inhibition. Mol. Psychiatry 2021, 26 (12), 7076–7090. 10.1038/s41380-021-01214-x. [DOI] [PubMed] [Google Scholar]
- Dunn D.; Hostetler G.; Iqbal M.; Messina-McLaughlin P.; Reiboldt A.; Lin Y. G.; Gruner J.; Bacon E. R.; Ator M. A.; Chatterjee S. Wake-promoting agents: Search for next generation modafinil: Part I. Bioorg. Med. Chem. Lett. 2012, 22 (6), 2312–2314. 10.1016/j.bmcl.2011.12.099. [DOI] [PubMed] [Google Scholar]
- Dunn D.; Hostetler G.; Iqbal M.; Messina-McLaughlin P.; Reiboldt A.; Lin Y. G.; Gruner J.; Bacon E. R.; Ator M. A.; Chatterjee S. Wake-promoting agents: search for next generation modafinil: part II. Bioorg. Med. Chem. Lett. 2012, 22 (6), 2315–2317. 10.1016/j.bmcl.2012.01.064. [DOI] [PubMed] [Google Scholar]
- Wager T. T.; Hou X.; Verhoest P. R.; Villalobos A. Moving beyond rules: the development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties. ACS Chem. Neurosci. 2010, 1 (6), 435–449. 10.1021/cn100008c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T. T.; Hou X.; Verhoest P. R.; Villalobos A. Central Nervous System Multiparameter Optimization Desirability: Application in Drug Discovery. ACS Chem. Neurosci. 2016, 7 (6), 767–775. 10.1021/acschemneuro.6b00029. [DOI] [PubMed] [Google Scholar]
- Bisgaard H.; Larsen M. A.; Mazier S.; Beuming T.; Newman A. H.; Weinstein H.; Shi L.; Loland C. J.; Gether U. The binding sites for benztropines and dopamine in the dopamine transporter overlap. Neuropharmacology 2011, 60 (1), 182–190. 10.1016/j.neuropharm.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loland C. J.; Norregaard L.; Litman T.; Gether U. Generation of an activating Zn(2+) switch in the dopamine transporter: mutation of an intracellular tyrosine constitutively alters the conformational equilibrium of the transport cycle. Proc. Natl. Acad. Sci. U.S.A. 2002, 99 (3), 1683–1688. 10.1073/pnas.032386299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola J. B.; Bonifazi A.; Cao J.; Ku T.; Haraczy A. J.; Lam J.; Rais R.; Coggiano M. A.; Tanda G.; Newman A. H. Structure-activity relationships for a series of (Bis(4-fluorophenyl)methyl)sulfinylethyl-aminopiperidines and -piperidine amines at the dopamine transporter: Bioisosteric replacement of the piperazine improves metabolic stability. Eur. J. Med. Chem. 2020, 208, 112674. 10.1016/j.ejmech.2020.112674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G.; Hersey M.; Hempel B.; Xi Z. X.; Newman A. H. Modafinil and its structural analogs as atypical dopamine uptake inhibitors and potential medications for psychostimulant use disorder. Curr. Opin. Pharmacol. 2021, 56, 13–21. 10.1016/j.coph.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A. H.; Ku T.; Jordan C. J.; Bonifazi A.; Xi Z. X. New Drugs, Old Targets: Tweaking the Dopamine System to Treat Psychostimulant Use Disorders. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 609–628. 10.1146/annurev-pharmtox-030220-124205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. M. S.; Burch R. L. The Principles of Humane Experimental Technique. Med. J. Aust. 1960, 1 (13), 500. 10.5694/j.1326-5377.1960.tb73127.x. [DOI] [Google Scholar]
- Flecknell P. Replacement, reduction and refinement. ALTEX 2002, 19 (2), 73–78. [PubMed] [Google Scholar]
- Gorzalczany S. B.; Rodriguez Basso A. G. Strategies to apply 3Rs in preclinical testing. Pharmacol Res. Perspect 2021, 9 (5), e00863 10.1002/prp2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Hernandez G. A.; Casiraghi A.; Rudin D.; Luethi D.; Ku T. C.; Guthrie D. A.; Straniero V.; Valoti E.; Schutz G. J.; Sitte H. H.; Newman A. H. Illuminating the norepinephrine transporter: fluorescent probes based on nisoxetine and talopram. RSC Med. Chem. 2021, 12 (7), 1174–1186. 10.1039/D1MD00072A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen A. V.; Andreassen T. F.; Loland C. J. A conserved salt bridge between transmembrane segments 1 and 10 constitutes an extracellular gate in the dopamine transporter. J. Biol. Chem. 2014, 289 (50), 35003–35014. 10.1074/jbc.M114.586982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durdagi S.; Guo J.; Lees-Miller J. P.; Noskov S. Y.; Duff H. J. Structure-guided topographic mapping and mutagenesis to elucidate binding sites for the human ether-a-go-go-related gene 1 potassium channel (KCNH2) activator NS1643. J. Pharmacol. Exp. Ther. 2012, 342 (2), 441–452. 10.1124/jpet.111.189159. [DOI] [PubMed] [Google Scholar]
- Gaulton A.; Bellis L. J.; Bento A. P.; Chambers J.; Davies M.; Hersey A.; Light Y.; McGlinchey S.; Michalovich D.; Al-Lazikani B.; Overington J. P. ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012, 40 (D1), D1100–D1107. 10.1093/nar/gkr777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J.; Obejero-Paz C. A.; Myatt G.; Kuryshev Y. A.; Bruening-Wright A.; Verducci J. S.; Brown A. M. MICE models: superior to the HERG model in predicting Torsade de Pointes. Sci. Rep. 2013, 3, 2100. 10.1038/srep02100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.