Abstract
Homeostasis of fluid and electrolytes is a tightly controlled physiological process. Failure of this process is a hallmark of hypertension, chronic kidney disease, heart failure, and other acute and chronic diseases. While the kidney remains the major player in the control of whole-body fluid and electrolyte homeostasis, recent discoveries point toward more peripheral mechanisms leading to sodium storage in tissues, such as skin and muscle, and a link between this sodium and a range of diseases, including the conditions above. In this review, we describe multiple facets of sodium and fluid balance from traditional concepts to novel discoveries. We examine the differences between acute disruption of sodium balance and the longer term adaptation in chronic disease, highlighting areas that cannot be explained by a kidney-centric model alone. The theoretical and methodological challenges of more recently proposed models are discussed. We acknowledge the different roles of extracellular and intracellular spaces and propose an integrated model that maintains fluid and electrolyte homeostasis and can be distilled into a few elemental players: the microvasculature, the interstitium, and tissue cells. Understanding their interplay will guide a more precise treatment of conditions characterized by sodium excess, for which primary aldosteronism is presented as a prototype.
Keywords: body fluids, electrolytes, homeostasis, hypertension, water
Historically, studies and public policies on cardiovascular disease and particularly hypertension have focused on sodium (Na+) intake, while relatively less attention has been devoted to differences in its handling. Old assumptions on the constancy of the internal environment have now been challenged, revealing that Na+ balance can be dynamic and over time not even perfectly balanced.
Approximately 10 years ago, Bhave and Neilson1 reviewed the mechanisms of body fluid and electrolyte dynamics in relation to body compartments and reconnected historical principles with novel insights. In this review, we will expand on those dynamics, presenting new technologies and evidence, but also old experimental data that provide ground to our current interpretations. In particular, we will discuss the following: (1) the variability of day-to-day Na+ balance; (2) the concept of long-term uneven Na+ balance in cardiovascular and renal disease, long established but nowadays directly visualized as Na+ excess in tissues; (3) the evolving interpretation for such tissue signal; (4) the importance of interstitial fluid balance, microvascular interface, and intracellular compartment. In these regard, insights from primary aldosteronism (PA), a prototypic salt-sensitive disease leading to hypertension but also to cardiorenovascular damage in excess of blood pressure (BP) values, will be presented.
ROLLERCOASTER BALANCE
Urinary Na+ excretion has traditionally been assumed to invariably reflect intake. However, the day-to-day validity of such equivalence has been recently disproved by long-term balance studies2,3: the daily Na+ excretion showed remarkable oscillations around the amount of salt targeted by the dietitians (Figures S1 and S2), in addition to weekly and monthly periodicity related to rhythmic hormonal control.3 The daily Na+ excretion from 1 single 24-hour urine collection predicted the recorded Na+ intake within a predefined±25 mmol 2-sided interval only in 49% of cases. Reassuringly, the average daily Na+ excretion provided an accurate estimate of mean salt intake, thus reflecting the ultimate achievement of a steady-state balance, and repeated 24-hour collections (but not nocturnal only4 or spot urine5) improved the precision.3,6 In keeping with a variability of 24-hour collections, half of the subjects included in a single-center cohort study switched between tertiles of estimated Na+ intake at up to 15-year follow-up collections, compared with a reference baseline. With the limit of a noninterventional design and lack of control on dietary changes, disease, and medications over time, this reclassification significantly affected the observed relationship between Na+ intake and long-term cardiovascular and renal outcomes.7 Averaging multiple collections strengthened the association,7 as later confirmed.8
Should we abandon the use of 24-hour urine collections for the estimation of daily Na+ intake in patients? We should not, because of 2-fold considerations. First, single 24-hour urine collections from adequately sized cohorts offer a precise estimate of group—albeit not of individual—Na+ intake,9 which is relevant for researchers and study designs. Second, if the goal of sensible clinicians is to detect true excess intake and not a precise value, to avoid false positives and consequent unnecessary and potentially stressful changes in dietary habits, the focus should be on a 1-sided end, rather than 2-sided CI. This clinical approach requires less precision: a 270 mmol Na+/day, for example, is unlikely to reflect anything but high Na+ intake, which is universally recognized as a cardiovascular risk factor.10 In the setting of our hypertension clinics where dietary advice and medications are controlled,11 24-hour urine collection remains a valuable and inexpensive tool to decipher the biochemical screening of secondary forms,12 to estimate adherence to lifestyle recommendations and to provide semiquantitative metrics for positive patient reinforcement upon clinically significant reductions at follow-up, provided that data are interpreted with the patient and cum grano salis—much welcome in this occasion.
TANGRAM
Ultra-Long-Term or Life-Long Balance May Obey Different Rules
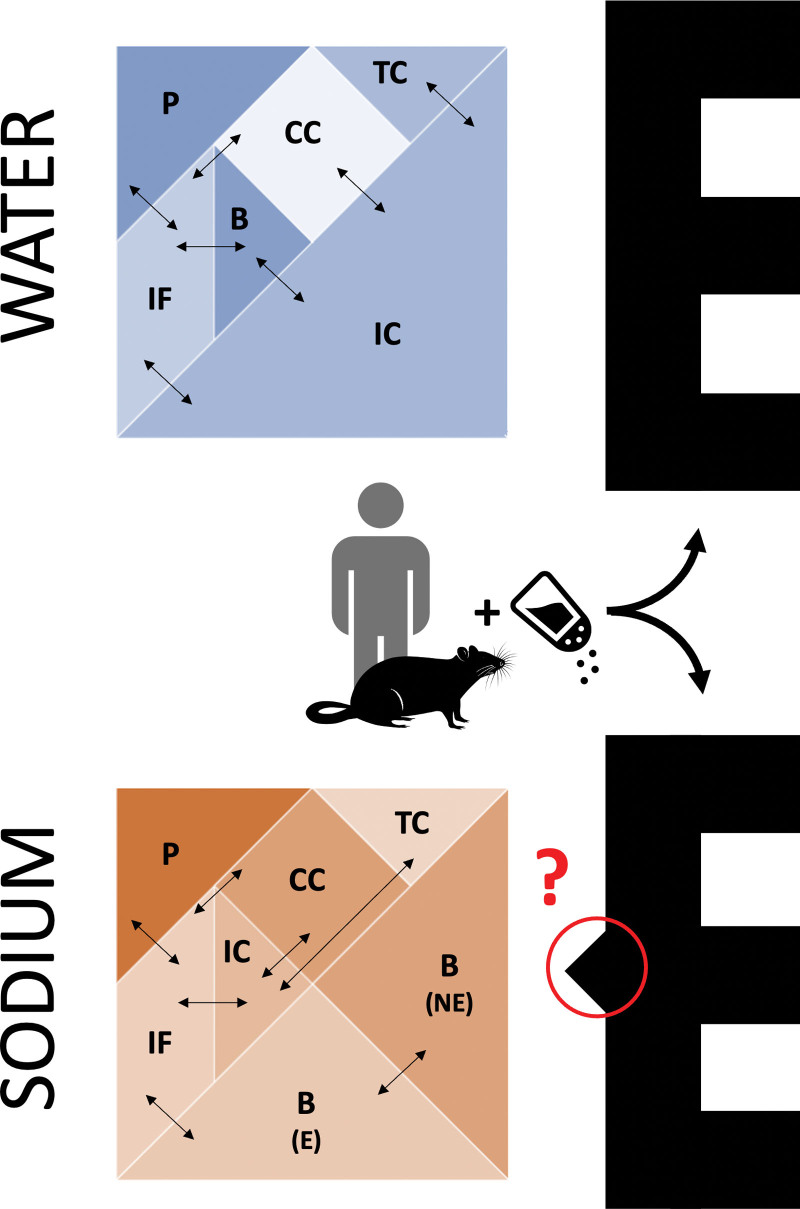
Long-term human balance studies, in keeping with previous experimental reports of positive Na+ (but not water) balance on extremely high Na+ intake,13 found considerable changes in total body Na+ without parallel changes in body weight.2,14 Similarly, multiple studies in rodents identified a dissociation between Na+ and water tissue content.15–17 The degree of Na+-associated fluid retention upon high Na+ intake was first shown to differ in animal models of normotension, salt-resistant, and salt-sensitive hypertension, with the former showing the highest water-independent Na+ storage capacity.15 Subsequent body composition studies, conducted by desiccation and ashing of whole rat carcasses or specific parts (ie, bones, quadriceps muscles, and skin), suggested the skin as the main depot for excess Na+ accumulation.16–19 If one sees the analogy of body fluid and electrolyte compartmentalization20 with a Tangram, an old dissection puzzle consisting of a few pieces that are variably combined to form different shapes, these unphysiological findings of a water-free Na+ excess would seem like one of those Tangram paradoxes with seemingly redundant (or missing, depending on one’s perspective) pieces (Figure 1).
Figure 1.
The Tangram of water and sodium. The Tangram shapes (left) depict the traditional distribution of body water and sodium in compartments.20 Experimental salt loading of humans and rats led to a puzzling excess of sodium compared with water,13–17 which reminds of Tangram paradoxes (E shapes): 2 figures composed with the same 7 pieces, one of which incomprehensibly seems to be a subset of the other (solution shown in Figure S3). B indicates bone; CC, dense connective tissue and cartilage; E, exchangeable fraction; IC, intracellular; IF, interstitial/lymphatic fluid; NE, nonexchangeable fraction; P, plasma; and TC, transcellular.
The most obvious answer to the question “where is the salt?”21 pointed to the extracellular space, that is, the compartment where 98% of total body Na+ is confined.20 A volume-independent hypertonic extracellular accumulation of Na+ appeared most likely, as volume-paralleled extracellular expansion was at odds with the unchanged extracellular volume measured across different Na+ intake phases in the original reports.13 Although the magnitude of the increase in Na+ intake between experimental groups and the inulin-based measure of extracellular volume have been criticized,22 the hypothesis of a hypertonic interstitium was strengthened by evidence of TonEBP (tonicity-responsive enhancer-binding protein) activation in the skin resident mononuclear phagocytic cells of salt-loaded rodents.18 TonEBP-mediated signaling included Vascular Endothelial Growth Factor-C (VEGF-C) secretion, VEGF receptor 3 activation, and plastic expansion of the lymphatic vascular network, to provide enhanced local Na+ excess clearance.19 Disruption of this pathway resulted not only in skin Na+ accumulation but also in salt-sensitive hypertension.19,23 These findings led to conclude that water-independent binding of Na+ to the negatively charged glycosaminoglycan network, particularly represented in the skin interstitium and expanded by dietary NaCl loading,24 could explain the puzzling tangram appendage (Figure 1), that is, where the retained sodium was being stored. The plasticity of glycosaminoglycans in regulating tissue Na+ binding and storage was supported by subsequent rat25,26 and human27 data, including distinct responses in animals and patients with genetically altered glycosaminoglycan structure.28,29 The concept of glycosaminoglycan binding and osmotic inactivation of Na+ is, however, problematic because this would rely on repulsion and thereby excretion of chloride (Cl−),30 found to be increased rather than reduced in rat skin during high-salt conditions.19 Moreover, experiments designed to assess glycosaminoglycan binding of Na+ in skin indicated that such binding was negligible.31 Recent observations in rats and mice also showed no increase in skin glycosaminoglycans by salt loading, or even a decrease with mineralocorticoid (deoxycorticosterone acetate [DOCA]) treatment, despite significant tissue Na+ storage.31,32 These heterogeneous observations may be explained by methodological differences or by additional players such as inflammation and mechanical stress (shown to induce glycosaminoglycan production in cardiac fibroblasts33 and skin34) rather than a solely Na+-dependent control of glycosaminoglycans.35 While the implication of interstitial mucopolysaccharides in the regulation of circulation dates back to Guyton et al,36 how this links to tissue Na+ and the direction of the reported associations still remain unclear.
I WAS BLIND BUT NOW I SEE (JOHN 9:25)
The development of 23Na magnetic resonance spectroscopy and high-magnetic field imaging (MRI) in the last decade has given us the chance of seeing Na+.
23NaMRI, originally validated against direct chemical analysis of tissues and calibrated with phantoms containing NaCl at different concentrations,37 revealed excess skin and muscle Na+ content in patients with resistant hypertension or PA,38,39 diabetes,40–42 heart failure,43,44 but also systemic inflammatory conditions,45–48 lipedema,49 and obesity, but only in the presence of high circulating inflammatory markers.50 Chronic kidney disease (CKD), a highly salt-sensitive condition, has been the most extensively studied: patients on maintenance hemodialysis, particularly those with concomitant diabetes,51 harbor high tissue Na+ content,52–54 comparable to heart failure.55 Tissue Na+ in patients with CKD was higher than in controls even before the end stage56 and correlated with left ventricular mass better than total body overhydration or BP.57 A similar association of tissue Na+ with target organ remodeling, independent of age, gender, diuretic use, and 24-hour ambulatory BP, has been found in diabetes for retinal vessels.58 When Bhave and Neilson1 contended that only about 5% of essential hypertension in American patients may involve alterations in (tissue) Na+ storage, that is, those on a diet >300 Na+ mEq/day, they could not know this pandemic scale of tissue Na+ excess, even exceeding values found in hypertensives, as later revealed by 23NaMRI.41
Nonetheless, a few related technical aspects are worth additional considerations. Similar to most reported chemical analyses of homogenized tissues, only recently coupled with a reliable extracellular volume tracer to provide some compartmental information,31 current 23NaMRI can only measure whole-tissue Na+. In fact, it was recently claimed that 23NaMRI protocols used in the clinical setting predominantly reveal signals from free (dissolved) ions,42,59 thus lending scarce support to the hypothesis of excess glycosaminoglycan-bound Na+ in cardiovascular disease; however, whether it is possible to reliably discriminate the amount of Na+ that is free or bound (and this definition may extend to the intracellularly constrained pool) remains much debated.59 For sure, and at odds with the suggested hypertonicity, all authors reported that whole-tissue Na+ levels for the above pathologies are ≤40 mmol/L, far below the Na+ concentration typically found in plasma,1 but also in lymph or interstitial fluid eluate.60 This may reflect the presence of a relatively Na+-poor intracellular fraction in all tissues, which would dilute the signal from the interstitium in the final whole-tissue readout.61,62 Unfortunately, the attempt to separate the intracellular from extracellular fractions is limited by MRI spatial resolution and by a similar relaxation time of Na+ in the 2 compartments63 or by toxicity of extracellular-limited shifting agents.64 To date, multinuclear, multicompartment modeling, which has shown promise, is still in its infancy and not devoid of limitations.59,65 The feasibility of tissue Na+ imaging down to a cellular scale has recently been proven with the alternative approach of X-ray fluorescence spectromicroscopy, but the technique is destructive and yielded extracellular and intracellular concentrations opposite to what is expected from physiology, likely due to the chemical treatment used for the sample preparation.66 Therefore, while waiting for validated and clinically applicable advancements, the available 23NaMRI data should be interpreted as total tissue Na+.
ON THE SHOULDERS OF GIANTS
Our predecessors were blind but knowledgeable. Not only did they deduce morphological information on tissues and their constitutive compartments from purely chemical measurements since the 40s but they also knew that tissue electrolytes change with aging, toward excess Na+ and Cl− (and loss of K+).67–70 Moreover, in vivo studies from the 70s and 80s performed with nuclear whole-body counting, revealed high total and exchangeable Na+ (NaE) retained somewhere in the body of patients with many of the conditions in which 23NaMRI was later applied, including some forms of hypertension. Those studies, at the time conducted in patients with overt increase in BP but on no treatment or in adequate washout, showed that excess body Na+ (or Cl−) does not necessarily involve all patients with hypertension at any stage of the disease.71,72 In particular, NaE was significantly increased in PA but was normal in patients presumed to have essential hypertension and in those with unilateral renal artery stenosis or even below normal in young hypertensives aged ≤35 years.73 Nevertheless, the correlation of NaE with arterial pressure was positive and significant in almost all patients with hypertension,71,73 as for NaE and total body Na+ (r=0.91; P<0.001).71 Modern 23NaMRI studies overall confirmed these findings, suggesting that young, noncomorbid subjects with early-stage hypertension may not show any increase in tissue Na+, at variance with resistant hypertension or PA,38,41,74 although definitive conclusions are prevented by limitations including sample size, lack of adequate controls, and of conclusive screening for secondary hypertension.
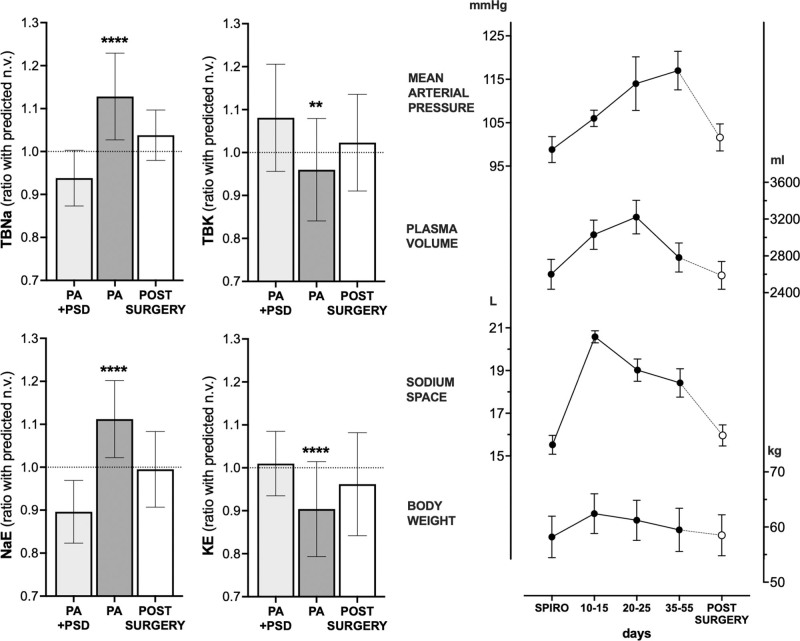
PA, a prototypic form of salt-dependent hypertension, has offered valuable insights into these aspects over the years. Conn studied isotope dilution over 28 days in 6 patients with PA and reported evidence of a diminished NaE pool that did not readily equilibrate within 24 hours.75 This was interpreted as diminished bone sodium, in keeping with the later recognition of a chronic bone-resorptive state, driven by the hypercalciuria and hyperparathyroidism, typical of PA.76,77 Such a diminished slowly exchangeable pool could not be confirmed by later approaches of noninvasive whole-body counting,78 but these were hampered by a one-off, relative rather than absolute, overall complex and likely less sensitive nature of the methodology, compared with a 4-week daily blood dilution measurement. Nevertheless, they sufficed to provide compelling evidence of high total body Na+ and NaE in PA, which were reduced by spironolactone or amiloride and by curative surgery (Figure 2, left).78 Almost 30 years before seeing tissue Na+37 we knew it was there, somewhere.
Figure 2.
Sodium, potassium, and fluid balance in primary aldosteronism. Left, Nuclear whole-body counting, revealing high total body Na+ (TBNa) and exchangeable Na (NaE) in patients with an aldosterone-producing adenoma without treatment (primary aldosteronism [PA]); both were reduced by spironolactone or amiloride (potassium-sparing diuretics; PSD) and by adrenalectomy. Total body K+ (TBK) and exchangeable K+ (KE) revealed opposite trends. Data are presented as mean±SD. Right, Changes induced in 5 patients with PA by withdrawal of spironolactone and by adrenalectomy. **P<0.01, ****P<0.0001 vs normal values. Drawn from Williams et al.78 Copyright © 1984, Lippincott-Raven Publishers. Data are presented as mean±SEM. Adapted from Wenting et al79 with permission. Copyright ©1977, Wolters Kluwer Health.
SHRINKING BUCKETS
Recent work, only apparently unrelated with aspects pertaining Na+ localization, linked excess sodium to a metabolic shift toward catabolism. The authors of the original observation noted a decrease in water intake, without changes in urine volume, during high compared with low salt intake in the aforementioned human long-term balance studies.80 Parallel experiments in rodents exposed to (admittedly extreme) salt loading, including pair-feeding approaches, revealed surplus generation of endogenous water by muscle protein catabolism and hepatic ureagenesis, coupled with reduced free water clearance by urea-driven water reabsorption in the kidneys.81 Analogy was made with animals experiencing dormancy during conditions of aridity and high temperatures, suggesting a multiorgan water conservation system to prevent natriuresis-induced water loss during high Na+ intake. More recently, the same authors reported that these mechanisms operate also in other experimental models prone to dehydration, like impaired urine concentration ability in 5/6 nephrectomy,82 vasopressin antagonism,83 and psoriatic skin barrier defect.84 Others independently confirmed that mild water deficit achieved by chronic restriction shifted metabolism toward catabolic water production, increased energy expenditure, and high food intake, ultimately shortening life span in mice.85 In a retrospective analysis of patients with essential hypertension, we identified similar water-preserving mechanisms upon high Na+ intake, arguably sustained by energy-consuming renal changes and characterized by peripheral metabolomic signatures suggestive of protein catabolism.86 Similarly, a high-salt diet decreased free water clearance and increased the excretion of amino acids involved in the urea cycle in a randomized trial including 20 lean and 20 abdominally obese individuals.87 In fact, a secondary analysis of the DASH (Dietary Approaches to Stop Hypertension)-sodium randomized trial could not confirm different energy requirements across different dietary sodium levels, but weight did vary despite the attempt for controlled energy intake and measures for body composition were missing.88
Collectively, evidence suggests that under conditions of salt excess, the cellular mass may shrink because of a water-preserving catabolic state. In free-living conditions, different energetic sources, either endogenous (muscle mass) or exogenous (excess food), can be exploited, thus complicating absolute and relative assessments. Moreover, water balance depends not only on intake, diuresis, and catabolism but also on water in ingested food and exchange via other routes, including respiration, feces, and skin,84,89 all substantively affected by environmental and lifestyle factors.90
All these considerations are key for interpreting any Tangram compartment puzzle: no surprise that watery volumes or weights were missing, if one accounts for the parallel (subclinical) loss of cellular mass with salt loading. This contention still lacks conclusive experimental confirmation; however, in the representative model offered by PA, simultaneous assessment of the whole-body elemental composition revealed a significant potassium deficit (Figure 2, left), and total body potassium is an old but still one of the most precise measures of cell mass.1,91
TO BE OR NOT TO BE
Changes in the relative proportion of extracellular and intracellular volumes can profoundly impact on whole-tissue Na+ content and concentration.61 However, uncertainties about cell mass (and intracellular volume, accordingly) are not the sole factor affecting the syllogism that long-term divergence of Na+ and water balance would equal hypertonic accumulation.
Our chemical analysis of multiple tissues in salt-loaded rats and of skin biopsies from patients with hypertension, by us and others,62,92 did not support the hypothesis of a hypertonic Na+ excess, since tissue water largely paralleled tissue Na+. We interpreted the findings as a systemic expansion of the extracellular volume, that is, subtle isotonic edema. Interestingly, the isolation of interstitial fluid and lymph draining the skin of rats during salt accumulation induced by a high-salt diet or deoxycorticosterone pellet implantation revealed Na+ concentration that was not different from plasma.60 While this cannot exclude the preferential binding of Na+ to the glycosaminoglycan network in the ECM (extracellular matrix), its quantitative relevance has been challenged,31 as discussed above. Moreover, the stoichiometry of any Na+ binding in excess of water, which is similarly attracted by glycosaminoglycans, remains unclear. In fact, in agreement with previous data,60,62,93 high-salt diet caused an increase in skin water content; the sum of cations remained within physiological ranges in the whole tissue and only modestly increased in the dermis, in parallel with increased Na+ concentration in the serum.31 This increase is well below those reported in both high salt and control animals in the original studies that suggested Na+ hypertonicity.18 Moreover, a chemical analysis coupled with extracellular volume tracking with 51Cr-EDTA confirmed that the extracellular space undergoes expansion with salt loading, particularly in the loose dermis.31 Even in dermis, extracellular Na+ concentration never exceeded ≈120 mmol/L, when intracellular Na+ was conveniently assumed to be fixed at 10 mmol/L (which may have magnified the extracellular estimates if true intracellular values were higher; see below).
Does this evidence dismantle the concept of hypertonic tissue Na+ accumulation? No, it does not. First, except for the specialized renal medulla, there is currently no firm evidence to confirm or exclude that local gradients of Na+ exist in tissues. If present, they would likely to be smaller than initially suggested, but lack of magnitude does not equal lack of biological relevance: excess Na+ can modulate multiple immune cells and polarize them toward an inflammatory phenotype, as extensively reviewed elsewhere.94,95 Furthermore, even mild increases reflecting repeated (dietary) insults96 or long-term mishandling may eventually be pathogenic over a life span. Recently, serum Na+ values as low as the upper limits of normal were associated with long-term risk of developing left ventricular hypertrophy and heart failure in a population-based prospective cohort study,97 consistent with old cellular studies.98 Second, the epidermis of salt-loaded rats indeed showed hyperosmolarity but due to osmolytes other than Na+, like urea.60 This may link with water loss at the epidermal surface89 or to counter-mechanisms preserving body water in conditions of water deficit and (relative) Na+ excess.81,84,99 However, it seems unlikely that this surface hyperosmolarity can substantially affect deeper cells, since the interstitial fluid Na+ (and protein) concentration is similar in normal and high-salt conditions.35,60 This contention was proven by the evidence of similar, or even reduced, shift of fluids from blood capillaries into the interstitium in human heart failure compared with age- and sex-matched controls.100
One last key, previously suggested1 but generally neglected aspect to consider is the shift of Na+ inside the cells. Recent experimental evidence revealed that skeletal and cardiac muscle of salt-loaded rats indeed accumulate Na+, without changes in total Na++K+ concentration31,62 but with marked increases in intracellular Na+ concentration.31 Also skin, where ≈25% of the fluid is intracellular,31 is a potential compartment for Na+/K+ exchange and skin cells may act as Na+ reservoir that may appear as 'bound' irrespective of sulfated glycosaminoglycans. In fact, the concept is not new in the field of hypertension and there are old reports of excess intracellular Na+ in smooth muscle, as well as circulating cells.101 Unfortunately, definite conclusions were limited by methodological inconsistencies, which possibly still impact the herein discussed field. We are now aware of multiple molecular mechanisms and pathogenic correlates of excess sodium entering immune cells95,96 to support those early descriptive findings. In addition, recent evidence extends the phenomenon to body cell mass at large, including skeletal and cardiac muscle cells, with obvious electromechanical and energetic implications for their function. This makes our Tangram even more complicated.
FROM A UNIQUE HUMAN MODEL TOWARD A UNIFYING VIEW
PA does not generally present with overt signs of fluid overload, that is, detectable edema. This is explained by the so-called aldosterone escape, whereby administration of large doses of aldosterone does cause an initial decrease in urinary sodium excretion, but this phase is followed by a gradual increase to eventually match intake, thus attaining a new equilibrium and avoiding overt Na+ and water retention.102–104 This process depends on increased renal perfusion pressure, high sodium delivery to the distal nephron that overrides the usual mineralocorticoid-driven reabsorption, and increased natriuretic peptides.105 At least 2 of these mechanisms are driven by volume expansion that a quick clinical look may miss but that original investigators spotted as slight periorbital puffiness.102 In fact, in our experience of systematically searching for and subtyping PA,12 patients with hypertension who temporarily undergo washout from drugs like mineralocorticoid receptor antagonists do often report some degree of subjective swelling. A small study, conducted on 5 patients with overt PA submitted to a protocol of spironolactone washout in a metabolic ward, revealed that sodium space and exchangeable sodium rose until 10 to 15 days and declined afterward, although eventually remaining higher than during spironolactone treatment (Figure 2, right). Plasma, blood volumes, and body weight returned to values that are only minimally, but not significantly, higher than baseline. All parameters normalized at long-term follow-up after surgery.80 Unfortunately, the study did not track K+, which (1) is missing in patients with PA at whole-body counting (Figure 2, left)78; (2) when expressed as either plasma concentration, exchangeable K+, or total body K+, correlated inversely and significantly with BP in patients with hypertension71; (3) at variance with Na+, never reached a new equilibrium between excess excretion and intake in the early escape experiments.103 Although it would be key to know whether the cell mass shrank in those 5 patients on a fixed diet over the course of the almost-2-month washout, evidence for a parallel weight loss in PA remains anecdotal,103 conflicting for plasma volume,106–108 but certainly conclusive for an expanded NaE, Cl−, and extracellular fluid volume.106,107,109,110 The phenomenon may not be restricted to a prior history of hypertension80 and recapitulate the cardiovascular continuum that spans from a variety of subclinical states to overt interstitial congestion, or heart failure, to which PA demonstrates high risk of progression.111 Similar to PA, all these pre-heart failure states (eg, resistant hypertension, diabetes, CKD) feature excess tissue Na+ at 23NaMRI. Additionally, in the context of metabolic syndrome or CKD, absolute or relative cell mass reduction is both a determinant and a result of disease.112,113 Whether this is also paralleled by intracellular Na+ accumulation remains to be established, but the high 23Na signal from skeletal muscle,38,41,53,55 in addition to the dermis, would suggest so.
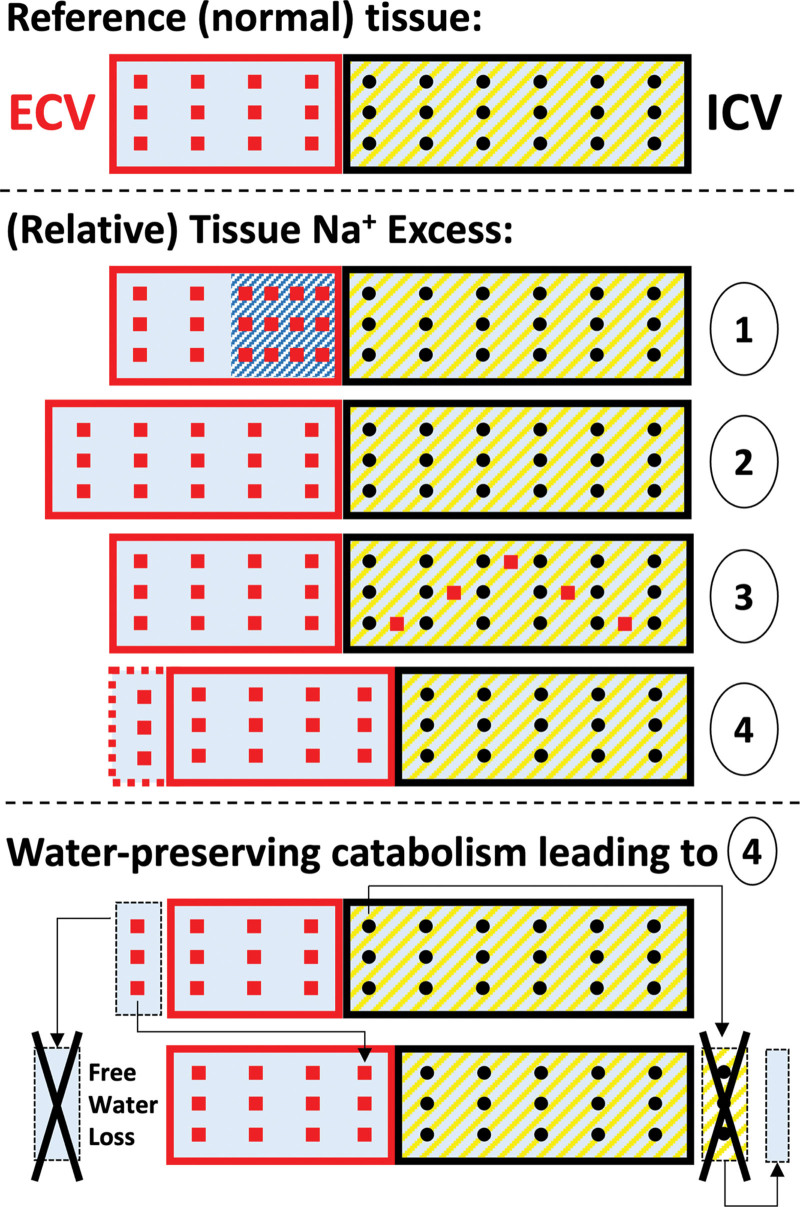
In summary, our interpretation of the tissue Na+ excess deserves a reappraisal of the Tangram: (1) small interstitial hypertonic niches, (2) more or less clinically visible edema, (3) intracellular Na+ accumulation, (4) or relative cell mass loss (driven by aging, whole-body sodium-water imbalance, and the ensuing water-preserving catabolic state) may all present with the final readout of excess tissue Na+ signal (Figure 3).
Figure 3.
Reappraisal of the Tangram for interpretation of tissue Na excess. Top, Physiological reference tissue, composed of extracellular and intracellular volumes (ECV and ICV), rich in Na+ (squares) and K+ (dots), respectively. Middle, Different pathophysiological patterns resulting, at whole-tissue analysis (eg, in 23NaMRI), in tissue Na+ excess: (1) hypertonic tissue Na+ accumulation, whereby Na+ would bind glycosaminoglycans (shaded) in the extracellular matrix in excess of water; (2) absolute expansion of the ECV, that is, edema; (3) accumulation of Na+ inside the cells, as for muscle; (4) relative but not absolute ECV predominance due to shrinking ICV (with or without additional edema). These patterns may coexist in different disease states. Bottom, Suggested mechanism described in long-term/experimental excess Na+ intake80,81 and other water-losing conditions,82–84 by which free water deficit or loss would induce a catabolic state and a loss of cellular mass and of K+, resulting in relative tissue Na+ excess, in the attempt to generate endogenous water moieties from the breakdown of proteins. Loss is depicted as a black X.
OPEN MIC! MICROVASCULATURE, INTERSTITIUM, AND CELLS
We propose a small functional unit to interpret excess tissue Na+ in hypertension and cardiovascular disease. This MIC unit includes (1) the blood and lymphatic microvasculature (M) down to capillaries, which are responsible for fluid extravasation and removal, respectively; (2) the interstitium (I), a highly dynamic interface; (3) parenchymal cells (C), which are impacted by but also active determinants of the extracellular—and ultimately whole body—Na+ content (Figure S4). Each component of the MIC unit has either been discussed above (shrinking buckets) or reviewed elsewhere in relation to Na+ handling by the blood vessel wall and its permeability,114 the role of lymphatics in interstitial homeostasis, the composition and biophysics of the interstitial matrix, and the local forces that impact stromal and immune cells.1,115–118
Despite the unique sodium handling by salt-sensitive and salt-resistant subjects,119–121 all these players have received little experimental attention due to a difficult investigation and the wrong belief that there was little left to discover.1 This hasty conclusion disregards historical evidence. Tarazi et al122,123 found that the ratio of plasma volume (PV) to interstitial fluid (IF) volume was significantly lower in uncomplicated, untreated essential hypertensive patients compared with normotensive subjects. The difference was independent of diminished PV, rather indicating a shift of extracellular fluid from the intravascular to the interstitial compartments. In patients with variable degrees of CKD, salt loading consistently expanded the extracellular volume; however, the PV/IF ratio decreased in those with mild estimated Glomerular Filtration Rate (eGFR) reduction and increased in those with more severe impairment. PV/IF change directly correlated with the change in BP (Figure S5).124 In rats, undergoing sequential removal of both kidneys, Lucas and Floyer125 found changes in the PV/IF ratio similar to those in patients with more severe CKD but also a marked increase in interstitial tissue pressure and a fall in interstitial compliance after saline infusion. A similar pattern was found in one-kidney one-clip rats,126 suggesting modulation of those parameters and their determinants by the renin-angiotensin-aldosterone system. Later studies (in which both PV and IF volume were directly measured, rather than calculated from changes in total extracellular volume) confirmed different changes in PV during experimental dehydration and fluid load but not different interstitial compliance in one-kidney one-clip rats compared with one-kidney sham-clipped normotensive controls.127 However, the interstitial pressure and volume at baseline were higher in hypertensive compared with control rats and their change after peritoneal dialysis or the change in the interstitial colloid osmotic pressure after saline load differed. Unfortunately, there was no follow-up to these old studies. All in all, available data suggest different tissue-capillary filtration forces and dynamics in different patients of the cardiovascular-renal spectrum of hypertensive disease.
SUMMARY AND CLINICAL PERSPECTIVES
There is no polished surface (Supplemental Material S6) in the field of sodium balance. The old intracellular-extracellular 2-compartment model of fluid and electrolyte homeostasis was first expanded to 3 compartments, in which the interstitium featured as a separately regulated space. Our body’s Na+ balance was found to disregard the need for strict equilibrium not only on a day-to-day basis but also in the ultra-long term: Na+ accumulates in tissues with aging, cardiorenovascular and inflammatory diseases. Investigators from the 70s could already guess it, but recent technological advancement, namely 23NaMRI, enabled us to see the invisible. At odds with its initial intended use for the extracellular space, the anatomic approach of 23NaMRI led us to further rethink the 3-compartment model and dignify the intracellular space. For too long regarded as a constant in the equation, intracellular volume emerged as (1) a key determinant of the architecture of tissues and of their total chemical composition; (2) the immediate target of catabolic processes triggered by conditions of water and Na+ imbalance; (3) one additional key site for tissue Na+ accumulation, particularly in muscles but also skin, with electromechanical implications that remain to be unraveled. Finally, we realized that at least part of the interstitial Na+ storage is not independent of water, in keeping with old beliefs.
Even reshuffling this Tangram, thanks to a decade of progress built on the shoulders of giants, still does not fill many of the blanks. We can now see and better interpret tissue Na+ excess based on evidence-based pathophysiological patterns (Figure 3); however, we do not know which (or which combination) best applies to each timing of each disease. We have proposed the MIC unit to help researchers, and hopefully clinicians in the future, to disentangle the crucial interplay of physics, biomechanics, and energetic balance at such a microscopic scale and understand their full therapeutic implications.
One last, but compelling and overarching question remains: does sodium and water retention, even at subclinical scale, affect organ function, translate into organ damage, and ultimately affect prognosis? Data from patients with PA, herein referenced as the epitome for the discussed aspects, would suggest so. Although traditionally considered a benign form of hypertension because of the undetectable renin levels,128 extensive work from us and others has robustly associated PA with excess left ventricular hypertrophy, LV fibrosis, vascular remodeling, microalbuminuria, endothelial dysfunction, and with a high risk of stroke, myocardial infarction, heart failure, and atrial fibrillation.112 Of note, surgical cure of PA was associated with a decrease of incident atrial fibrillation and regression of left ventricular hypertrophy via reverse inward remodeling,129 in line with the concept that removal of the mineralocorticoid-mediated salt-retaining excess is associated with a decrease of body fluid volumes. Such evidence from this salt-dependent and reversible disease model may guide the investigation of the molecular mechanisms implicated in the pandemic of tissue Na+ excess. In perspective, it may be relevant to more precise treatment of many other cardiorenovascular diseases and possibly beyond.
ARTICLE INFORMATION
Sources of Funding
G. Rossitto receive support from the NextGenerationEU-funded program STARS@UNIPD 2021 (starting grant; POLYPHEMUS-CVD); G. Rossitto and G.P. Rossi from the University of Padua, and the Foundation for Advanced Research in Hypertension and Cardiovascular Diseases; C. Delles from the British Heart Foundation (Center of Research Excellence; RE/18/6/34217); H. Wiig from the Research Council of Norway (project number 262079), the Norwegian Health Association, and from the Western Norway Regional Health Authority (project number 912168).
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BP
- blood pressure
- CKD
- chronic kidney disease
- IF
- interstitial fluid
- NaE
- exchangeable Na+
- PA
- primary aldosteronism
- PV
- plasma volume
This manuscript is part of the Salt Review Series.
For Sources of Funding and Disclosures, see page 497.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.123.19569.
REFERENCES
- 1.Bhave G, Neilson EG. Body fluid dynamics: back to the future. J Am Soc Nephrol. 2011;22:2166–2181. doi: 10.1681/ASN.2011080865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rakova N, Jüttner K, Dahlmann A, Schröder A, Linz P, Kopp C, Rauh M, Goller U, Beck L, Agureev A, et al. Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell Metab. 2013;17:125–131. doi: 10.1016/j.cmet.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 3.Lerchl K, Rakova N, Dahlmann A, Rauh M, Goller U, Basner M, Dinges DF, Beck L, Agureev A, Larina I, et al. Agreement between 24-hour salt ingestion and sodium excretion in a controlled environment. Hypertension. 2015;66:850–857. doi: 10.1161/HYPERTENSIONAHA.115.05851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luft FC, Fineberg NS, Sloan RS. Estimating dietary sodium intake in individuals receiving a randomly fluctuating intake. Hypertension. 1982;4:805–808. doi: 10.1161/01.hyp.4.6.805 [DOI] [PubMed] [Google Scholar]
- 5.He FJ, Ma Y, Campbell NRC, MacGregor GA, Cogswell ME, Cook NR. Formulas to estimate dietary sodium intake from spot urine alter sodium-mortality relationship. Hypertension. 2019;74:572–580. doi: 10.1161/HYPERTENSIONAHA.119.13117 [DOI] [PubMed] [Google Scholar]
- 6.Sun Q, Bertrand KA, Franke AA, Rosner B, Curhan GC, Willett WC. Reproducibility of urinary biomarkers in multiple 24-h urine samples. Am J Clin Nutr. 2017;105:159–168. doi: 10.3945/ajcn.116.139758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olde Engberink RHG, van den Hoek TC, van Noordenne ND, van den Born B-JH, Peters-Sengers H, Vogt L. Use of a single baseline versus multiyear 24-hour urine collection for estimation of long-term sodium intake and associated cardiovascular and renal risk. Circulation. 2017;136:917–926. doi: 10.1161/CIRCULATIONAHA.117.029028 [DOI] [PubMed] [Google Scholar]
- 8.Ma Y, He FJ, Sun Q, Yuan C, Kieneker LM, Curhan GC, MacGregor GA, Bakker SJL, Campbell NRC, Wang M, et al. 24-hour urinary sodium and potassium excretion and cardiovascular risk. N Engl J Med. 2022;386:252–263. doi: 10.1056/NEJMoa2109794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cogswell ME, Frieden TR. Dietary sodium and cardiovascular disease risk. N Engl J Med. 2016;375:2407–2408. doi: 10.1056/NEJMc1612304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Donnell M, Mente A, Alderman MH, Brady AJB, Diaz R, Gupta R, López-Jaramillo P, Luft FC, Lüscher TF, Mancia G, et al. Salt and cardiovascular disease: insufficient evidence to recommend low sodium intake. Eur Heart J. 2020;41:3363–3373. doi: 10.1093/eurheartj/ehaa586 [DOI] [PubMed] [Google Scholar]
- 11.Rossi GP, Bisogni V, Rossitto G, Maiolino G, Cesari M, Zhu R, Seccia TM. Practice recommendations for diagnosis and treatment of the most common forms of secondary hypertension. High Blood Press Cardiovasc Prev. 2020;27:547–560. doi: 10.1007/s40292-020-00415-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi GP. Primary aldosteronism: JACC State-of-the-art review. J Am Coll Cardiol. 2019;74:2799–2811. doi: 10.1016/j.jacc.2019.09.057 [DOI] [PubMed] [Google Scholar]
- 13.Heer M, Baisch F, Kropp J, Gerzer R, Drummer C. High dietary sodium chloride consumption may not induce body fluid retention in humans. Am J Physiol Renal Physiol. 2000;278:F585–F595. doi: 10.1152/ajprenal.2000.278.4.F585 [DOI] [PubMed] [Google Scholar]
- 14.Titze J, Maillet A, Lang R, Gunga HC, Johannes B, Gauquelin-Koch G, Kihm E, Larina I, Gharib C, Kirsch KA. Long-term sodium balance in humans in a terrestrial space station simulation study. Am J Kidney Dis. 2002;40:508–516. doi: 10.1053/ajkd.2002.34908 [DOI] [PubMed] [Google Scholar]
- 15.Titze J, Krause H, Hecht H, Dietsch P, Rittweger J, Lang R, Kirsch KA, Hilgers KF. Reduced osmotically inactive Na storage capacity and hypertension in the Dahl model. Am J Physiol Renal Physiol. 2002;283:F134–F141. doi: 10.1152/ajprenal.00323.2001 [DOI] [PubMed] [Google Scholar]
- 16.Titze J, Lang R, Ilies C, Schwind KH, Kirsch KA, Dietsch P, Luft FC, Hilgers KF. Osmotically inactive skin Na+ storage in rats. Am J Physiol Renal Physiol. 2003;285:F1108–F1117. doi: 10.1152/ajprenal.00200.2003 [DOI] [PubMed] [Google Scholar]
- 17.Titze J, Bauer K, Schafflhuber M, Dietsch P, Lang R, Schwind KH, Luft FC, Eckardt K-U, Hilgers KF. Internal sodium balance in DOCA-salt rats: a body composition study. Am J Physiol Renal Physiol. 2005;289:F793–F802. doi: 10.1152/ajprenal.00096.2005 [DOI] [PubMed] [Google Scholar]
- 18.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Müller DN, Derer W, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C–dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960 [DOI] [PubMed] [Google Scholar]
- 19.Wiig H, Schröder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123:2803–2815. doi: 10.1172/JCI60113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelman IS, Leibman J. Anatomy of body water and electrolytes. Am J Med. 1959;27:256–277. doi: 10.1016/0002-9343(59)90346-8 [DOI] [PubMed] [Google Scholar]
- 21.Titze J. Sodium balance is not just a renal affair. Curr Opin Nephrol Hypertens. 2014;23:101–105. doi: 10.1097/01.mnh.0000441151.55320.c3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bie P. Mechanisms of sodium balance: total body sodium, surrogate variables, and renal sodium excretion. Am J Physiol Regul Integr Comp Physiol. 2018;315:R945–R962. doi: 10.1152/ajpregu.00363.2017 [DOI] [PubMed] [Google Scholar]
- 23.Machnik A, Dahlmann A, Kopp C, Goss J, Wagner H, van Rooijen N, Eckardt KU, Müller DN, Park JK, Luft FC, et al. Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension. 2010;55:755–761. doi: 10.1161/HYPERTENSIONAHA.109.143339 [DOI] [PubMed] [Google Scholar]
- 24.Titze J, Shakibaei M, Schafflhuber M, Schulze-Tanzil G, Porst M, Schwind KH, Dietsch P, Hilgers KF. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am J Physiol Heart Circ Physiol. 2004;287:H203–H208. doi: 10.1152/ajpheart.01237.2003 [DOI] [PubMed] [Google Scholar]
- 25.Schafflhuber M, Volpi N, Dahlmann A, Hilgers KF, Maccari F, Dietsch P, Wagner H, Luft FC, Eckardt KU, Titze J. Mobilization of osmotically inactive Na+ by growth and by dietary salt restriction in rats. Am J Physiol Renal Physiol. 2007;292:F1490–F1500. doi: 10.1152/ajprenal.00300.2006 [DOI] [PubMed] [Google Scholar]
- 26.Sugár D, Agócs R, Tatár E, Tóth G, Horváth P, Sulyok E, Szabó AJ. The contribution of skin glycosaminoglycans to the regulation of sodium homeostasis in rats. Physiol Res. 2018;67:777–785. doi: 10.33549/physiolres.933463 [DOI] [PubMed] [Google Scholar]
- 27.Fischereder M, Michalke B, Schmöckel E, Habicht A, Kunisch R, Pavelic I, Szabados B, Schönermarck U, Nelson PJ, Stangl M. Sodium storage in human tissues is mediated by glycosaminoglycan expression. Am J Physiol Renal Physiol. 2017;313:F319–F325. doi: 10.1152/ajprenal.00703.2016 [DOI] [PubMed] [Google Scholar]
- 28.Olde Engberink RHG, de Vos J, van Weert A, Zhang Y, van Vlies N, van den Born BJH, Titze JM, van Bavel E, Vogt L. Abnormal sodium and water homeostasis in mice with defective heparan sulfate polymerization. PLoS One. 2019;14:e0220333. doi: 10.1371/journal.pone.0220333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wenstedt EFE, Oppelaar JJ, Besseling S, Rorije NMG, Olde Engberink RHG, Oosterhof A, van Kuppevelt TH, van den Born BJH, Aten J, Vogt L. Distinct osmoregulatory responses to sodium loading in patients with altered glycosaminoglycan structure: a randomized cross-over trial. J Transl Med. 2021;19:38. doi: 10.1186/s12967-021-02700-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farber SJ, Schubert M, Schuster N. The binding of cations by chondroitin sulfate. J Clin Invest. 1957;36:1715–1722. doi: 10.1172/JCI103573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thowsen IM, Karlsen TV, Nikpey E, Haslene-Hox H, Skogstrand T, Randolph GJ, Zinselmeyer BH, Tenstad O, Wiig H. Na+ is shifted from the extracellular to the intracellular compartment and is not inactivated by glycosaminoglycans during high salt conditions in rats. J Physiol. 2022;600:2293–2309. doi: 10.1113/JP282715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thowsen IM, Reikvam T, Skogstrand T, Samuelsson AM, Müller DN, Tenstad O, Alitalo K, Karlsen T, Wiig H. Genetic engineering of lymphangiogenesis in skin does not affect blood pressure in mouse models of salt-sensitive hypertension. Hypertension. 2022;79:2451–2462. doi: 10.1161/HYPERTENSIONAHA.122.19777 [DOI] [PubMed] [Google Scholar]
- 33.Engebretsen KVT, Lunde IG, Strand ME, Waehre A, Sjaastad I, Marstein HS, Skrbic B, Dahl CP, Askevold ET, Christensen G, et al. Lumican is increased in experimental and clinical heart failure, and its production by cardiac fibroblasts is induced by mechanical and proinflammatory stimuli. FEBS J. 2013;280:2382–2398. doi: 10.1111/febs.12235 [DOI] [PubMed] [Google Scholar]
- 34.Agócs R, Pap D, Sugár D, Tóth G, Turiák L, Veréb Z, Kemény L, Tulassay T, Vannay A, Szabó AJ. Cyclooxygenase-2 modulates glycosaminoglycan production in the skin during salt overload. Front Physiol. 2020;11:561722. doi: 10.3389/fphys.2020.561722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiig H. Regulation of fluid volume from the outside: a role of glycosaminoglycans in the skin interstitium? Circ Heart Fail. 2018;11:e005135. doi: 10.1161/CIRCHEARTFAILURE.118.005135 [DOI] [PubMed] [Google Scholar]
- 36.Guyton AC, Coleman TG, Granger HJ. Circulation: overall regulation. Annu Rev Physiol. 1972;34:13–46. doi: 10.1146/annurev.ph.34.030172.000305 [DOI] [PubMed] [Google Scholar]
- 37.Kopp C, Linz P, Wachsmuth L, Dahlmann A, Horbach T, Schöfl C, Renz W, Santoro D, Niendorf T, Müller DN, et al. 23Na magnetic resonance imaging of tissue sodium. Hypertension. 2012;59:167–172. doi: 10.1161/HYPERTENSIONAHA.111.183517 [DOI] [PubMed] [Google Scholar]
- 38.Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Müller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, et al. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013;61:635–640. doi: 10.1161/HYPERTENSIONAHA.111.00566 [DOI] [PubMed] [Google Scholar]
- 39.Christa M, Weng AM, Geier B, Wörmann C, Scheffler A, Lehmann L, Oberberger J, Kraus BJ, Hahner S, Störk S, et al. Increased myocardial sodium signal intensity in Conn’s syndrome detected by 23Na magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 2019;20:263–270. doi: 10.1093/ehjci/jey134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karg MV, Bosch A, Kannenkeril D, Striepe K, Ott C, Schneider MP, Boemke-Zelch F, Linz P, Nagel AM, Titze J, et al. SGLT-2-inhibition with dapagliflozin reduces tissue sodium content: a randomised controlled trial. Cardiovasc Diabetol. 2018;17:5. doi: 10.1186/s12933-017-0654-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kannenkeril D, Karg MV, Bosch A, Ott C, Linz P, Nagel AM, Uder M, Schmieder RE. Tissue sodium content in patients with type 2 diabetes mellitus. J Diabetes Complications. 2019;33:485–489. doi: 10.1016/j.jdiacomp.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 42.Hanson P, Philp CJ, Randeva HS, James S, O’Hare JP, Meersmann T, Pavlovskaya GE, Barber TM. Sodium in the dermis colocates to glycosaminoglycan scaffold, with diminishment in type 2 diabetes mellitus. JCI Insight. 2021;6:e145470. doi: 10.1172/jci.insight.145470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammon M, Grossmann S, Linz P, Kopp C, Dahlmann A, Garlichs C, Janka R, Cavallaro A, Luft FC, Uder M, et al. 23Na magnetic resonance imaging of the lower leg of acute heart failure patients during diuretic treatment. PLoS One. 2015;10:e0141336. doi: 10.1371/journal.pone.0141336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolwelter J, Kannenkeril D, Linz P, Jung S, Nagel AM, Bosch A, Ott C, Bramlage P, Nöh L, Schiffer M, et al. The SGLT2 inhibitor empagliflozin reduces tissue sodium content in patients with chronic heart failure: results from a placebo-controlled randomised trial. Clin Res Cardiol. 2023;112:134–144. doi: 10.1007/s00392-022-02119-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huhn K, Linz P, Pemsel F, Michalke B, Seyferth S, Kopp C, Chaudri MA, Rothhammer V, Dörfler A, Uder M, et al. Skin sodium is increased in male patients with multiple sclerosis and related animal models. Proc Natl Acad Sci USA. 2021;118:e2102549118. doi: 10.1073/pnas.2102549118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maifeld A, Wild J, Karlsen TV, Rakova N, Wistorf E, Linz P, Jung R, Birukov A, Gimenez-Rivera VA, Wilck N, et al. Skin sodium accumulates in psoriasis and reflects disease severity. J Invest Dermatol. 2022;142:166–178.e8. doi: 10.1016/j.jid.2021.06.013 [DOI] [PubMed] [Google Scholar]
- 47.Kopp C, Beyer C, Linz P, Dahlmann A, Hammon M, Jantsch J, Neubert P, Rosenhauer D, Müller DN, Cavallaro A, et al. Na+ deposition in the fibrotic skin of systemic sclerosis patients detected by 23Na-magnetic resonance imaging. Rheumatology (Oxford, England). 2017;56:674. doi: 10.1093/rheumatology/kex149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carranza-León DA, Oeser A, Marton A, Wang P, Gore JC, Titze J, Stein CM, Chung CP, Ormseth MJ. Tissue sodium content in patients with systemic lupus erythematosus: association with disease activity and markers of inflammation. Lupus. 2020;29:455–462. doi: 10.1177/0961203320908934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crescenzi R, Donahue PMC, Petersen KJ, Garza M, Patel N, Lee C, Beckman JA, Donahue MJ. Upper and lower extremity measurement of tissue sodium and fat content in patients with lipedema. Obesity (Silver Spring). 2020;28:907–915. doi: 10.1002/oby.22778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ertuglu LA, Sahinoz M, Alsouqi A, Deger SM, Guide A, Stewart TG, Pike M, Robinson-Cohen C, Akwo E, Pridmore M, et al. High tissue-sodium associates with systemic inflammation and insulin resistance in obese individuals. Nutr Metab Cardiovasc Dis. 2023;33:1398–1406. doi: 10.1016/j.numecd.2023.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kopp C, Linz P, Maier C, Wabel P, Hammon M, Nagel AM, Rosenhauer D, Horn S, Uder M, Luft FC, et al. Elevated tissue sodium deposition in patients with type 2 diabetes on hemodialysis detected by 23Na magnetic resonance imaging. Kidney Int. 2018;93:1191–1197. doi: 10.1016/j.kint.2017.11.021 [DOI] [PubMed] [Google Scholar]
- 52.Dahlmann A, Dörfelt K, Eicher F, Linz P, Kopp C, Mössinger I, Horn S, Büschges-Seraphin B, Wabel P, Hammon M, et al. Magnetic resonance-determined sodium removal from tissue stores in hemodialysis patients. Kidney Int. 2015;87:434–441. doi: 10.1038/ki.2014.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qirjazi E, Salerno FR, Akbari A, Hur L, Penny J, Scholl T, McIntyre CW. Tissue sodium concentrations in chronic kidney disease and dialysis patients by lower leg sodium-23 magnetic resonance imaging. Nephrol Dial Transplant. 2021;36:1234–1243. doi: 10.1093/ndt/gfaa036 [DOI] [PubMed] [Google Scholar]
- 54.Sahinoz M, Tintara S, Deger SM, Alsouqi A, Crescenzi RL, Mambungu C, Vincz A, Mason O, Prigmore HL, Guide A, et al. Tissue sodium stores in peritoneal dialysis and hemodialysis patients determined by 23-sodium magnetic resonance imaging. Nephrol Dial Transplant. 2020;36:1307–1317. doi: 10.1093/ndt/gfaa350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lemoine S, Salerno FR, Akbari A, McKelvie RS, McIntyre CW. Tissue sodium storage in patients with heart failure: a new therapeutic target? Circ Cardiovasc Imaging. 2021;14:e012910. doi: 10.1161/CIRCIMAGING.121.012910 [DOI] [PubMed] [Google Scholar]
- 56.Mitsides N, McHugh D, Swiecicka A, Mitra R, Brenchley P, Parker GJM, Mitra S. Extracellular resistance is sensitive to tissue sodium status; implications for bioimpedance-derived fluid volume parameters in chronic kidney disease. J Nephrol. 2020;33:119–127. doi: 10.1007/s40620-019-00620-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider MP, Raff U, Kopp C, Scheppach JB, Toncar S, Wanner C, Schlieper G, Saritas T, Floege J, Schmid M, et al. Skin sodium concentration correlates with left ventricular hypertrophy in CKD. J Am Soc Nephrol. 2017;28:1867–1876. doi: 10.1681/ASN.2016060662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kannenkeril D, Jung S, Harazny J, Striepe K, Ott C, Dahlmann A, Kopp C, Schiffer M, Linz P, Nagel AM, et al. Tissue sodium content correlates with hypertrophic vascular remodeling in type 2 diabetes. J Diabetes Complications. 2021;35:108055. doi: 10.1016/j.jdiacomp.2021.108055 [DOI] [PubMed] [Google Scholar]
- 59.Burstein D, Springer CS. Sodium MRI revisited. Magn Reson Med. 2019;82:521–524. doi: 10.1002/mrm.27738 [DOI] [PubMed] [Google Scholar]
- 60.Nikpey E, Karlsen TV, Rakova N, Titze JM, Tenstad O, Wiig H. High-salt diet causes osmotic gradients and hyperosmolality in skin without affecting interstitial fluid and lymph. Hypertension. 2017;69:660–668. doi: 10.1161/HYPERTENSIONAHA.116.08539 [DOI] [PubMed] [Google Scholar]
- 61.Rossitto G, Touyz RM, Petrie MC, Delles C. Much ado about N…atrium: modelling tissue sodium as a highly sensitive marker of subclinical and localized oedema. Clin Science (Lond). 2018;132:2609–2613. doi: 10.1042/CS20180575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rossitto G, Mary S, Chen JY, Boder P, Chew KS, Neves KB, Alves RL, Montezano AC, Welsh P, Petrie MC, et al. Tissue sodium excess is not hypertonic and reflects extracellular volume expansion. Nat Commun. 2020;11:4222. doi: 10.1038/s41467-020-17820-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Foy BD, Burstein D. Interstitial sodium nuclear magnetic resonance relaxation times in perfused hearts. Biophys J. 1990;58:127–134. doi: 10.1016/S0006-3495(90)82358-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wimperis S. Relaxation of quadrupolar nuclei measured via multiple-quantum filtration. In: Harris RK, ed. Encyclopedia of Magnetic Resonance. John Wiley & Sons, Ltd; 2011:emrstm0462.pub2. [Google Scholar]
- 65.Ianniello C, Moy L, Fogarty J, Schnabel F, Adams S, Axelrod D, Axel L, Brown R, Madelin G. Multinuclear MRI to disentangle intracellular sodium concentration and extracellular volume fraction in breast cancer. Sci Rep. 2021;11:5156. doi: 10.1038/s41598-021-84616-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Artyukov I, Arutyunov G, Bobrov M, Bukreeva I, Cedola A, Dragunov D, Feshchenko R, Fratini M, Mitrokhin V, Sokolova A, et al. The first observation of osmotically neutral sodium accumulation in the myocardial interstitium. Sci Rep. 2021;11:22025. doi: 10.1038/s41598-021-01443-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lowry OH. Histochemical changes associated with aging: I methods and calculations. J Biol Chem. 1942;143:257–269. doi: 10.1016/S0021-9258(18)72684-7 [Google Scholar]
- 68.Lowry OH, Hastings AB, Hull TZ, Brown AN. Histochemical changes associated with aging: II. Skeletal and cardiac muscle in the rat. J Biol Chem. 1942;143:271–280. doi: 10.1016/S0021-9258(18)72685-9 [Google Scholar]
- 69.Lowry OH, Hastings AB. Histochemical changes associated with aging; liver, brain, and kidney in the rat. J Gerontol. 1946;1:345–357. doi: 10.1093/geronj/1.3_part_1.345 [DOI] [PubMed] [Google Scholar]
- 70.Simms HS, Stolman A. Changes in human tissue electrolytes in senescence. Science. 1937;86:269–270. doi: 10.1126/science.86.2229.269 [DOI] [PubMed] [Google Scholar]
- 71.Beretta-Piccoli C, Davies DL, Boddy K, Brown JJ, Cumming AM, East BW, Fraser R, Lever AF, Padfield PL, Semple PF, et al. Relation of arterial pressure with body sodium, body potassium and plasma potassium in essential hypertension. Clin Sci (Lond). 1982;63:257–270. doi: 10.1042/cs0630257 [DOI] [PubMed] [Google Scholar]
- 72.Williams ED, Boddy K, Brown JJ, Cumming AM, Davies DL, Harvey IR, Haywood JK, Lever AF, Robertson JI. Whole body elemental composition in patients with essential hypertension. Eur J Clin Invest. 1982;12:321–325. doi: 10.1111/j.1365-2362.1982.tb02239.x [DOI] [PubMed] [Google Scholar]
- 73.Davies DL, McElroy K, Atkinson AB, Brown JJ, Cumming AM, Fraser R, Leckie BJ, Lever AF, Mackay A, Morton JJ, et al. Relationship between exchangeable sodium and blood pressure in different forms of hypertension in man. Clin Science (Lond). 1979;57(5):69s–75s. doi: 10.1042/cs057069s [DOI] [PubMed] [Google Scholar]
- 74.Alsouqi A, Deger SM, Sahinoz M, Mambungu C, Clagett AR, Bian A, Guide A, Stewart TG, Pike M, Robinson-Cohen C, et al. Tissue sodium in patients with early stage hypertension: a randomized controlled trial. J Am Heart Assoc. 2022;11:e022723. doi: 10.1161/JAHA.121.022723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Streeten DH, Rapoport A, Conn JW. Existence of a slowly exchangeable pool of body sodium in normal subjects and its diminution in patients with primary aldosteronism. J Clin Endocrinol Metab. 1963;23:928–937. doi: 10.1210/jcem-23-9-928 [DOI] [PubMed] [Google Scholar]
- 76.Resnick LM, Laragh JH. Calcium metabolism and parathyroid function in primary aldosteronism. Am J Med. 1985;78:385–390. doi: 10.1016/0002-9343(85)90328-6 [DOI] [PubMed] [Google Scholar]
- 77.Maniero C, Fassina A, Seccia TM, Toniato A, Iacobone M, Plebani M, De Caro R, Calò LA, Pessina AC, Rossi GP. Mild hyperparathyroidism: a novel surgically correctable feature of primary aldosteronism. J Hypertens. 2012;30:390–395. doi: 10.1097/HJH.0b013e32834f0451 [DOI] [PubMed] [Google Scholar]
- 78.Williams ED, Boddy K, Brown JJ, Cumming AM, Davies DL, Harvey IR, Haywood JK, Lever AF, Robertson JI. Body elemental composition, with particular reference to total and exchangeable sodium and potassium and total chlorine, in untreated and treated primary hyperaldosteronism. J Hypertens. 1984;2:171–176. doi: 10.1097/00004872-198404000-00008 [DOI] [PubMed] [Google Scholar]
- 79.Wenting GH, Man in ’t Veld AJ, Verhoeven RP, Derkx FH, Schalekamp DH. Volume-pressure relationships during development of mineralocorticoid hypertension in man. Circ Res. 1977;40:I163–I170. [PubMed] [Google Scholar]
- 80.Rakova N, Kitada K, Lerchl K, Dahlmann A, Birukov A, Daub S, Kopp C, Pedchenko T, Zhang Y, Beck L, et al. Increased salt consumption induces body water conservation and decreases fluid intake. J Clin Invest. 2017;127:1932–1943. doi: 10.1172/JCI88530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kitada K, Daub S, Zhang Y, Klein JD, Nakano D, Pedchenko T, Lantier L, LaRocque LM, Marton A, Neubert P, et al. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J Clin Invest. 2017;127:1944–1959. doi: 10.1172/JCI88532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kovarik JJ, Morisawa N, Wild J, Marton A, Takase-Minegishi K, Minegishi S, Daub S, Sands JM, Klein JD, Bailey JL, et al. Adaptive physiological water conservation explains hypertension and muscle catabolism in experimental chronic renal failure. Acta Physiol (Oxf). 2021;232:e13629. doi: 10.1111/apha.13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kidoguchi S, Kitada K, Fujisawa Y, Nakano D, Yokoo T, Titze J, Nishiyama A. Tolvaptan induces body fluid loss and subsequent water conservation in normal rats. J Pharmacol Sci. 2022;149:115–123. doi: 10.1016/j.jphs.2022.04.008 [DOI] [PubMed] [Google Scholar]
- 84.Wild J, Jung R, Knopp T, Efentakis P, Benaki D, Grill A, Wegner J, Molitor M, Garlapati V, Rakova N, et al. Aestivation motifs explain hypertension and muscle mass loss in mice with psoriatic skin barrier defect. Acta Physiol (Oxf). 2021;232:e13628. doi: 10.1111/apha.13628 [DOI] [PubMed] [Google Scholar]
- 85.Allen MD, Springer DA, Burg MB, Boehm M, Dmitrieva NI. Suboptimal hydration remodels metabolism, promotes degenerative diseases, and shortens life. JCI Insight. 2019;4:e130949. doi: 10.1172/jci.insight.130949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rossitto G, Maiolino G, Lerco S, Ceolotto G, Blackburn G, Mary S, Antonelli G, Berton C, Bisogni V, Cesari M, et al. High sodium intake, glomerular hyperfiltration, and protein catabolism in patients with essential hypertension. Cardiovasc Res. 2021;117:1372–1381. doi: 10.1093/cvr/cvaa205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schütten MT, Kusters YH, Houben AJ, Niessen HE, Op ’t Roodt J, Scheijen JL, van de Waardenburg MP, Schalkwijk CG, de Leeuw PW, Stehouwer CD. Glucocorticoids affect metabolic but not muscle microvascular insulin sensitivity following high versus low salt intake. JCI Insight. 2020;5:e127530. doi: 10.1172/jci.insight.127530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Juraschek SP, Miller ER, Chang AR, Anderson CAM, Hall JE, Appel LJ. Effects of sodium reduction on energy, metabolism, weight, thirst, and urine volume: results from the DASH (Dietary Approaches to Stop Hypertension)-Sodium trial. Hypertension. 2020;75:723–729. doi: 10.1161/HYPERTENSIONAHA.119.13932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen JY, Chew KS, Mary S, Boder P, Bagordo D, Rossi GP, Touyz RM, Delles C, Rossitto G. Skin-specific mechanisms of body fluid regulation in hypertension. Clin Science (Lond). 2023;137:239–250. doi: 10.1042/CS20220609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamada Y, Zhang X, Henderson MET, Sagayama H, Pontzer H, Watanabe D, Yoshida T, Kimura M, Ainslie PN, Andersen LF, et al. ; International Atomic Energy Agency (IAEA) Doubly Labeled Water (DLW) Database Consortium§. Variation in human water turnover associated with environmental and lifestyle factors. Science. 2022;378:909–915. doi: 10.1126/science.abm8668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murphy AJ, Ellis KJ, Kurpad AV, Preston T, Slater C. Total body potassium revisited. Eur J Clin Nutr. 2014;68:153–154. doi: 10.1038/ejcn.2013.262 [DOI] [PubMed] [Google Scholar]
- 92.Chachaj A, Puła B, Chabowski M, Grzegrzółka J, Szahidewicz-Krupska E, Karczewski M, Janczak D, Dzięgiel P, Podhorska-Okołów M, Mazur G, et al. Role of the lymphatic system in the pathogenesis of hypertension in humans. Lymphat Res Biol. 2018;16:140–146. doi: 10.1089/lrb.2017.0051 [DOI] [PubMed] [Google Scholar]
- 93.Karlsen TV, Nikpey E, Han J, Reikvam T, Rakova N, Castorena-Gonzalez JA, Davis MJ, Titze JM, Tenstad O, Wiig H. High-salt diet causes expansion of the lymphatic network and increased lymph flow in skin and muscle of rats. Arterioscler Thromb Vasc Biol. 2018;38:2054–2064. doi: 10.1161/ATVBAHA.118.311149 [DOI] [PubMed] [Google Scholar]
- 94.Miyauchi H, Geisberger S, Luft FC, Wilck N, Stegbauer J, Wiig H, Dechend R, Jantsch J, Kleinewietfeld M, Kempa S, et al. Sodium as an important regulator of immunometabolism. Hypertension. 2024;81:426–435 doi: 10.1161/HYPERTENSIONAHA.123.19489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Elijovich F, Kleyman TR, Laffer CL, Kirabo A. Immune mechanisms of dietary salt-induced hypertension and kidney disease: Harry Goldblatt Award for Early Career Investigators 2020. Hypertension. 2021;78:252–260. doi: 10.1161/HYPERTENSIONAHA.121.16495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Geisberger S, Bartolomaeus H, Neubert P, Willebrand R, Zasada C, Bartolomaeus T, McParland V, Swinnen D, Geuzens A, Maifeld A, et al. Salt transiently inhibits mitochondrial energetics in mononuclear phagocytes. Circulation. 2021;144:144–158. doi: 10.1161/CIRCULATIONAHA.120.052788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dmitrieva NI, Liu D, Wu CO, Boehm M. Middle age serum sodium levels in the upper part of normal range and risk of heart failure. Eur Heart J. 2022;43:3335–3348. doi: 10.1093/eurheartj/ehac138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gu JW, Anand V, Shek EW, Moore MC, Brady AL, Kelly WC, Adair TH. Sodium induces hypertrophy of cultured myocardial myoblasts and vascular smooth muscle cells. Hypertension. 1998;31:1083–1087. doi: 10.1161/01.hyp.31.5.1083 [DOI] [PubMed] [Google Scholar]
- 99.Ogura T, Kitada K, Morisawa N, Fujisawa Y, Kidoguchi S, Nakano D, Kobara H, Masaki T, Titze J, Nishiyama A. Contributions of renal water loss and skin water conservation to blood pressure elevation in spontaneously hypertensive rats. Hypertens Res. 2023;46:32–39. doi: 10.1038/s41440-022-01044-6 [DOI] [PubMed] [Google Scholar]
- 100.Rossitto G, Mary S, McAllister C, Neves KB, Haddow L, Rocchiccioli JP, Lang NN, Murphy CL, Touyz RM, Petrie MC, et al. Reduced lymphatic reserve in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2020;76:2817–2829. doi: 10.1016/j.jacc.2020.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simon G. Is intracellular sodium increased in hypertension? Clin Sci (Lond). 1989;76:455–461. doi: 10.1042/cs0760455 [DOI] [PubMed] [Google Scholar]
- 102.August JT, Nelson DH, Thorn GW. Response of normal subjects to large amounts of aldosterone. J Clin Invest. 1958;37:1549–1555. doi: 10.1172/JCI103747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rovner DR, Conn JW, Knopf RF, Cohen EL, Hsueh MT. Nature of renal escape from the sodium-retaining effect of aldosterone in primary aldosteronism and in normal subjects. J Clin Endocrinol Metab. 1965;25:53–64. doi: 10.1210/jcem-25-1-53 [DOI] [PubMed] [Google Scholar]
- 104.Luft FC, Grim CE, Willis LR, Higgins JT, Weinberger MH. Natriuretic response to saline infusion in normotensive and hypertensive man. The role of renin suppression in exaggerated natriuresis. Circulation. 1977;55:779–784. doi: 10.1161/01.cir.55.5.779 [DOI] [PubMed] [Google Scholar]
- 105.Schrier RW. Aldosterone “escape” vs “breakthrough”. Nat Rev Nephrol. 2010;6:61. doi: 10.1038/nrneph.2009.228 [DOI] [PubMed] [Google Scholar]
- 106.Biglieri EG, Forsham PH. Studies on the expanded extracellular fluid and the responses to various stimuli in primary aldosteronism. Am J Med. 1961;30:564–576. doi: 10.1016/0002-9343(61)90080-8 [Google Scholar]
- 107.Novak LP, Strong CG, Hunt JC. Body composition in primary and secondary hypertension. In: Genest J, Koiw E, eds. Hypertension 1972. Springer-Verlag; 1972:444–459. [Google Scholar]
- 108.Tarazi RC, Ibrahim MM, Bravo EL, Dustan HP. Hemodynamic characteristics of primary aldosteronism. N Engl J Med. 1973;289:1330–1335. doi: 10.1056/NEJM197312202892502 [DOI] [PubMed] [Google Scholar]
- 109.Lebel M, Schalekamp MA, Beevers DG, Brown JJ, Davies DL, Fraser R, Kremer D, Lever AF, Morton JJ, Robertson JI, et al. Sodium and the renin-angiotensin system in essential hypertension and mineralocorticoid excess. Lancet. 1974;2:308–309. doi: 10.1016/s0140-6736(74)91690-0 [DOI] [PubMed] [Google Scholar]
- 110.Chobanian AV, Burrows BA, Hollander W. Body fluid and electrolyte composition in arterial hypertension. II. Studies in mineralocorticoid hypertension. J Clin Invest. 1961;40:416–422. doi: 10.1172/JCI104269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50. doi: 10.1016/S2213-8587(17)30319-4 [DOI] [PubMed] [Google Scholar]
- 112.Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819–829. doi: 10.1016/S2213-8587(14)70034-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sabatino A, Cuppari L, Stenvinkel P, Lindholm B, Avesani CM. Sarcopenia in chronic kidney disease: what have we learned so far? J Nephrol. 2021;34:1347–1372. doi: 10.1007/s40620-020-00840-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wenstedt EFE, Olde Engberink RHG, Vogt L. Sodium handling by the blood vessel wall: critical for hypertension development. Hypertension. 2018;71:990–996. doi: 10.1161/HYPERTENSIONAHA.118.10211 [DOI] [PubMed] [Google Scholar]
- 115.Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73:1–78. doi: 10.1152/physrev.1993.73.1.1 [DOI] [PubMed] [Google Scholar]
- 116.Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92:1005–1060. doi: 10.1152/physrev.00037.2011 [DOI] [PubMed] [Google Scholar]
- 117.Rutkowski JM, Swartz MA. A driving force for change: interstitial flow as a morphoregulator. Trends Cell Biol. 2007;17:44–50. doi: 10.1016/j.tcb.2006.11.007 [DOI] [PubMed] [Google Scholar]
- 118.Tschumperlin DJ, Ligresti G, Hilscher MB, Shah VH. Mechanosensing and fibrosis. J Clin Invest. 2018;128:74–84. doi: 10.1172/JCI93561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rossitto G, Delles C. Does excess tissue sodium storage regulate blood pressure? Curr Hypertens Rep. 2022;24:115–122. doi: 10.1007/s11906-022-01180-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Laffer CL, Scott RC, Titze JM, Luft FC, Elijovich F. Hemodynamics and salt-and-water balance link sodium storage and vascular dysfunction in salt-sensitive subjects. Hypertension. 2016;68:195–203. doi: 10.1161/HYPERTENSIONAHA.116.07289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dustan HP, Valdes G, Bravo EL, Tarazi RC. Excessive sodium retention as a characteristic of salt-sensitive hypertension. Am J Med Sci. 1986;292:67–74. doi: 10.1097/00000441-198608000-00001 [DOI] [PubMed] [Google Scholar]
- 122.Tarazi RC, Dustan HP, Frohlich ED. Relation of plasma to interstitial fluid volume in essential hypertension. Circulation. 1969;40:357–366. doi: 10.1161/01.cir.40.3.357 [DOI] [PubMed] [Google Scholar]
- 123.Tarazi RC. Hemodynamic role of extracellular fluid in hypertension. Circ Res. 1976;38:73–83. doi: 10.1161/01.res.38.6.73 [DOI] [PubMed] [Google Scholar]
- 124.Koomans HA, Roos JC, Boer P, Geyskes GG, Mees EJ. Salt sensitivity of blood pressure in chronic renal failure Evidence for renal control of body fluid distribution in man. Hypertension. 1982;4:190–197. doi: 10.1161/01.hyp.4.2.190 [DOI] [PubMed] [Google Scholar]
- 125.Lucas J, Floyer MA. Renal control of changes in the compliance of the interstitial space: a factor in the aetiology of renoprival hypertension. Clin Sci. 1973;44:397–416. doi: 10.1042/cs0440397 [DOI] [PubMed] [Google Scholar]
- 126.Lucas J, Floyer MA. Changes in body fluid distribution and interstitial tissue complicance during the development and reversal of experimental renal hypertension in the rat. Clin Sci Mol Med. 1974;47:1–11. doi: 10.1042/cs0470001 [DOI] [PubMed] [Google Scholar]
- 127.Wiig H, Reed RK. Interstitial compliance and transcapillary fluid balance in renal hypertensive rats. Acta Physiol Scand. 1986;127:407–417. doi: 10.1111/j.1748-1716.1986.tb07921.x [DOI] [PubMed] [Google Scholar]
- 128.Laragh J. Laragh’s lessons in pathophysiology and clinical pearls for treating hypertension. Am J Hypertens. 2001;14:186–194. doi: 10.1016/s0895-7061(00)01317-0 [DOI] [PubMed] [Google Scholar]
- 129.Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, Mantero F, Pessina AC. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62:62–69. doi: 10.1161/HYPERTENSIONAHA.113.01316 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.