Abstract
The primary cilium is a solitary, sensory organelle with many roles in bone development, maintenance, and function. In the osteogenic cell lineage, including skeletal stem cells, osteoblasts, and osteocytes, the primary cilium plays a vital role in the regulation of bone formation, and this has made it a promising pharmaceutical target to maintain bone health. While the role of the primary cilium in the osteogenic cell lineage has been increasingly characterized, little is known about the potential impact of targeting the cilium in relation to osteoclasts, a hematopoietic cell responsible for bone resorption. The objective of this study was to determine whether osteoclasts have a primary cilium and to investigate whether or not the primary cilium of macrophages, osteoclast precursors, serves a functional role in osteoclast formation. Using immunocytochemistry, we showed the macrophages have a primary cilium, while osteoclasts lack this organelle. Furthermore, we increased macrophage primary cilia incidence and length using fenoldopam mesylate and found that cells undergoing such treatment showed a significant decrease in the expression of osteoclast markers tartrate-resistant acid phosphatase, cathepsin K, and c-Fos, as well as decreased osteoclast formation. This work is the first to show that macrophage primary cilia resorption may be a necessary step for osteoclast differentiation. Since primary cilia and preosteoclasts are responsive to fluid flow, we applied fluid flow at magnitudes present in the bone marrow to differentiating cells and found that osteoclastic gene expression by macrophages was not affected by fluid flow mechanical stimulation, suggesting that the role of the primary cilium in osteoclastogenesis is not a mechanosensory one. The primary cilium has been suggested to play a role in bone formation, and our findings indicate that it may also present a means to regulate bone resorption, presenting a dual benefit of developing ciliary-targeted pharmaceuticals for bone disease.
Keywords: Primary cilium, Osteoclast, Macrophage, Fenoldopam
Introduction
Bone is maintained by the careful orchestration and balance of bone formation by osteoblasts and resorption by osteoclasts. In aging, the healthy balance of formation and resorption to maintain skeletal integrity is impeded by reduced osteoblast differentiation and proliferation, and increased maturation of osteoclasts [Fang et al., 2022]. Imbalanced metabolic activity is a hallmark of osteoporosis, a skeletal disease characterized by significant bone loss, deterioration of bone microarchitecture, and reduced bone strength, which affects more than half of the US population over 50 years old [Office of the Surgeon General, 2004; Blume and Curtis, 2011; Wright et al., 2014]. Fragility fractures, most frequently occurring in the hip or spine, are common complications of osteoporosis that result in a significant loss of quality of life and a great economic burden. There are over 1.5 million fractures in the USA annually, an incidence greater than the number of strokes, heart attacks, and breast cancer diagnoses combined.
There are two main categories of therapeutics to treat low bone mass – anabolics (e.g., parathyroid hormone analog, anti-sclerostin antibody) that promote new bone formation and antiresorptive agents (e.g., bisphosphonates, RANKL inhibitors) that inhibit osteoclast activity [Tu et al., 2018]. Anabolic agents show promise but are, either, only approved for a certain subset of patients or for a limited duration of use, which is inadequate for a multi-decade disease. Bisphosphonates, the most common agent used to prevent bone loss, bind to the surfaces of bones and promote the apoptosis of osteoclasts [Drake et al., 2008]. While such treatment can prove effective for some patients, a wide range of short-term and long-term adverse side effects persist, such as an increased rate of mandibular necrosis, atypical fractures, esophageal cancer, and atrial fibrillation [Marx, 2003; Black et al., 2010; Reyes et al., 2016]. Thus, there is a great need to identify more efficacious therapeutics for osteoporosis.
Bone has an innate ability to respond to mechanical loading; taking advantage of this mechanotransduction system could lead to the identification of new targeting strategies. Several modes of cell mechanosensing have been proposed, including by the primary cilium, a dynamic, immotile organelle that assembles and disassembles from the centrosome in a cell cycle-dependent manner. Primary cilia have been shown to form a mechano-/chemical-signaling nexus capable of coordinating signaling pathways, and their function has been tightly linked to ciliary structure. Not only are shortened or absent primary cilia characteristic of diseases such as autosomal dominant polycystic kidney disease and Bardet-Biedl syndrome [Satir and Christensen, 2008; Copelovitch and Kaplan, 2012], but a number of aforementioned ciliopathies also exhibit skeletal patterning defects and even low bone mass [Xiao and Quarles, 2010; Nguyen and Jacobs, 2013]. Several studies have previously identified the osteocyte primary cilium as a promising pharmaceutical target to exploit the body’s natural response to physical loading [Malone et al., 2007]. Our group and others have shown in vitro that fenoldopam, a dopamine D1-like receptor agonist that increases primary cilia length [Kathem et al., 2014; Upadhyay et al., 2014], heightens the mechanoresponse of osteocyte-like cells when exposed to fluid flow-induced shear stress [Spasic and Jacobs, 2017], and promotes the differentiation of osteoblast precursors [Corrigan et al., 2019]. Recently, we have shown that fenoldopam treatment increases osteogenic paracrine signaling of mechanical-stimulated osteocytes and load-induced bone formation in mice [Spasic et al., 2022], but little is known about the role of the primary cilium in osteoclast biology.
Osteoclasts are large, multinucleated cells found on the bone surface and possess the built-in machinery to degrade and digest the mineral and organic phases of the bone matrix. They are derived via the fusion of macrophages, which form a multinucleated cell with a characteristic actin ring in the cell cortex [Väänänen et al., 2000]. Hematopoietic mononuclear cells are reported to have a primary cilium [Singh et al., 2016], but it is not known what happens as an osteoclast forms or what effect increasing primary cilia length has on osteoclast maturation. Fluid flow stimulation upregulates preosteoclast cell signaling, particularly nitric oxide and prostaglandin E2 production [McAllister et al., 2000], indicating a role for preosteoclast mechanotransduction. Loss of primary cilia in other cells reduced flow-stimulated prostaglandin E2 and NO release [Malone et al., 2007; Cabral and Garvin, 2011], but whether the primary cilium as a mechanosensor plays a role in osteoclast formation is unknown. The objective of this study was to investigate whether the primary cilium is maintained in osteoclast formation, if lengthening the primary cilium with fenoldopam alters osteoclast formation, and if mechanical stimulation of the primary cilium with fluid flow impacts osteoclast maturation. With increasing evidence that the primary cilium may be a potential therapeutic target to treat bone metabolic disease, it is critical to understand its role not only in the osteogenic mesenchymal lineage [Tummala et al., 2010; Delaine-Smith et al., 2014] but also in the bone-resorbing hematopoietic lineage cells.
Materials and Methods
Cell Culturing
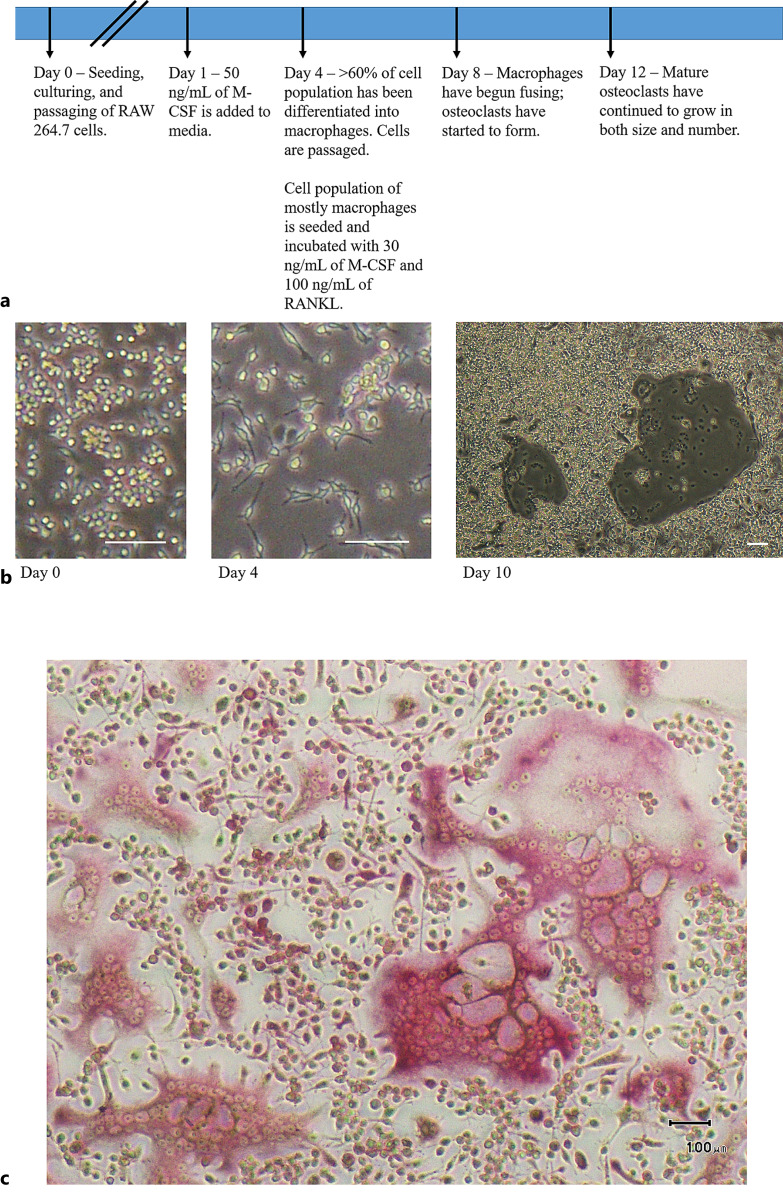
RAW 264.7 cells (adherent, immortalized cell line of monocyte/macrophage mixture) (ATCC) were seeded onto Petri dishes (Corning) at 1,000 cells per cm2 in DMEM (Gibco) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin for standard passaging and maintenance of the cell line. To differentiate this heterogeneous group into a population of mostly macrophages, which are cellular precursors to osteoclasts, macrophage colony-stimulating factor (M-CSF) (Shenandoah Biotechnology) was added at a concentration of 50 ng/mL for 72 h. To differentiate these macrophages further into osteoclasts via cell fusion, these cells were incubated for an additional 4–8 days in the same medium as used for culturing the RAW 264.7 cells but including 30 ng/mL of M-CSF and 100 ng/mL of receptor activator of nuclear factor-kappa Β ligand (RANKL) (Shenandoah Biotechnology). Cells were washed with phosphate buffered saline (Gibco) with fresh media applied every 72 h. As a confirmation of osteoclast differentiation, tartrate-resistant acid phosphatase staining was performed following manufactures directions (Cosmo Bio). A timeline of culturing and changes in cell morphology is shown in Figure 1.
Fig. 1.
Differentiation of RAW 264.7 cells into osteoclasts. a Timeline of cell passaging and differentiation process. b Representative phase-contrast micrographs demonstrating the distinct phases of osteoclast differentiation. The first image of panel b shows RAW 264.7 cells on day 0; the second image is captured on day 4, after RAW 264.7 cells have been incubated with M-CSF to differentiate into macrophages; the third image is captured from day 10, with two mature osteoclasts visible. c Positive TRAP staining demonstrating successful differentiation. Scale bar, 100 μm. TRAP, tartrate-resistant acid phosphatase.
Cilia Lengthening
Fenoldopam mesylate (Sigma) was used at 10 μm diluted in dimethyl sulfoxide (DMSO) (Sigma) and culture medium, as previously described [Upadhyay et al., 2014]. This, or the DMSO vehicle control, was added to cells for 16 h [Spasic and Jacobs, 2017]. After treatment, cells were washed with PBS and incubated in fresh medium supplemented with M-CSF and RANKL for an additional 4–8 days before being fixed or lysed.
Oscillatory Fluid Flow
Macrophages were exposed to oscillatory fluid flow as a mechanical stimulus. Cells were seeded onto four-well plates for 24 h. Flow was applied using a rocker platform (Boekel Scientific, 260350) placed in a cell culture incubator, for 12 h at 0.75 Hz, a rocker edge height change (2x amplitude) of 11 mm and 3.5 mL of medium per well. This resulted in a 0.16 Pa peak wall shear stress calculated according to Zhou et al. [2010]. Cells continued to grow for 4–8 days in M-CSF and RANKL before being lysed.
Immunocytochemistry
For primary cilia imaging and analysis, cells cultured on round glass-bottom dishes (MatTek) were fixed in 10% formalin and treated with anti-acetylated α-tubulin primary antibody, 1:10, from a C3B9 hybridoma cell line (Sigma). Cilia were visualized with Alexa-Fluor 568 secondary antibody, 1:500 (Life Technologies), and imaged with a 100× oil objective on an Olympus Fluoview FV1000 confocal microscope. Nuclei were stained with NucBlue (Invitrogen). Cilia lengths were analyzed using ImageJ from maximum projections in 2D. Cells were also treated with 488 phalloidin (Invitrogen) to visualize the F-actin cytoskeleton and pericentrin (Abcam) to visualize the centrosome and its alignment with respect to the primary cilium.
mRNA Expression
After fenoldopam treatment, cells were washed with PBS, and total mRNA was isolated using TriZol (Life Technologies). Total mRNA was converted to cDNA by TaqMan reverse transcriptase (Applied Biosystems). Gene expression was analyzed by quantitative real-time PCR using primers and probes (Life Technologies) for analysis of tartrate-resistant acid phosphatase, Acp5 (Mm00475698_m1), cathepsin k (Ctsk) (Mm00484039_m1), Nfatc1 (Mm01265944_m1), Fos (Mm00487425_m1), Dcstamp (Mm04209236_m1), and Gapdh (4351309). Samples and standards were run in triplicate; all gene expression was normalized to Gapdh endogenous control, and data were analyzed using the delta-delta Ct method.
Osteoclast Surface Area Quantification
The surface area of osteoclasts formed was quantified as a standard secondary measure to compare rates of osteoclast formation for treated and untreated groups [Lemieux et al., 2011]. Samples were again cultured on glass-bottom dishes and fixed in 10% formalin. Samples were visualized on an Olympus CKX41 microscope, and images were captured using a DP20 digital microscope camera that includes a 2-megapixel CCD. These images were collected and inputted into ImageJ with a ratio of 9.0333 pixels per micrometer. The outer edge of each osteoclast was traced, with the corresponding output being converted to surface area in µm2. The total osteoclast surface area of a given frame divided by the total surface area of that glass coverslip yields a quantified percentage surface area to compare osteoclast formation across conditions.
Statistical Analysis
Data were analyzed using Student’s t test, Welch’s t test for samples of unequal variances, or Mann-Whitney test for data not following a normal distribution. Quantitative data are reported graphically as mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Sample size, n, represents biological replicates.
Results
Primary Cilia Are Present in Osteoclast Precursors but Absent from Mature Osteoclasts
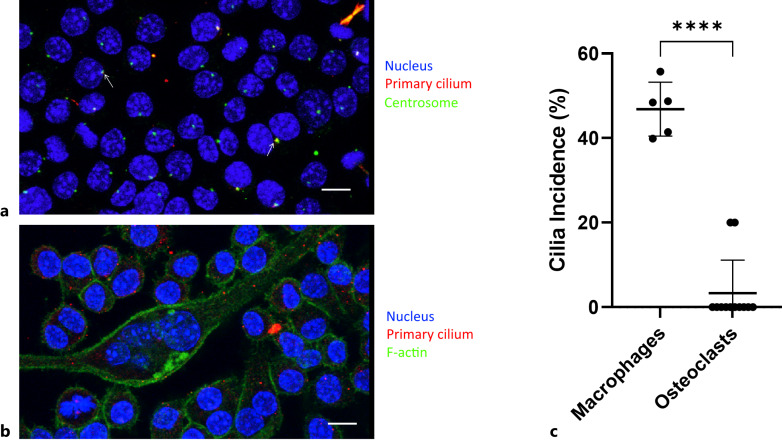
In order to test our hypothesis that the primary cilium directly affects osteoclast formation, we first verified the presence of primary cilia on macrophages. We utilized immunocytochemistry to image both centrosomes (anti-pericentrin), from which primary cilia form, and primary cilia (anti-acetylated alpha-tubulin) on RAW 264.7 cells after incubating in M-CSF. Macrophages were found to have a primary cilium incidence of 46.8% ± 6.4% (shown in Fig. 2). However, cilia were predominantly absent from differentiated multinucleated mature osteoclasts (greater than three nuclei), with an incidence of just 3.3%, and exclusively found in the immature osteoclast population, defined as fused macrophages possessing fewer than three nuclei. Distinct multinuclear and cortical actin staining was used to visually distinguish osteoclasts from their immature osteoclasts.
Fig. 2.
Macrophages possess primary cilia, but mature osteoclasts do not. Maximum projection of confocal image z-stack. a Primary cilia were found on macrophages. Blue = DAPI (nuclei), red = acetylated alpha-tubulin (primary cilia), green = γ-tubulin (centrosomes). Arrows highlight examples of centrosome/primary cilium overlay. b Primary cilia were mostly absent from differentiated osteoclasts, shown via distinct multinuclear and cortical actin staining. Blue = DAPI (nuclei), green = phalloidin (actin), red = acetylated alpha-tubulin (primary cilia). Scale bar, 10 μm. c Macrophages were found to have a primary cilia incidence of 46.8% (n = 5), while differentiated osteoclasts were found to have a cilia incidence of just 3.3% (n = 12).
Fenoldopam Increases Macrophage Primary Cilium Incidence and Length
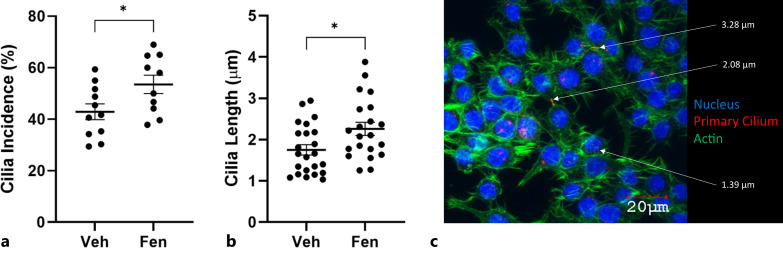
Although the procedure had been previously carried out on other cell types, we verified that the macrophage primary cilium could in fact be lengthened. Cells were cultured in fenoldopam mesylate – an FDA-approved compound known to extend cilia length – for 16 h [Spasic and Jacobs, 2017]. Immunocytochemistry was used to visualize primary cilia and assess changes in both cilia incidence and length. Fenoldopam was shown to induce significant increases in primary cilia incidence (from 42.9% to 53.5%) and length (from 1.75 μm to 2.26 μm) compared to vehicle control (shown in Fig. 3).
Fig. 3.
Fenoldopam effectively increases macrophage primary cilia incidence and length. Macrophages were treated with fenoldopam mesylate or DMSO vehicle control. Fenoldopam-treated cells showed a significant increase in both cilia incidence (a) (from 42.9% to 53.5%; n = 11, 10) and cilia length (b) (from 1.75 μm to 2.26 μm; n = 23, 21) compared to control samples. c Example primary cilia length measurements are shown.
Macrophage Primary Cilium Lengthening Inhibits Osteoclastogenesis
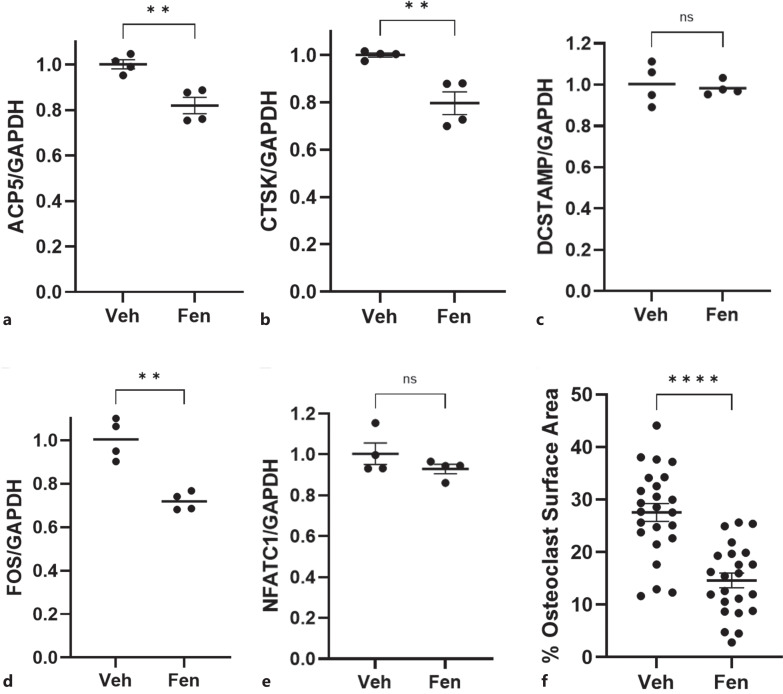
After confirming that the primary cilia of immature osteoclasts could in fact be elongated using fenoldopam treatment, we examined the effect of this lengthening on osteoclast formation. Differentiation was quantified at the mRNA level by analyzing Acp5, Ctsk, Dcstamp, c-Fos, and Nfatc1 expression and presented as fold changes relative to our housekeeping gene, Gapdh. Acp5 is the gene that codes for tartrate-resistant acid phosphatase, which degrades skeletal phosphoproteins; Ctsk codes for cathepsin K, which catabolizes elastin and collagen; Dcstamp codes for dendritic cell-specific transmembrane protein and is essential for cell-cell fusion in osteoclasts; c-Fos is a gene that is necessary for osteoclast differentiation; Nfatc1, nuclear factor of activated T cells 1, is a gene that cooperates with other transcriptional partners to activate osteoclast-specific genes. Macrophages treated with fenoldopam had an 18.2% decrease in Acp5 expression, a 20.3% decrease in CTSK expression, and a 28.5% reduction in c-Fos, compared to non-lengthened DMSO controls (shown in Fig. 4a, b, d). No change was detected in Dcstamp or Nfatc1 expression (Fig. 4c, e). These fenoldopam-treated macrophages also exhibited a markedly decreased rate of fusion, evident by the osteoclast surface area coverage decreasing by almost half, from 27.6% surface coverage in the DMSO control group to 14.6% in the fenoldopam-treated samples (shown in Fig. 4f).
Fig. 4.
Macrophage primary cilium lengthening inhibits osteoclastogenesis. mRNA quantification of genes involved in osteoclast differentiation or function showed that fenoldopam treatment prior to osteoclast differentiation treatment resulted in an 18.2% decrease in expression of tartrate-resistant acid phosphatase (Acp5) (a), a 20.3% decrease in expression of cathepsin K (Ctsk) (b), no change in dendritic cell-specific transmembrane protein (Dcstamp) (c), a 28.5% decrease in c-Fos (d), and no change in nuclear factor of activated T cells, cytoplasmic 1 (Nfatc1) (e), when compared to the DMSO vehicle control (n = 4). f Samples treated with fenoldopam also formed osteoclasts at a lesser rate, indicated by a 13% drop in osteoclast surface area coverage (n = 24, 23).
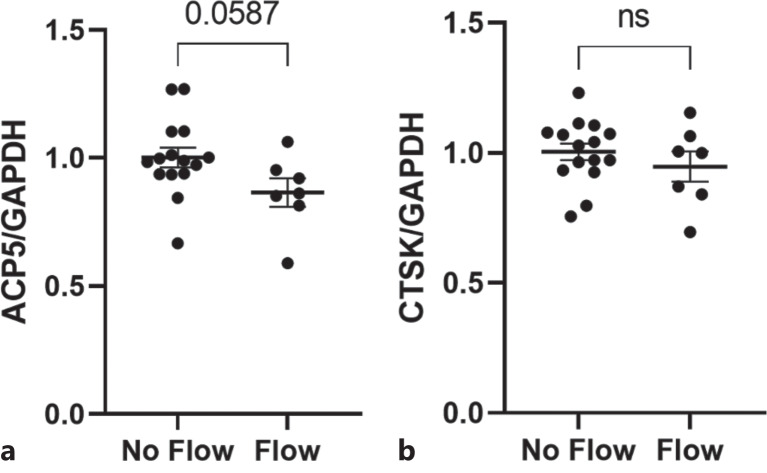
Fluid Flow/Mechanical Stimulation Does Not Affect Osteoclastogenesis
Given that primary cilia are believed to play a role in the ability of other bone cells to direct cell function when mechanically stimulated [Delaine-Smith et al., 2014; Lee et al., 2015], we sought to determine whether or not the same was true of macrophages by subjecting the cells to oscillatory fluid flow. The results were again quantified at the mRNA level by analyzing Acp5 and Ctsk expression, presented as normalized fold changes (Fig. 5). Neither expression levels of the genes tested showed a significant change between the flow and static groups, indicating that the progression of osteoclastogenesis in response to mechanical loading does not rely on the primary cilium as a mechanosensor.
Fig. 5.
Mechanical stimulation of macrophage primary cilia did not result in a significant change in osteoclastogenesis. Macrophages were subjected to oscillatory fluid flow prior to being incubated with M-CSF and RANKL to induce osteoclast differentiation. Mechanically stimulated cells did not demonstrate a significant change in osteoclastogenesis when compared to the static control samples (n = 15, 7).
Discussion
Our results demonstrated for the first time that osteoclasts do not form primary cilia. Interestingly, they are present on macrophage and immature osteoclasts (less than three nuclei), indicating that loss of primary cilia may be a key event in the timeline of osteoclastogenesis. In fact, we show that by treating with fenoldopam, macrophage primary cilia incidence and length are increased, and osteoclast differentiation is decreased when cultured with M-CSF and RANKL. Since the primary cilium is shown to function both as a biochemical signaling nexus and mechanosensor, we applied fluid flow to mechanically stimulate the primary cilium and found it had no effect on osteoclast differentiation as measured by gene expression levels. This indicates the primary cilium likely doesn’t play a role in osteoclast differentiation from a mechanosensing perspective.
We quantified the primary cilia incidence of macrophages at ∼47%, while bone marrow aspirate mononuclear cells have been reported to have a primary cilia incidence around 97–99% [Singh et al., 2016]. The difference is likely due to the need for cell cycle-dependent disassembly of the primary cilium in proliferating cells in culture [Sánchez and Dynlacht, 2016]. We observed that primary cilia are virtually absent as macrophages fuse to form mature osteoclasts, as they were only found sparingly on cells (∼3%) at the earliest stage of fusion, and none were found on mature osteoclasts (cells with greater than three nuclei). We also did not observe more than one cilium on an immature osteoclast. This finding suggests that the loss of the primary cilium is either a byproduct of fusion or a checkpoint on osteoclast maturation.
To see if the primary cilium acts as a checkpoint on osteoclast maturation, we treated with an agent, fenoldopam, known to lengthen and increase the incidence of primary cilia [Spasic and Jacobs, 2017; Corrigan et al., 2019]. Applied to macrophages, fenoldopam treatment resulted in a statistically significant increase in both macrophage cilia incidence and length. These length changes were of a similar magnitude to those shown, previously, to alter mechanosensitivity in bone cells [Spasic and Jacobs, 2017]. When cilia-lengthened macrophages were then induced in the osteoclast differentiation medium, we found reduced osteoclastogenesis, evident by Acp5 and Ctsk gene expression levels, as well as a decrease by almost half in the osteoclast surface area. Additionally, c-Fos, a gene necessary for osteoclast formation [Asagiri and Takayanagi, 2007], was downregulated by approximately 28% with fenoldopam treatment. While there was no significant change in gene expression of Dcstamp, necessary for cell fusion [Yagi et al., 2005], or Nfatc1, a regulator of a number of osteoclast-specific genes [Kim and Kim, 2014], a downward trend in expression of osteoclastic genes with fenoldopam could be observed in the data. Therefore, it seems that the primary cilium resorption is not just a byproduct of osteoclast maturation but may be an important step in the process. A role for the primary cilium in macrophage fusion cannot be ruled out as this process is not yet fully understood [Yao et al., 2021] and is further complicated by the fact that osteoclasts may undergo successive rounds of fusion and fission [Jansen et al., 2012; McDonald et al., 2021]. Furthermore, a distinct role of the primary cilium in macrophages and immature osteoclasts is not yet known, but the primary cilium has been shown to be important for migration during tissue repair, a potential role in osteoclast formation as well [Christensen et al., 2008].
The primary cilium has been shown to be intimately related to actin and microtubule function in the cytosol, which are both important to osteoclast differentiation. In chondrocytes, inhibited primary cilia formation results in increased F-actin staining intensity and reduced cell stiffness [Wang et al., 2016]; in an osteocyte-like cell line, an inhibition of primary cilia formation altered microtubule dynamics in response to physical stimuli [Espinha et al., 2014]. As osteoclasts undergo maturation, there is an observable morphological change from spindly macrophages to rounded preosteoclasts [Takeshita et al., 2000] and finally large mature osteoclasts with a distinct actin cortical ring. Additionally, osteoclast differentiation is also highly dependent on Rho-GTPases [He et al., 2022], an actin cytoskeleton regulator, and substrate-stiffness-dependent cytoskeletal reorganization is critical to osteoclast maturation [Wang et al., 2022]. It is possible the primary cilium plays a role in the coordination of cytoskeletal changes that are dependent on cilia resorption. A further potential role of the primary cilium in osteoclast formation is regulating cell state. In fact, progression of cells to a G1/S phase seems critical for osteoclast maturation [Meiyanto et al., 2001; Watanabe et al., 2022], and cilia disassembly is initiated in the G1 phase and cilia are mostly absent in the S phase [Sánchez and Dynlacht, 2016]. These changes are also potentially linked to cytoskeletal regulation.
Osteoclasts are shown to respond to substrate strain (i.e., substrate deformation), but reports on whether strain promotes or inhibits osteoclast formation are contradictory and seem to depend on strain levels and duration of loading [Kurata et al., 2001; Xu et al., 2012; Wang et al., 2022]. One report has shown that fluid flow stimulates preosteoclast to release several cytokines [McAllister et al., 2000], but this was not extended to determine effects on osteoclast differentiation or formation. Since the primary cilium is shown to be a potent mechanosensor [Malone et al., 2007; Delaine-Smith et al., 2014; Lee et al., 2015], we sought to determine if it played a mechanosensing role in osteoclast formation. Since primary cilia extend from the apical surface of most cultured cells and preosteoclasts are responsive to fluid flow [McAllister et al., 2000], we applied fluid flow to mechanically stimulate the cells. We showed no effect of mechanical stimulation on osteoclast maturation, indicating that fluid flow stimulation may not play a role in osteoclast mechanobiology; this is of particular note when compared to substrate deformation – the most commonly applied form of mechanical stimulation in osteoclast studies –a mechanism unlikely to directly stimulate the primary cilium. Here we applied a modest level of shear stress (0.16 Pa), which we deemed appropriate as it is within the range of bone marrow shear stress (0.02–0.26 Pa) found computationally in trabecular bone volumes [Birmingham et al., 2013], it has been shown to be adequate to stimulate other cells in culture [Spasic et al., 2022], and the primary cilium is shown to deflect at fluid shear as low as 0.03 Pa [Malone et al., 2007]. It is important to note that a higher range of shear stresses and flow stimulation may elicit a mechanobiological response.
Although we have demonstrated the capability to lengthen macrophage primary cilia and inhibit osteoclast formation using fenoldopam, the protocol was adapted from previous studies experimenting with osteocyte primary cilia. While the primary cilium itself seems to be structurally preserved across cell types, the specific way in which fenoldopam lengthens primary cilia is not yet fully understood. Used as a vasodilator in acute emergency settings, fenoldopam is a dopamine D1-like receptor agonist and is shown to stimulate adenylyl cyclase activity [Murphy et al., 2001; Spasic and Jacobs, 2017]. In fact, adenylyl cyclase activity and increased cAMP levels have been shown to also decrease osteoclastogenesis [Yoon et al., 2011]. In our study, the application of fenoldopam was removed prior to incubation with differentiation media for a course of 4–8 days, likely minimizing any potential effects of initially elevated cAMP. Though, given the role of G-protein-coupled receptors, adenylyl cyclases, and cAMP in osteoclast formation [Yoon et al., 2011; Ramaswamy et al., 2018], this warrants future exploration. Other cilia-modifying approaches are available. For instance, tubastatin is an HDAC6-specific deacetylase inhibitor that causes an increase in primary cilia microtubule acetylation, which both stabilizes the primary cilium and increases its stiffness [Xiang et al., 2017]. Additionally, lithium chloride, an antidepressant agent, is shown to increase primary cilia length in a range of contexts [Miyoshi et al., 2009; Mehran et al., 2016; Thompson et al., 2016; Spasic and Jacobs, 2017; Corrigan et al., 2019]. We chose to explore the use of fenoldopam as it has shown promise as a bone anabolic agent [Spasic and Jacobs, 2017; Corrigan et al., 2019; Spasic et al., 2022], yet its role on osteoclasts was not known.
While our results certainly suggest that fenoldopam treatment may provide an anti-resorption effect, which would be synergistic with fenoldopam’s reported anabolic potential [Spasic et al., 2022], its long-term effect on whole-bone has not yet been examined. Fenoldopam has been clinically approved to treat extreme hypertension and is administered for a maximum of 48 h [Murphy et al., 2001], and there remains limited data on its long-term effect beyond initial reports in mice [Spasic et al., 2022]. Together, our data indicate that primary cilium disassembly may be an important step in osteoclast formation. The primary cilium hosts a distinct pool of receptors and can act as a unique biochemical signaling nexus, distinct from the cytosol, and whether cilia disassembly alters biochemical signaling or removes a cytoskeletal remodeling checkpoint similar to its role in mitosis, the particular contribution of the primary cilium to osteoclast formation remains unknown. Given the importance of the primary cilium in the osteogenic role of mesenchymal lineage cells, cilia-targeted treatment strategies could have a two-pronged benefit of promoting bone formation and inhibiting bone resorption. Currently, no such treatment strategy for low bone mass exists and the combination of antiresorptive and anabolic therapies remains in the research phase [Chan et al., 2016; Tu et al., 2018]. This work is a critical first step in understanding the role of the primary cilium in osteoclast biology and, importantly, provides further support for the potential of ciliotherapies in treating bone disease.
Acknowledgment
We would like to thank the principal investigator of this study, the late Dr. Christopher R. Jacobs, for his invaluable contributions to this work as well as his role as a mentor and pioneer in the field of cellular mechanics and the study of primary cilia in bone.
Statement of Ethics
All experiments conducted were carried out on an established cell line. Ethical approval is not required for this study in accordance with local and national guidelines.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was made possible by funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health (R01 – AR062177) and the National Science Foundation Graduate Research Fellowship (DGE – 1644869). This project has received funding from the European Union’s Horizon, 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No. 748305 (S.W.V.).
Author Contributions
M.M.S., M.P.D., S.W.V., and C.R.J. designed the experiments. M.M.S. performed all experiments. M.M.S. and M.P.D. planned and performed data analysis and wrote the manuscript. S.W.V. provided discussion and manuscript input. M.M.S., M.P.D., and S.W.V. approved the final manuscript. C.R.J. died prior to final manuscript preparation.
Funding Statement
This work was made possible by funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health (R01 – AR062177) and the National Science Foundation Graduate Research Fellowship (DGE – 1644869). This project has received funding from the European Union’s Horizon, 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No. 748305 (S.W.V.).
Data Availability Statement
All data generated and analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007 Feb;40(2):251–64. 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Birmingham E, Grogan JA, Niebur GL, McNamara LM, McHugh PE. Computational modelling of the mechanics of trabecular bone and marrow using fluid structure interaction techniques. Ann Biomed Eng. 2013 Apr 1;41(4):814–26. 10.1007/s10439-012-0714-1. [DOI] [PubMed] [Google Scholar]
- Black DM, Kelly MP, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, et al. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med. 2010 May 13;362(19):1761–71. 10.1056/nejmoa1001086. [DOI] [PubMed] [Google Scholar]
- Blume SW, Curtis JR. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos Int. 2011 Jun;22(6):1835–44. 10.1007/s00198-010-1419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral PD, Garvin JL. Luminal flow regulates NO and O2(-) along the nephron. Am J Physiol Ren Physiol. 2011 May;300(5):F1047–53. 10.1152/ajprenal.00724.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CKY, Mason A, Cooper C, Dennison E. Novel advances in the treatment of osteoporosis. Br Med Bull. 2016 Sep;119(1):129–42. 10.1093/bmb/ldw033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen ST, Pedersen SF, Satir P, Veland IR, Schneider L. The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr Top Dev Biol. 2008;85:261–301. 10.1016/S0070-2153(08)00810-7. [DOI] [PubMed] [Google Scholar]
- Copelovitch L, Kaplan BS. Developmental abnormalities of the kidneys. In: Avery’s Diseases of the Newborn. 9th ed; 2012. p. 1182–90. [Google Scholar]
- Corrigan MA, Ferradaes TM, Riffault M, Hoey DA. Ciliotherapy treatments to enhance biochemically- and biophysically-induced mesenchymal stem cell osteogenesis: a comparison study. Cell Mol Bioeng. 2019 Feb;12(1):53–67. 10.1007/s12195-018-00561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaine-Smith RM, Sittichokechaiwut A, Reilly GC. Primary cilia respond to fluid shear stress and mediate flow-induced calcium deposition in osteoblasts. FASEB J. 2014 Jan;28(1):430–9. 10.1096/fj.13-231894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008 Sep 1;83(9):1032–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinha LC, Hoey DA, Fernandes PR, Rodrigues HC, Jacobs CR. Oscillatory fluid flow influences primary cilia and microtubule mechanics. Cytoskelet Hob. 2014 Jul;71(7):435–45. 10.1002/cm.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Deng Z, Liu J, Chen S, Deng Z, Li W. The mechanism of bone remodeling after bone aging. Clin Interv Aging. 2022 Apr 5;17:405–15. 10.2147/CIA.S349604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Zhang K, Cao Y, Liu G, Zou H, Song R, et al. Effect of cadmium on Rho GTPases signal transduction during osteoclast differentiation. Environ Toxicol. 2022 Mar 8;37(7):1608–17. 10.1002/tox.23510. [DOI] [PubMed] [Google Scholar]
- Jansen I, Vermeer JA, Bloemen V, Stap J, Everts V. Osteoclast fusion and fission. Calcif Tissue Int. 2012 Jun;90(6):515–22. 10.1007/s00223-012-9600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathem SH, Mohieldin AM, Abdul-Majeed S, Ismail SH, Altaei QH, Alshimmari IK, et al. Ciliotherapy: a novel intervention in polycystic kidney disease. J Geriatr Cardiol. 2014 Mar;11(1):63–73. 10.3969/j.issn.1671-5411.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim N. Regulation of NFATc1 in osteoclast differentiation. J Bone Metab. 2014 Nov;21(4):233–41. 10.11005/jbm.2014.21.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata K, Uemura T, Nemoto A, Tateishi T, Murakami T, Higaki H, et al. Mechanical strain effect on bone-resorbing activity and messenger RNA expressions of marker enzymes in isolated osteoclast culture. J Bone Miner Res. 2001 Apr;16(4):722–30. 10.1359/jbmr.2001.16.4.722. [DOI] [PubMed] [Google Scholar]
- Lee KL, Guevarra MD, Nguyen AM, Chua MC, Wang Y, Jacobs CR. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia. 2015 May 29;4:7. 10.1186/s13630-015-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux JM, Wu G, Morgan JA, Kacena MA. DMSO regulates osteoclast development in vitro. Vitro Cell Dev Biol Anim. 2011 Mar;47(3):260–7. 10.1007/s11626-011-9385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone AMD, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007 Aug 14;104(33):13325–30. 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003 Sep;61(9):1115–7. 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- McAllister TN, Du T, Frangos JA. Fluid shear stress stimulates prostaglandin and nitric oxide release in bone marrow-derived preosteoclast-like cells. Biochem Biophys Res Commun. 2000;270(2):643–8. 10.1006/bbrc.2000.2467. [DOI] [PubMed] [Google Scholar]
- McDonald MM, Khoo WH, Ng PY, Xiao Y, Zamerli J, Thatcher P, et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell. 2021 Mar 4;184(7):1940–347.e13. 10.1016/j.cell.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehran NA, Mallow JF, Himmel NJ, Blount MA. Lithium modulates cilia length in renal collecting duct cells. FASEB J. 2016 Apr;30(1):1219–4. [Google Scholar]
- Meiyanto E, Hoshijima M, Ogawa T, Ishida N, Takeya T. Osteoclast differentiation factor modulates cell cycle machinery and causes a delay in s phase progression in RAW264 cells. Biochem Biophys Res Commun. 2001 Mar 23;282(1):278–83. 10.1006/bbrc.2001.4564. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Kasahara K, Miyazaki I, Asanuma M. Lithium treatment elongates primary cilia in the mouse brain and in cultured cells. Biochem Biophys Res Commun. 2009 Oct 30;388(4):757–62. 10.1016/j.bbrc.2009.08.099. [DOI] [PubMed] [Google Scholar]
- Murphy MB, Murray C, Shorten GD. Fenoldopam: a selective peripheral dopamine-receptor agonist for the treatment of severe hypertension. N Engl J Med. 2001 Nov 22;345(21):1548–57. 10.1056/NEJMra010253. [DOI] [PubMed] [Google Scholar]
- Nguyen AM, Jacobs CR. Emerging role of primary cilia as mechanosensors in osteocytes. Bone. 2013 Jun;54(2):196–204. 10.1016/j.bone.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of the Surgeon General (US) . Bone health and osteoporosis: a report of the surgeon general. Rockville (MD): Office of the Surgeon General (US); 2004. [PubMed] [Google Scholar]
- Ramaswamy G, Fong J, Brewer N, Kim H, Zhang D, Choi Y, et al. Ablation of Gsα signaling in osteoclast progenitor cells adversely affects skeletal bone maintenance. Bone. 2018 Apr;109:86–90. 10.1016/j.bone.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes C, Hitz M, Prieto-Alhambra D, Abrahamsen B. Risks and benefits of bisphosphonate therapies. J Cell Biochem. 2016 Jan;117(1):20–8. 10.1002/jcb.25266. [DOI] [PubMed] [Google Scholar]
- Sánchez I, Dynlacht B. Cilium assembly and disassembly. Nat Cell Biol. 2016 Jun 28;18(7):711–7. 10.1038/ncb3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Structure and function of mammalian cilia. Histochem Cell Biol. 2008 Jun;129(6):687–93. 10.1007/s00418-008-0416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Chaudhry P, Merchant A. Primary cilia are present on human blood and bone marrow cells and mediate Hedgehog signaling. Exp Hematol. 2016 Dec;44(12):1181–7.e2. 10.1016/j.exphem.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasic M, Jacobs CR. Lengthening primary cilia enhances cellular mechanosensitivity. Eur Cell Mater. 2017 Feb 20;33:158–68. 10.22203/eCM.v033a12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasic M, Duffy MP, Jacobs CR. Fenoldopam sensitizes primary cilia-mediated mechanosensing to promote osteogenic intercellular signaling and whole bone adaptation. J Bone Miner Res. 2022 Mar 1;37(5):972–82. 10.1002/jbmr.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita S, Kaji K, Kudo A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res. 2000 Aug;15(8):1477–88. 10.1359/jbmr.2000.15.8.1477. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Wiles A, Poole CA, Knight MM. Lithium chloride modulates chondrocyte primary cilia and inhibits Hedgehog signaling. FASEB J. 2016 Feb;30(2):716–26. 10.1096/fj.15-274944. [DOI] [PubMed] [Google Scholar]
- Tu KN, Lie JD, Wan CKV, Cameron M, Austel AG, Nguyen JK, et al. Osteoporosis: a review of treatment options. P T. 2018 Feb;43(2):92–104. [PMC free article] [PubMed] [Google Scholar]
- Tummala P, Arnsdorf EJ, Jacobs CR. The role of primary cilia in mesenchymal stem cell differentiation: a pivotal switch in guiding lineage commitment. Cell Mol Bioeng. 2010 Sep 1;3(3):207–12. 10.1007/s12195-010-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay VS, Muntean BS, Kathem SH, Hwang JJ, Aboualaiwi WA, Nauli SM. Roles of dopamine receptor on chemosensory and mechanosensory primary cilia in renal epithelial cells. Front Physiol. 2014 Feb 26;5:72. 10.3389/fphys.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väänänen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J Cell Sci. 2000 Feb;113(Pt 3):377–81. 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wann A, Thompson C, Hassen A, Wang W, Knight MM. IFT88 influences chondrocyte actin organization and biomechanics. Osteoarthritis Cartilage. 2016 Mar;24(3):544–54. 10.1016/j.joca.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Xie J, Zhou C, Lai W. Substrate stiffness regulates the differentiation profile and functions of osteoclasts via cytoskeletal arrangement. Cell Prolif. 2022 Jan;55(1):e13172. 10.1111/cpr.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Okada H, Hirose J, Omata Y, Matsumoto T, Matsumoto M, et al. Transcription factor hematopoietically expressed homeobox protein (hhex) negatively regulates osteoclast differentiation by controlling cyclin-dependent kinase inhibitors. JBMR Plus. 2022 Feb 14;6(4):e10608. 10.1002/jbm4.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014 Nov;29(11):2520–6. 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang W, Guo F, Cheng W, Zhang J, Huang J, Wang R, et al. HDAC6 inhibition suppresses chondrosarcoma by restoring the expression of primary cilia. Oncol Rep. 2017 Jul;38(1):229–36. 10.3892/or.2017.5694. [DOI] [PubMed] [Google Scholar]
- Xiao ZS, Quarles LD. Role of the polycytin-primary cilia complex in bone development and mechanosensing. Ann N Y Acad Sci. 2010 Mar;1192(1):410–21. 10.1111/j.1749-6632.2009.05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XY, Guo C, Yan YX, Guo Y, Li RX, Song M, et al. Differential effects of mechanical strain on osteoclastogenesis and osteoclast-related gene expression in RAW264.7 cells. Mol Med Rep. 2012 Aug;6(2):409–15. 10.3892/mmr.2012.908. [DOI] [PubMed] [Google Scholar]
- Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005 Aug 1;202(3):345–51. 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Cai X, Ren F, Ye Y, Wang F, Zhang C, et al. The macrophage-osteoclast axis in osteoimmunity and osteo-related diseases. Front Immunol. 2021;12(664871). 10.3389/fimmu.2021.664871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SH, Ryu JY, Lee Y, Lee ZH, Kim HH. Adenylate cyclase and calmodulin-dependent kinase have opposite effects on osteoclastogenesis by regulating the PKA-NFATc1 pathway. J Bone Miner Res. 2011 Jun;26(6):1217–29. 10.1002/jbmr.310. [DOI] [PubMed] [Google Scholar]
- Zhou X, Liu D, You L, Wang L. Quantifying fluid shear stress in a rocking culture dish. J Biomech. 2010 May 28;43(8):1598–602. 10.1016/j.jbiomech.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.