Abstract
Background
Abdominal aortic calcification (AAC) is an independent risk factor for cardiovascular disease. We aim to examine the associations between Life's Essential 8 (LE8), the recently updated measurement of cardiovascular health (CVH), and AAC among participants aged ≥40 years.
Methods and Results
This population‐based cross‐sectional study used data from the National Health and Nutrition Examination Survey in 2013 to 2014. AAC (AAC score>0) and severe AAC (AAC score>6) were quantified by the Kauppila score system. Multiple linear, multivariable logistic, and restricted cubic spline models were used to assess the associations. A total of 2369 participants were included with a mean AAC score of 1.41 (0.13). Participants in the high‐cardiovascular‐health group had lower AAC scores, lower prevalence of AAC, and lower prevalence of severe AAC. After the adjustment of potential confounders (age, sex, race and ethnicity, education levels, marital status, poverty income ratio, estimated glomerular filtration rate, serum creatinine, serum uric acid, serum phosphorus, and serum total calcium), higher cardiovascular health was significantly associated with lower risk of AAC. Meanwhile, elevated nicotine exposure score, blood glucose score, and blood pressure score within the LE8 components were significantly associated with lower risk of AAC. Also, nonlinear dose–response relationships were observed. Subgroup analyses (age strata, sex, poverty income ratio, education levels, marital status) indicated the inverse associations of LE8 and AAC were generally similar in different populations.
Conclusions
LE8 was negatively and nonlinearly related to the risk of AAC among middle‐aged and older populations. Meanwhile, LE8 components should prioritize higher scores for nicotine exposure, blood glucose, and blood pressure evaluations.
Keywords: abdominal aortic calcification, cardiovascular health, Life's Essential 8, NHANES
Subject Categories: Lifestyle, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- AAC
abdominal aortic calcification
- CVH
cardiovascular health
- LE8
Life's Essential 8
- NHANES
National Health and Nutrition Examination Survey
- PIR
poverty income ratio
Clinical Perspective.
What Is New?
This was the first study to elucidate novel inverse dose–response relationships between cardiovascular health and both abdominal aortic calcification and severe abdominal aortic calcification by employing Life's Essential 8 to assess cardiovascular health levels.
Life's Essential 8 may be negatively and nonlinearly related to the risk of abdominal aortic calcification and severe abdominal aortic calcification among middle‐aged and older populations.
What Are the Clinical Implications?
These findings suggest that Life's Essential 8 may have clinical applications as a functional and utilitarian composite indicator for improving vascular health.
Differences in the underlying salutary value of cardiovascular health constituents need to be fully recognized.
Abdominal aortic calcification (AAC) represents a common vascular condition characterized by disrupted mineral metabolism of calcium and phosphorus, as well as abnormal deposition of mineralized plaques in the arterial wall, and its incidence increases progressively with age. 1 , 2 Extensive studies have demonstrated that AAC was an independent risk factor for the incidence of cardiovascular events, as well as a reliable biomarker for atherosclerotic cardiovascular disease (CVD). 3 , 4 , 5 A retrospective study has revealed that computed tomography–based AAC was a robust predictor of CVD. 6 A quantifiable methodology for grading a calcified abdominal aorta was developed by Kauppila and colleagues (Kauppila AAC score), using lateral radiographs of the lumbar region to quantifiably estimate the extent of AAC. 7 Owing to its accuracy and excellent predictive power, the quantifiable methodology has been extensively used in prior investigations and has been shown to forecast all‐cause death and CVD independently. 8 , 9 , 10 , 11
The American Heart Association initiated Life's Essential 8 (LE8) as a measure of quantifying cardiovascular health (CVH) to enhance CVH of the general population. 12 LE8 is a scoring system that is sensitive to interindividual differences and intraindividual variation. Meanwhile, LE8 has emerged as a powerful tool for assessing CVH, and extensive studies have demonstrated a significant, progressive, negative link between LE8 score and CVD, all‐cause death, and various non‐CVD outcomes. 13 , 14 , 15 A prospective study has demonstrated that maintaining a high CVH, defined by LE8 score, is significantly associated with increased life expectancy among US adults. 16 Given the intimate association between AAC and CVD, promoting LE8 may be a crucial management strategy for mitigating the risk of AAC and severe AAC. Nevertheless, as of yet, no research has evaluated the associations between LE8 and both AAC and severe AAC.
Thus, this cross‐sectional investigation used information from the National Health and Nutrition Examination Survey (NHANES) to examine the potential relationships between LE8 and both AAC and severe AAC in middle‐aged and older populations. These findings may provide new strategies for preventing and managing AAC and severe AAC in clinical practice.
Methods
All data are publicly available and can be accessed at the NHANES (https://www.cdc.gov/nchs/nhanes/index.htm). Relevant R code is available upon reasonable request to the corresponding author.
Study Population
NHANES focuses on estimating the prevalence of primary illnesses and disease‐specific risk factors in the United States. A detailed description of the survey can be obtained at http://www.cdc.gov/nchs/nhanes.htm. Sophisticated multiperiod probability‐based sampling methods were used by NHANES to obtain samples that were representative of the nation. The National Center for Health Statistics Research Ethics Review Board approved all NHANES protocols, and all participants provided written informed consent. This cross‐sectional investigation used NHANES data from 2013 to 2014 and adhered to the standards for Strengthening the Reporting of Observational Studies in Epidemiology. 17
This investigation was limited to adults aged ≥40 years, with no available AAC score data for participants aged <40 years. After excluding participants aged <40 years (n=6360), those with missing AAC score data (n=675), missing CVH indicators data (n=558), and missing relevant covariates data (n=213) from a total of 10 175 participants in the 2013 to 2014 NHANES cycle, a total of 2369 participants aged ≥40 years were eventually included (Figure 1).
Figure 1. Flowchart of the screening process for the selection of the study population.
AAC indicates abdominal aortic calcification; CVH, cardiovascular health; and NHANES, National Health and Nutrition Examination Survey.
Measurements of LE8
The American Heart Association has recently updated Life's Simple 7 to LE8 to quantify CVH, which includes 4 health behaviors and 4 health factors, as a way to significantly enhance guidance on improving CVH in the general population. 12 The elaborated description of the calculation of scores for each metric of LE8 using NHANES data can be viewed in Table S1. 18 Overall, each of the LE8 indicators was rated on a scale of 0 to 100 scores, and the unweighted mean of these 8 indicators was calculated to obtain the total LE8 score. The American Heart Association recommended that participants with LE8 scores ≥80 were categorized as the high CVH, those with LE8 scores of 50 to 79 were classified as moderate CVH, and those with LE8 scores <50 were grouped as low CVH. 12
The Healthy Eating Index–2015 was utilized to estimate the diet metric. 19 Table S2 generalizes the constituents and criteria for scoring the Healthy Eating Index–2015. The Healthy Eating Index–2015 score was computed by employing information from the first 24‐hour dietetic recall interview completed during the NHANES mobile examination center visit, and if two 24‐hour recalls were available, the first one that provided diet data was used. Information on physical activity, nicotine exposure, and sleep health, as well as diabetes and medication history, was obtained through self‐report questionnaires. During the physical examination, participants were professionally measured for blood pressure, height, and weight. Researchers collected blood samples for analysis of blood lipids, plasma glucose, and glycated hemoglobin at central laboratories.
Evaluations of AAC and Severe AAC
The Kauppila AAC score was calculated from dual‐energy x‐ray absorptiometry scans of the lateral lumbar spine. 7 The AAC score was used to assess the severity of AAC, with higher scores indicating a more pronounced degree of calcification. The Kauppila scoring system divided the wall of the abdominal aorta into 4 continuous segments that correspond directly to the L1 to L4 vertebral region. Each segment was given a score (0–6) according to the degree of calcium deposition, and the sum of the scores from all segments yielded the final AAC score (0–24). AAC was considered present when the total AAC score was >0, and severe AAC was determined by a total AAC score that exceeded 6, which have been extensively employed as thresholds in previous investigations. 20 , 21
Covariates Assessment
During the home interviews, trained interviewers collected demographic information using computer‐assisted personal interviewing. Age was separated into 2 categories: 40 to 60 years and >60 years. Race and ethnicity were categorized into 5 groups: Mexican American, Non‐Hispanic White, Non‐Hispanic Black, Other Hispanic, and other race/multiracial. The poverty income ratio (PIR) was estimated by dividing monthly family income by the poverty level and then classified into 3 groups: ≤1.30, 1.31 to 3.50, and >3.50. Education levels were categorized into 3 groups: less than high school, high school, and more than high school. Single/separated or coupled were the 2 marital status categories.
The serum samples were transported to the central laboratory for evaluation to obtain serum creatinine, serum uric acid, serum phosphorus, and serum total calcium. The Chronic Kidney Disease Epidemiology Collaboration equation was used to calculate the estimated glomerular filtration rate. 22
Statistical Analysis
The data were analyzed following the analytical guidelines and recommended survey weights for NHANES data. Baseline characteristics were described using weighted mean with SE for continuous variables and unweighted frequencies with weighted percentages for categorical variables. Participants were divided into low‐CVH, moderate‐CVH, and high‐CVH groups on the basis of their LE8 scores, and differences in baseline characteristics were compared employing one‐way ANOVA for continuous variables and the chi‐square test for categorical variables.
Survey‐weighted multiple linear regression was used to explore the correlations between LE8 and its components with AAC score. Multiple logistic regression was also applied to investigate the connections between LE8 and its components with AAC and severe AAC. Additionally, restricted cubic spline regression (3 knots) was adopted to probe into underlying nonlinear relationships between LE8 score and its components with AAC. The nonlinearity was evaluated with the likelihood ratio test.
To further investigate the associations between LE8 and both AAC and severe AAC in different populations, subgroup analysis was implemented by age strata, sex, PIR levels, education levels, and marital status. The significance of interactions was estimated using P values for the interaction coefficients between LE8 and subgroup populations. Furthermore, we excluded participants with self‐reported histories of CVD (including coronary heart disease, angina, congestive heart failure, and heart attack; n=309) to evaluate the soundness of our results. All statistical analyses were performed using R version 4.2.1 software (R Foundation for Statistical Computing, Vienna, Austria). Statistical tests were 2‐sided, and statistical significance was assumed to be P<0.05.
Results
Baseline Characteristics
The basic characteristics of the included and excluded individuals are exhibited in Table S3. A total of 2369 middle‐aged and elderly adults were enrolled in this study. Based on low‐CVH, moderate‐CVH, and high‐CVH categories, Table 1 generalizes the baseline characteristics of the study population. Significant differences were found among different CVH groups in terms of age, age strata, sex, race and ethnicity, education levels, marital status, PIR, PIR levels, estimated glomerular filtration rate, serum creatinine, serum uric acid, AAC score, AAC, and severe AAC (all P values <0.05). Participants in the high‐CVH group were younger and more likely to be women; had higher education levels, higher PIR, higher estimated glomerular filtration rate, lower serum creatinine, and lower serum uric acid; and were coupled more than all other groups. Participants in the high‐CVH group had lower AAC scores (low CVH, 1.59 [0.18], moderate CVH, 1.58 [0.17], high CVH, 0.50 [0.10]), lower prevalence of AAC (low CVH, 31.06%; moderate CVH, 30.29%; high CVH, 11.93%), and lower prevalence of severe AAC (low CVH, 8.22%; moderate CVH, 8.77%; high CVH, 2.74%).
Table 1.
Baseline Characteristics of the Study Population*
| Characteristics | Total | Low CVH | Moderate CVH | High CVH | P value |
|---|---|---|---|---|---|
| Participant number | 2369 | 438 | 1637 | 294 | / |
| Age, y, mean (SE) | 57.15 (0.30) | 57.40 (0.60) | 57.97 (0.34) | 53.44 (0.68) | <0.001 |
| Age strata, n (%) | <0.001 | ||||
| 40–60 | 1362 (63.16) | 245 (60.65) | 901 (60.17) | 216 (78.42) | |
| >60 | 1007 (36.84) | 193 (39.35) | 736 (39.83) | 78 (21.58) | |
| Sex, n (%) | 0.010 | ||||
| Female | 1238 (51.93) | 227 (50.87) | 837 (50.14) | 174 (60.60) | |
| Male | 1131 (48.07) | 211 (49.13) | 800 (49.86) | 120 (39.40) | |
| Race and ethnicity, n (%) | <0.001 | ||||
| Mexican American | 286 (6.24) | 63 (7.89) | 200 (6.60) | 23 (3.13) | |
| Non‐Hispanic Black | 465 (9.87) | 118 (15.59) | 319 (9.90) | 28 (4.20) | |
| Non‐Hispanic White | 1092 (72.66) | 182 (66.05) | 752 (72.46) | 158 (79.93) | |
| Other Hispanic | 214 (4.27) | 47 (5.73) | 144 (4.07) | 23 (3.68) | |
| Other race/multiracial | 312 (6.96) | 28 (4.74) | 222 (6.97) | 62 (9.06) | |
| Education levels, n (%) | <0.001 | ||||
| Less than high school | 183 (4.19) | 50 (5.86) | 125 (4.44) | 8 (1.53) | |
| High school | 828 (30.89) | 202 (44.74) | 582 (32.97) | 44 (8.62) | |
| More than high school | 1358 (64.92) | 186 (49.40) | 930 (62.60) | 242 (89.85) | |
| Marital status, n (%) | 0.010 | ||||
| Coupled | 1505 (68.90) | 259 (62.69) | 1032 (68.86) | 214 (75.07) | |
| Single or separated | 864 (31.10) | 179 (37.31) | 605 (31.14) | 80 (24.93) | |
| PIR, mean (SE) | 3.22 (0.13) | 2.39 (0.14) | 3.19 (0.12) | 4.13 (0.15) | <0.001 |
| PIR levels, n (%) | <0.001 | ||||
| ≤1.30 | 703 (18.75) | 186 (30.79) | 479 (18.79) | 38 (6.90) | |
| 1.31–3.50 | 813 (33.03) | 174 (45.77) | 569 (33.99) | 70 (16.57) | |
| >3.50 | 853 (48.23) | 78 (23.43) | 589 (47.22) | 186 (76.52) | |
| eGFR, mL/min per 1.73 m2 | 84.68 (0.50) | 85.22 (0.73) | 84.00 (0.66) | 87.05 (0.95) | 0.020 |
| Serum creatinine, μmol/L | 81.29 (0.76) | 81.68 (1.32) | 82.16 (1.06) | 77.21 (1.14) | 0.010 |
| Serum uric acid, μmol/L | 318.95 (1.48) | 341.07 (7.08) | 320.83 (2.30) | 289.47 (5.40) | 0.002 |
| Serum phosphorus, mmol/L | 1.23 (0.01) | 1.24 (0.01) | 1.22 (0.01) | 1.24 (0.02) | 0.210 |
| Serum total calcium, mmol/L | 2.36 (0.00) | 2.37 (0.00) | 2.36 (0.00) | 2.35 (0.01) | 0.080 |
| AAC score, mean (SE) | 1.41 (0.13) | 1.59 (0.18) | 1.58 (0.17) | 0.50 (0.10) | <0.001 |
| AAC†, n (%) | 693 (27.47) | 148 (31.06) | 497 (30.29) | 48 (11.93) | <0.001 |
| Severe AAC‡, n (%) | 213 (7.72) | 44 (8.22) | 157 (8.77) | 12 (2.74) | 0.010 |
AAC indicates abdominal aortic calcification; CVH, cardiovascular health; eGFR, estimated glomerular filtration rate; and PIR, poverty income ratio.
Data are presented as weighted mean (SE) for continuous variables and unweighted frequencies (weighted percentages) for categorical variables; low CVH was defined as an LE8 score of 0 to 49, moderate CVH, 50 to 79, and high CVH, 80 to 100.
AAC was considered present when the total AAC score was >0.
Severe AAC was determined by the total AAC score that exceeded 6.
Associations Between LE8 and Its Components With AAC
Multivariable regression models were used to explore the relationships between LE8 and AAC. Model 1 was unadjusted for any covariates, and model 2 included adjustments for age (continuous), sex, race and ethnicity, education levels, and marital status. Model 3 was further adjusted for PIR (continuous), estimated glomerular filtration rate, serum creatinine, serum uric acid, serum phosphorus, and serum total calcium.
Results from the fully adjusted multiple linear regression in Table S4 illustrate an inverse link between LE8 and AAC score. The multivariable‐adjusted restricted cubic spline regression analysis revealed that there were inverse dose–response associations between LE8 score (P for nonlinearity=0.027; Figure 2A) and its components (Figure S1) with AAC score, and the minimum beneficial thresholds were found to be 62.9 (β‐coefficient=0). These results demonstrated that following higher CVH may have potential benefits in reducing AAC score. Further analysis (Table S4) revealed significant associations between higher nicotine exposure score (β=−0.07 [95% CI, −0.12 to −0.03]), blood glucose score (β=−0.09 [95% CI, −0.15 to −0.03]), blood pressure score (β=−0.05 [95% CI, −0.10 to 0.00]) of LE8 components, and lower AAC score in model 3. The newly included sleep score in LE8 components demonstrated no significant association with AAC scores. Additionally, the outcome of the subgroup analysis in Table S5 indicates a robust inverse association between LE8 components and AAC score among various subgroups of the population, and the results were consistent with the primary findings.
Figure 2. The nonlinear associations between LE8 and AAC.
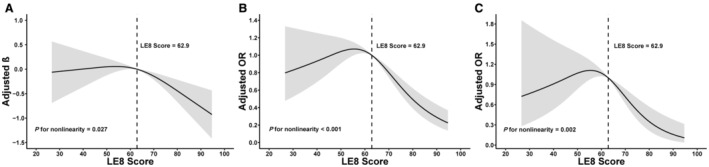
Dose–response relationships Life's Essential 8 score with AAC score (A), AAC (B), and severe AAC (C). β/OR (solid lines) and 95% CIs (shaded areas) were adjusted for age (as a continuous variable), sex, race and ethnicity, poverty income ratio (as a continuous variable), education levels, marital status, eGFR, serum creatinine, serum uric acid, serum phosphorus, and serum total calcium. Vertical dotted lines indicate the minimal threshold for the beneficial association with estimated β=0 or OR=1. AAC indicates abdominal aortic calcification; eGFR, estimated glomerular filtration rate; LE8, Life's Essential 8; and OR, odds ratio.
Multiple logistic regression in Table 2 reveals that compared with the low‐CVH group, the odds ratios (ORs) for AAC and severe AAC were 0.41 (95% CI, 0.22–0.74) and 0.38 (95% CI, 0.11–1.24), respectively, in the high‐CVH group. According to the findings in Table S6, when considering moderate CVH as the reference, the ORs for AAC and severe AAC in the high‐CVH group were 0.42 (95% CI, 0.26–0.70) and 0.42 (95% CI, 0.17–1.02), respectively. Additionally, the results in Table 3 indicate that elevated nicotine exposure score, blood glucose score, and blood pressure score within the LE8 component may potentially have beneficial effects in reducing the risk of AAC.
Table 2.
Associations Between LE8 and Both AAC and Severe AAC*
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| LE8 score† | ||||||
| Low (0–49) | Reference | / | Reference | / | Reference | / |
| Moderate (50–79) | 0.96 (0.62–1.50) | 0.861 | 0.91 (0.49–1.71) | 0.722 | 0.96 (0.60–1.55) | 0.867 |
| High (80–100) | 0.30 (0.19–0.47) | <0.001 | 0.37 (0.17–0.78) | 0.021 | 0.41 (0.22–0.74) | 0.006 |
| LE8 score‡ | ||||||
| Low (0–49) | Reference | / | Reference | / | Reference | / |
| Moderate (50–79) | 1.07 (0.67–1.72) | 0.749 | 0.86 (0.43–1.71) | 0.570 | 0.90 (0.55–1.49) | 0.670 |
| High (80–100) | 0.31 (0.11–0.90) | 0.034 | 0.36 (0.08–1.70) | 0.141 | 0.38 (0.11–1.24) | 0.100 |
AAC indicates abdominal aortic calcification; LE8, Life's Essential 8; and OR, odds ratio.
Model 1 was unadjusted for any covariates, and Model 2 included adjustments for age (continuous), sex, race and ethnicity, education levels, and marital status. Model 3 was further adjusted for poverty income ratio (continuous), estimated glomerular filtration rate, serum creatinine, serum uric acid, serum phosphorus, and serum total calcium.
The association between LE8 and AAC.
The association between LE8 and severe AAC.
Table 3.
Associations Between Components of LE8 and Both AAC and Severe AAC*
| OR† (95% CI) | P value | OR‡ (95% CI) | P value | |
|---|---|---|---|---|
| HEI‐2015 diet score | ||||
| Low (0–49) | Reference | / | Reference | / |
| Moderate (50–79) | 0.86 (0.67–1.10) | 0.207 | 1.17 (0.76–1.81) | 0.447 |
| High (80–100) | 0.67 (0.24–1.87) | 0.419 | 0.50 (0.14–1.79) | 0.262 |
| Physical activity score | ||||
| Low (0–49) | Reference | / | Reference | / |
| Moderate (50–79) | 1.64 (0.92–2.93) | 0.090 | 1.76 (0.62–4.95) | 0.265 |
| High (80–100) | 0.74 (0.53–1.05) | 0.091 | 1.21 (0.63–2.31) | 0.546 |
| Nicotine exposure score | ||||
| Low (0–49) | Reference | / | Reference | / |
| Moderate (50–79) | 0.61 (0.42–0.89) | 0.013 | 0.57 (0.31–1.05) | 0.070 |
| High (80–100) | 0.44 (0.26–0.73) | 0.003 | 0.27 (0.13–0.58) | 0.002 |
| Sleep health score | ||||
| Low (0–49) | Reference | / | Reference | … |
| Moderate (50–79) | 0.85 (0.60–1.20) | 0.337 | 1.28 (0.56–2.95) | 0.535 |
| High (80–100) | 0.81 (0.53–1.23) | 0.290 | 1.12 (0.54–2.34) | 0.748 |
| Body mass index score | ||||
| Low (0–49) | Reference | / | Reference | … |
| Moderate (50–79) | 1.32 (1.03–1.69) | 0.029 | 1.77 (1.02–3.06) | 0.043 |
| High (80–100) | 1.20 (0.83–1.74) | 0.31 | 0.96 (0.42–2.18) | 0.910 |
| Blood glucose score | ||||
| Low (0–49) | Reference | / | Reference | … |
| Moderate (50–79) | 0.63 (0.48–0.82) | 0.002 | 0.43 (0.28–0.65) | <0.001 |
| High (80–100) | 0.68 (0.47–0.99) | 0.044 | 0.36 (0.22–0.56) | <0.001 |
| Blood lipids score | ||||
| Low (0–49) | Reference | / | Reference | … |
| Moderate (50–79) | 0.73 (0.46–1.15) | 0.157 | 0.68 (0.39–1.16) | 0.143 |
| High (80–100) | 0.82 (0.56–1.18) | 0.260 | 0.88 (0.52–1.51) | 0.629 |
| Blood pressure score | ||||
| Low (0–49) | Reference | / | Reference | … |
| Moderate (50–79) | 0.79 (0.57–1.10) | 0.151 | 0.91 (0.58–1.42) | 0.648 |
| High (80–100) | 0.79 (0.53–1.18) | 0.237 | 0.48 (0.34–0.67) | <0.001 |
AAC indicates abdominal aortic calcification; HEI, Healthy Eating Index; LE8, Life's Essential 8; and OR, odds ratio.
Adjusted for age (continuous), sex, race and ethnicity, education levels, marital status, poverty income ratio (continuous), estimated glomerular filtration rate, serum creatinine, serum uric acid, serum phosphorus, and serum total calcium.
The association between components of LE8 and AAC.
The association between components of LE8 and severe AAC.
The multivariable‐adjusted restricted cubic spline regression analysis reveals that there were significant nonlinear associations between LE8 score and both AAC (P for nonlinearity <0.001; Figure 2B) and severe AAC (P for nonlinearity=0.002; Figure 2C), and the minimum beneficial thresholds were found to be 62.9 (OR, 1). Furthermore, Figures S2 and S3 illustrate the dose–response relationships between the LE8 component and the risk of AAC and severe AAC. The findings indicate that maintaining an LE8 score of ≥62.9 may be associated with a lower risk of AAC and severe AAC.
Subgroup and Sensitivity Analysis
The outcome of the subgroup analysis in Table S7 indicates a robust inverse association between LE8 and both AAC and severe AAC among various subgroups of the population, and the results are consistent with the primary findings. The outcome of the sensitivity analysis was also robust (Table 4). After excluding participants with histories of CVD from sensitivity analyses, the fully adjusted multivariable logistic regression models demonstrated that ORs for AAC and severe AAC were 0.43 (95% CI, 0.24–0.78) and 0.32 (95% CI, 0.08–1.18) in high CVH separately, relative to the low CVH.
Table 4.
Sensitivity Analysis for the Associations Between LE8 and Both AAC and Severe AAC
| Excluding participants with history of CVD | ||
|---|---|---|
| OR* (95% CI) | P value | |
| LE8 score† | ||
| Low (0–49) | Reference | / |
| Moderate (50–79) | 0.99 (0.63, 1.55) | 0.945 |
| High (80–100) | 0.43 (0.24– 0.78) | 0.009 |
| LE8 score‡ | ||
| Low (0–49) | Reference | / |
| Moderate (50–79) | 0.77 (0.44–1.33) | 0.322 |
| High (80–100) | 0.32 (0.08–1.18) | 0.083 |
AAC indicates abdominal aortic calcification; LE8, Life's Essential 8; and OR, odds ratio.
Adjusted for age (continuous), sex, race and ethnicity, education levels, marital status, poverty income ratio (continuous), estimated glomerular filtration rate, serum creatinine, serum uric acid, serum phosphorus, and serum total calcium.
The association between LE8 and AAC.
The association between LE8 and severe AAC.
Discussion
In this nationwide investigation, a significant relationship was discovered between higher LE8 scores and lower AAC scores, along with significant associations between higher nicotine exposure scores, blood glucose scores, blood pressure scores, and lower AAC scores. Additionally, the study found that individuals in the high‐CVH group had lower risks of developing AAC and severe AAC. Subgroup and sensitivity analyses demonstrated that the negative associations between LE8 and both AAC and severe AAC were broadly consistent with the overall results.
For now, this is the first study to elucidate novel inverse dose–response relationships between CVH and both AAC and severe AAC by employing LE8 to assess CVH levels. The ORs associated with AAC and severe AAC remained stable in the lower range of LE8 scores but decreased sharply in the higher range, suggesting that adherence to a higher LE8 score may confer greater protection against AAC and severe AAC.
Furthermore, participants with higher scores for nicotine exposure, blood pressure, and blood glucose had lower AAC scores, which was consistent with previous research findings. Research in 2021 revealed that activation of α7 and α3 nicotinic acetylcholine receptors by nicotine increased intracellular Ca2+ and initiated calcification of human vascular smooth muscle cells by upward Nox5 activity, resulting in oxidative stress–mediated extracellular vesicle release. 23 , 24 This research provided evidence that nicotine induces Nox5‐mediated procalcific processes as a novel mechanism of increased atherosclerotic calcification. Another clinical study demonstrated that pulse pressure was a robust and important correlate for calcified atherosclerosis in different vascular beds. 25 Several studies also demonstrated that sustained hyperglycemia in patients with diabetes can lead to the abundant production and cumulation of advanced glycation end products. 26 , 27 The binding of advanced glycation end products with their respective receptors can activate multiple cellular signaling pathways, thereby promoting vascular calcification. 27 In addition, hyperglycemia can upregulate the expression of runt‐related transcription factor‐2 by increasing oxidative stress, thus stimulating the calcification of vascular smooth muscle cells. 28 , 29 , 30 These findings imply that avoiding nicotine exposure and maintaining healthy blood glucose and blood pressure levels may have a beneficial impact on reducing the risk of AAC.
Although the underlying mechanism between LE8 and AAC remain elusive, extensive investigations have revealed the crucial roles that metabolic syndrome and lifestyles play in the development of AAC, 31 , 32 , 33 both of which were underlying health factors and indicators of healthy behaviors in LE8. Elevated chronic inflammation and oxidative stress during metabolic syndrome stimulate the synthesis of tumor necrosis factor‐α and interleukins. 34 The release of microparticles containing bone morphogenetic protein‐2 by endothelial cells following stimulation with tumor necrosis factor‐α facilitates osteogenesis when phagocytosed by vascular smooth muscle cells, implicating chronic inflammation in promoting arterial calcification. 35 Additionally, bone morphogenetic protein‐2 functions as a proinflammatory cytokine that stimulates endothelial activation, hinting at the possibility that these focal inflammatory disturbances might feed into a self‐perpetuating cycle that aggravates vascular calcification. 36 As a cytokine secreted by activated macrophages, interleukin‐1β is involved in regulating phosphate metabolism. It increases the expression of tissue‐nonspecific alkaline phosphatase in vascular smooth muscle cells, independently of runt‐related transcription factor‐2, leading to a decrease in extracellular pyrophosphate levels and driving vascular calcification. 37 Therefore, these findings may provide potential mechanistic explanations for the role of LE8 in the development of AAC.
Our finding was consistent with the current knowledge that aortic calcification is inversely associated with CVH levels. A US‐based multiethnic cohort revealed that higher Life's Simple 7 levels were associated with 57%, 56%, 70%, and 54% lower risk of incident aortic valve calcification, mitral annulus calcification, ascending thoracic aorta calcification, and descending thoracic aorta calcification, respectively. 38 However, as a predecessor to LE8, Life's Simple 7 has certain limitations. For instance, Life's Simple 7 is less sensitive to individual variations and cannot be used to assess dose–response effects. The updated LE8, on the other hand, addresses these shortcomings. 12 This study, by using LE8 as the definition for CVH, not only adds notable evidence of the relationship between CVH and AAC but also broadens the scope of health effects relevant to optimal CVH on AAC and severe AAC. In brief, our findings demonstrate that emphasizing optimal CVH may reduce the burden of AAC.
The results of this study may offer important guidance for managing and preventing the risk of AAC and severe AAC. Furthermore, CVH and AAC were studied concerning the dose–response relationship, and the minimum threshold for the salutary association was identified. Nevertheless, several limitations should be taken into account in this study. First, despite controlling for several potential confounding variables, the nature of the cross‐sectional investigation precluded us from concluding the causal relationship between LE8 and AAC. The longitudinal and causal relationship between LE8 and AAC risk needs further study. Second, the assessment of some indicators in the LE8 component was based on questionnaire surveys, which may easily lead to estimation errors. Third, the effect of nonrandom missing data on the results is unable to be excluded due to differences in baseline between included and excluded individuals. Finally, the generalizability of the associations identified in this study to younger individuals or populations from other countries remains uncertain and warrants additional research.
Conclusions
LE8 may be negatively and nonlinearly related to the risk of AAC among middle‐aged and older populations. Meanwhile, the LE8 component should prioritize higher scores for nicotine exposure, blood glucose, and blood pressure evaluations. These findings suggest that LE8, as practical and salutary comprehensive indicators for improving CVH, may be applied in clinical practice to help patients, as well as the general population, identify the risk of AAC early and minimize the burden of AAC.
Sources of Funding
This study was financially supported by the Joint Co‐construction Project of Henan Medical Science and Technology Research Plan (No. LHGJ20200342), the Key Research & Development and Promotion of Special Project (Scientific Problem Tackling) of Henan Province (No. 202102310122).
Disclosures
None.
Supporting information
Tables S1‐S7Figure S1‐S3
This manuscript was sent to Amgad Mentias, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.031146
For Sources of Funding and Disclosures, see page 8.
References
- 1. Quaglino D, Boraldi F, Lofaro FD. The biology of vascular calcification. Int Rev Cell Mol Biol. 2020;354:261–353. doi: 10.1016/bs.ircmb.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 2. Rodondi N, Taylor BC, Bauer DC, Lui LY, Vogt MT, Fink HA, Browner WS, Cummings SR, Ensrud KE. Association between aortic calcification and total and cardiovascular mortality in older women. J Intern Med. 2007;261:238–244. doi: 10.1111/j.1365-2796.2007.01769.x [DOI] [PubMed] [Google Scholar]
- 3. Bastos Gonçalves F, Voûte MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ, Verhagen HJ. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta‐analysis. Heart. 2012;98:988–994. doi: 10.1136/heartjnl-2011-301464 [DOI] [PubMed] [Google Scholar]
- 4. Harbaugh CM, Terjimanian MN, Lee JS, Alawieh AZ, Kowalsky DB, Tishberg LM, Krell RW, Holcombe SA, Wang SC, Campbell DA Jr, et al. Abdominal aortic calcification and surgical outcomes in patients with no known cardiovascular risk factors. Ann Surg. 2013;257:774–781. doi: 10.1097/SLA.0b013e31826ddd5f [DOI] [PubMed] [Google Scholar]
- 5. Leow K, Szulc P, Schousboe JT, Kiel DP, Teixeira‐Pinto A, Shaikh H, Sawang M, Sim M, Bondonno N, Hodgson JM, et al. Prognostic value of abdominal aortic calcification: a systematic review and meta‐analysis of observational studies. J Am Heart Assoc. 2021;10:e017205. doi: 10.1161/jaha.120.017205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Connor SD, Graffy PM, Zea R, Pickhardt PJ. Does nonenhanced CT‐based quantification of abdominal aortic calcification outperform the Framingham risk score in predicting cardiovascular events in asymptomatic adults? Radiology. 2019;290:108–115. doi: 10.1148/radiol.2018180562 [DOI] [PubMed] [Google Scholar]
- 7. Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25‐year follow‐up study. Atherosclerosis. 1997;132:245–250. doi: 10.1016/s0021-9150(97)00106-8 [DOI] [PubMed] [Google Scholar]
- 8. Mäkelä S, Asola M, Hadimeri H, Heaf J, Heiro M, Kauppila L, Ljungman S, Ots‐Rosenberg M, Povlsen JV, Rogland B, et al. Abdominal aortic calcifications predict survival in peritoneal dialysis patients. Perit Dial Int. 2018;38:366–373. doi: 10.3747/pdi.2017.00043 [DOI] [PubMed] [Google Scholar]
- 9. Choi SR, Lee YK, Cho AJ, Park HC, Han CH, Choi MJ, Koo JR, Yoon JW, Noh JW. Malnutrition, inflammation, progression of vascular calcification and survival: inter‐relationships in hemodialysis patients. PLoS One. 2019;14:e0216415. doi: 10.1371/journal.pone.0216415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bai J, Zhang A, Zhang Y, Ren K, Ren Z, Zhao C, Wang Q, Cao N. Abdominal aortic calcification score can predict all‐cause and cardiovascular mortality in maintenance hemodialysis patients. Ren Fail. 2023;45:2158869. doi: 10.1080/0886022x.2022.2158869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu J, Li H, Wang S. The kidney reabsorption‐related magnesium depletion score is associated with increased likelihood of abdominal aortic calcification among US adults. Nephrol Dial Transplant. 2023;38:1421–1429. doi: 10.1093/ndt/gfac218 [DOI] [PubMed] [Google Scholar]
- 12. Lloyd‐Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, et al. Life's essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/cir.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang X, Ma H, Li X, Heianza Y, Manson JE, Franco OH, Qi L. Association of Cardiovascular Health with Life Expectancy Free of cardiovascular disease, diabetes, cancer, and dementia in UK adults. JAMA Intern Med. 2023;183:340–349. doi: 10.1001/jamainternmed.2023.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun J, Li Y, Zhao M, Yu X, Zhang C, Magnussen CG, Xi B. Association of the American Heart Association's new "Life's essential 8" with all‐cause and cardiovascular disease‐specific mortality: prospective cohort study. BMC Med. 2023;21:116. doi: 10.1186/s12916-023-02824-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun Y, Yu Y, Zhang K, Yu B, Yu Y, Wang Y, Wang B, Tan X, Wang Y, Lu Y, et al. Association between Life's essential 8 score and risk of premature mortality in people with and without type 2 diabetes: a prospective cohort study. Diabetes Metab Res Rev. 2023;39:e3636. doi: 10.1002/dmrr.3636 [DOI] [PubMed] [Google Scholar]
- 16. Ma H, Wang X, Xue Q, Li X, Liang Z, Heianza Y, Franco OH, Qi L. Cardiovascular health and life expectancy among adults in the United States. Circulation. 2023;147:1137–1146. doi: 10.1161/circulationaha.122.062457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/s0140-6736(07)61602-x [DOI] [PubMed] [Google Scholar]
- 18. Lloyd‐Jones DM, Ning H, Labarthe D, Brewer L, Sharma G, Rosamond W, Foraker RE, Black T, Grandner MA, Allen NB, et al. Status of cardiovascular health in US adults and children using the American Heart Association's new "Life's essential 8" metrics: prevalence estimates from the National Health and nutrition examination survey (NHANES), 2013 through 2018. Circulation. 2022;146:822–835. doi: 10.1161/circulationaha.122.060911 [DOI] [PubMed] [Google Scholar]
- 19. Krebs‐Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, Wilson MM, Reedy J. Update of the healthy eating index: HEI‐2015. J Acad Nutr Diet. 2018;118:1591–1602. doi: 10.1016/j.jand.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lewis JR, Schousboe JT, Lim WH, Wong G, Wilson KE, Zhu K, Thompson PL, Kiel DP, Prince RL. Long‐term atherosclerotic vascular disease risk and prognosis in elderly women with abdominal aortic calcification on lateral spine images captured during bone density testing: a prospective study. J Bone Miner Res. 2018;33:1001–1010. doi: 10.1002/jbmr.3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson PW, Kauppila LI, O'Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.cir.103.11.1529 [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petsophonsakul P, Burgmaier M, Willems B, Heeneman S, Stadler N, Gremse F, Reith S, Burgmaier K, Kahles F, Marx N, et al. Nicotine promotes vascular calcification via intracellular Ca2+−mediated, Nox5‐induced oxidative stress, and extracellular vesicle release in vascular smooth muscle cells. Cardiovasc Res. 2022;118:2196–2210. doi: 10.1093/cvr/cvab244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Furmanik M, Chatrou M, van Gorp R, Akbulut A, Willems B, Schmidt H, van Eys G, Bochaton‐Piallat ML, Proudfoot D, Biessen E, et al. Reactive oxygen‐forming Nox5 links vascular smooth muscle cell phenotypic switching and extracellular vesicle‐mediated vascular calcification. Circ Res. 2020;127:911–927. doi: 10.1161/circresaha.119.316159 [DOI] [PubMed] [Google Scholar]
- 25. Jensky NE, Criqui MH, Wright MC, Wassel CL, Brody SA, Allison MA. Blood pressure and vascular calcification. Hypertension. 2010;55:990–997. doi: 10.1161/hypertensionaha.109.147520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121 [DOI] [PubMed] [Google Scholar]
- 27. Kay AM, Simpson CL, Stewart JA Jr. The role of AGE/RAGE signaling in diabetes‐mediated vascular calcification. J Diabetes Res. 2016;2016:6809703. doi: 10.1155/2016/6809703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a [DOI] [PubMed] [Google Scholar]
- 29. Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM, Javed A, Zhang K, Anderson PG, Chen Y. Smooth muscle cell‐specific runx2 deficiency inhibits vascular calcification. Circ Res. 2012;111:543–552. doi: 10.1161/circresaha.112.267237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Byon CH, Sun Y, Chen J, Yuan K, Mao X, Heath JM, Anderson PG, Tintut Y, Demer LL, Wang D, et al. Runx2‐upregulated receptor activator of nuclear factor κB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler Thromb Vasc Biol. 2011;31:1387–1396. doi: 10.1161/atvbaha.110.222547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang SW, Yang HF, Chen YY, Chen WL. Unraveling the link between metabolic syndrome and abdominal aortic calcification. Nutr Metab Cardiovasc Dis. 2021;31:464–471. doi: 10.1016/j.numecd.2020.10.003 [DOI] [PubMed] [Google Scholar]
- 32. Chen W, Eisenberg R, Mowrey WB, Wylie‐Rosett J, Abramowitz MK, Bushinsky DA, Melamed ML. Association between dietary zinc intake and abdominal aortic calcification in US adults. Nephrol Dial Transplant. 2020;35:1171–1178. doi: 10.1093/ndt/gfz134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bondonno NP, Lewis JR, Prince RL, Lim WH, Wong G, Schousboe JT, Woodman RJ, Kiel DP, Bondonno CP, Ward NC, et al. Fruit intake and abdominal aortic calcification in elderly women: a prospective cohort study. Nutrients. 2016;8:159. doi: 10.3390/nu8030159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13:851–863. doi: 10.5114/aoms.2016.58928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buendía P, Montes de Oca A, Madueño JA, Merino A, Martín‐Malo A, Aljama P, Ramírez R, Rodríguez M, Carracedo J. Endothelial microparticles mediate inflammation‐induced vascular calcification. Faseb J. 2015;29:173–181. doi: 10.1096/fj.14-249706 [DOI] [PubMed] [Google Scholar]
- 36. Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein‐2 induces proinflammatory endothelial phenotype. Am J Pathol. 2006;168:629–638. doi: 10.2353/ajpath.2006.050284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lencel P, Delplace S, Pilet P, Leterme D, Miellot F, Sourice S, Caudrillier A, Hardouin P, Guicheux J, Magne D. Cell‐specific effects of TNF‐α and IL‐1β on alkaline phosphatase: implication for syndesmophyte formation and vascular calcification. Lab Invest. 2011;91:1434–1442. doi: 10.1038/labinvest.2011.83 [DOI] [PubMed] [Google Scholar]
- 38. Ogunmoroti O, Osibogun O, Mathews L, Esuruoso OA, Ndumele CE, Okunrintemi V, Burke GL, Blumenthal RS, Budoff MJ, Michos ED. Favorable cardiovascular health is associated with lower prevalence, incidence, extent, and progression of extracoronary calcification: MESA. Circ Cardiovasc Imaging. 2022;15:e013762. doi: 10.1161/circimaging.121.013762 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S7Figure S1‐S3


