Abstract
Background
Chronic kidney disease (CKD) is closely associated with cardiovascular disease. We aimed to examine the association of Life's Essential 8 (LE8), the recently updated measurement of cardiovascular health, with the prevalence of CKD among US adults.
Methods and Results
This population‐based cross‐sectional study used data from the National Health and Nutrition Examination Survey from 2007 to 2018 and included adults aged ≥20 years. Multivariable logistic and restricted cubic spline models were used to assess the associations between LE8 and CKD. Among 24 960 participants, 4437 were determined to have CKD (weighted percentage, 14.11%). After the adjustment of potential confounders, higher LE8 scores were associated with reduced odds of CKD (odds ratio for each 10‐point increase, 0.79 [95% CI, 0.76–0.83]), and a nonlinear dose–response relationship was observed. Similar patterns were also identified in the associations of health behavior and health factor scores with CKD. Meanwhile, higher scores for blood glucose (odds ratio, for each 10‐point increase, 0.88 [95% CI, 0.87–0.90]) and blood pressure (odds ratio, for each 10‐point increase, 0.92 [95% CI, 0.91–0.94]) in the LE8 component are significantly associated with a lower prevalence of CKD. The inversed association of LE8 score and CKD was significantly stronger among middle‐aged, male, and coupled participants.
Conclusions
LE8 was negatively associated with the prevalence of CKD in a nonlinear fashion. Promoting adherence to optimal cardiovascular health levels may be beneficial to reduce the burden of CKD.
Keywords: cardiovascular health, chronic kidney disease, Life's essential 8, NHANES
Subject Categories: Lifestyle
Clinical Perspective.
What Is New?
This cross‐sectional study, which included a nationally representative sample of US adults, found that Life's Essential 8 was negatively associated with the prevalence of chronic kidney disease in a nonlinear fashion.
Subgroup analyses further revealed stronger negative associations between Life's Essential 8 scores and chronic kidney disease in middle‐aged, male, and coupled populations.
What Are the Clinical Implications?
These findings hint that Life's Essential 8 may have clinical application as a functional and utilitarian composite indicator for improving kidney health.
Recognizing the inherent variability in the components of Life's Essential 8 may have important clues for enhancing renal function.
Nonstandard Abbreviations and Acronyms
- AHA
American Heart Association
- ARIC
Atherosclerosis Risk in Communities
- CVH
cardiovascular health
- HEI
Healthy Eating Index
- LE8
Life's Essential 8
- LS7
Life's Simple 7
- NHANES
National Health and Nutrition Examination Survey
Chronic kidney disease (CKD) is caused by various underlying disease mechanisms that irreversibly impair the functional structure of the kidney. 1 Approximately 11% of the general US population is affected by CKD, which increases the risk of cardiovascular disease (CVD). 2 , 3 , 4 Meanwhile, over a third of US adults die of CVDs each year. 5 As CKD progresses, the susceptibility of CKD patients to CVD increases. Accumulating evidence has indicated that CKD significantly increases the risk of CVD. 6 , 7 , 8 , 9
The American Heart Association (AHA) initiated Life's Simple 7 (LS7) as a measure to enhance the cardiovascular health (CVH) of the general population. 10 LS7 has emerged as a powerful tool for assessing CVH, and extensive studies have demonstrated a significant, progressive, negative link between the ideal number of CVH indicators or overall CVH score and total CVD, CVD death, all‐cause death, and various non‐CVD outcomes. 11 , 12 , 13 , 14 The components of LS7 included 3 health behaviors (diet, physical activity, and exposure to cigarette smoking) and 4 health factors (body mass index, fasting blood glucose, total cholesterol, and blood pressure). Each metric was classified as poor, intermediate, or ideal on the basis of generally accepted clinical thresholds. 10 Overall, ideal CVH was defined as having all 7 metrics at ideal levels. Nevertheless, the use of LS7 evaluations for the complete range of health behaviors under current settings may be inappropriate due to some characteristics of the LS7 component definitions, and also the original definition of each component of LS7 cannot adequately and fully reflect the interindividual variation and intraindividual variability. 11
Therefore, the AHA recently introduced a revised ideal CVH measure, called Life's Essential 8 (LE8), which added sleep as an eighth CVH metric and updated the scoring of CVH metrics. 11 The LE8 is a scoring system that is more sensitive to interindividual differences and intraindividual variation, emphasizing mental health to maintain or enhance CVH. 15 , 16 Considering the tight link between CKD and CVD, facilitating LE8 may be a prophylactic and managerial measure to diminish the burden of CKD. Increasing studies have demonstrated that higher LS7 levels are associated with a reduced risk of CKD, 17 , 18 , 19 while the association between LE8 and CKD remains elusive.
This cross‐sectional study investigated whether LE8 scores were related to CKD risk among US adults on the basis of data from the National Health and Nutrition Examination Survey (NHANES). We also analyzed the relevance between LE8 scores and CKD at different levels of age strata, sex, race and ethnicity, education levels, poverty, and marital status.
Methods
All data are publicly available and can be accessed at the NHANES (https:// wwwn.cdc.gov/nchs/nhanes/Default.aspx). Relevant R code is available upon reasonable request to the corresponding author.
Study Participants
NHANES is a continuous cross‐sectional survey that focuses on estimating the prevalence of primary illnesses and disease‐specific risk factors in the United States. A detailed description of the survey is available at http://www.cdc.gov/nchs/nhanes.htm. NHANES employs a sophisticated multiperiod probability‐based sampling method to achieve a nationally representative sample. The National Center for Health Statistics Research Ethics Review Board approved all NHANES protocols, and all participants provided written informed consent. This cross‐sectional study used NHANES data from 2007 to 2018 and adhered to the guidelines for Strengthening the Reporting of Observational Studies in Epidemiology. 20
Among the 59 842 participants in NHANES 2007 to 2018, 34 770 were aged ≥20 years. Participants without complete data on serum creatinine or albumin‐to‐creatinine ratio (n=3506), those with missing data on CVH metrics (n=3931), and those with missing data on relevant demographic characteristics (n=2373) were eliminated. Ultimately, this study involved 24 960 adult participants (Figure S1).
Demographic Characteristics
During the home interviews, trained interviewers collected demographic information using computer‐assisted personal interviewing. Age was separated into 3 categories: 20 to 39 years, 40 to 59 years, and ≥60 years. Race and ethnicity were classified as Mexican American, Non‐Hispanic White, Non‐Hispanic Black, Other Hispanic, and other race/multiracial. The poverty ratio was estimated by dividing monthly family income by the poverty level and then classified into 4 groups: ≤1.30, 1.31 to 1.85, 1.86 to 3.50, and >3.50. 21 Education levels were categorized into 3 groups: less than high school, high school, and more than high school. Single/separated or coupled were the 2 marital status categories.
Diagnosis of CKD
In NHANES 2007 to 2016, serum and urinary creatinine were measured using the Jaffe rate methods, while in NHANES 2017 to 2018, serum and urinary creatinine were calculated with enzymatic methods. Urinary albumin was evaluated using a fluorescent immunoassay. The Chronic Kidney Disease Epidemiology Collaboration equation calculated the estimated glomerular filtration rate on the basis of serum creatinine as a sign of kidney function, 2 and albuminuria was calculated as the ratio of urine albumin to creatinine. According to current guidelines, CKD was defined as estimated glomerular filtration rate <60 mL/min per 1.73 m2 or albuminuria ≥30 mg/g, or both. 22
Measurement of LE8
The AHA has recently updated LS7 to LE8 to quantify CVH, which includes 4 health behaviors (diet, physical activity, nicotine exposure, and sleep health) and 4 health factors (body mass index, blood lipids, blood glucose, and blood pressure), as a way to significantly enhance guidance on improving CVH in the general population. The elaborated description of the calculation of scores for each metric of LE8 using NHANES data can be viewed in Table S1. 11 , 15 Overall, each of the 8 LE8 indicators was rated on a scale of 0 to 100 points, and the unweighted average of these 8 indicators was calculated to obtain the total LE8 score. The AHA recommended that participants with LE8 scores >80 were categorized as high CVH, those with LE8 scores of 50 to 79 were classified as moderate CVH, and those with LE8 scores <50 were grouped as low CVH.11 To further explore the association between the LE8 subscales and CKD, this study adopted the equal definition and assertion signs to classify LE8 subscale scores.
Healthy Eating Index 2015 was used to estimate the dietary metric. 23 Table S2 generalizes the constituents and criteria for scoring the Healthy Eating Index‐2015. The Healthy Eating Index‐2015 scores were calculated using information from the first 24‐hour dietetic recall interview completed during the mobile examination center visit, and if two 24‐hour recalls were available, the first one that provided diet data was used. Information on physical activity, nicotine exposure, and sleep health, as well as diabetes and medication history, was obtained through self‐report questionnaires. During the physical examination, participants were professionally measured for blood pressure, height, and weight. Body mass index was measured as weight in kilograms divided by the square of height in meters. Researchers collected blood samples for analysis of blood lipids, plasma glucose, and glycated hemoglobin at central laboratories.
Statistical Analysis
The data were analyzed following the analytical guidelines and recommended survey weights for NHANES data. Baseline characteristics were described using weighted means with SE for continuous variables and unweighted frequencies with weighted percentages for categorical variables. Participants were classified into CKD and non‐CKD groups on the basis of their CKD status, and baseline characteristics were compared using the t‐test for continuous variables and the Rao–Scott chi‐square test for categorical variables. Participants were categorized into 3 groups on the basis of LE8 scores, and age‐standardized prevalence estimates and SE were computed separately.
The independent association between LE8 and its components with CKD was investigated using survey‐weighted multivariable logistic regressions by adjusting for potential confounders. Additionally, the underlying nonlinear associations between LE8 and its subscales with CKD were examined by applying restricted cubic spline regression, and nonlinearity was evaluated with the likelihood ratio test.
To further investigate the association between LE8 and CKD in different populations, stratified analyses were implemented by age strata, sex, race and ethnicity, poverty ratio, education levels, and marital status. The significance of interactions was estimated using P values for the interaction coefficients between LE8 and subgroup populations. Furthermore, we excluded participants with self‐reported histories of CVD (including coronary heart disease, angina, congestive heart failure, and heart attack; n=2710) to evaluate the soundness of our results. All statistical analyses were performed using R version 4.2.1 software (R Foundation for Statistical Computing, Vienna, Austria). Statistical tests were 2‐sided, and statistical significance was assumed to be P<0.05.
Results
Baseline Characteristics
In this study, 24 960 adults aged ≥20 years were enrolled. Based on the prevalence of CKD, Table 1 generalizes the baseline characteristics of the study participants. The weighted mean (SE) age of the study population was 47.43 years (0.26), of which 12 660 participants were women (weighted percentage, 51.08%). The mean (SE) of the LE8 score was 65.83 (0.28), and the unweighted frequencies (weighted percentages) for low, medium, and high CVH were 4680 (18.75%), 16 591 (66.47%), and 3689 (14.78%), respectively. There were 4437 participants (weighted percentage, 14.11%) with CKD. Populations with CKD were older, more likely to be women, non‐Hispanic White, and single or separated, and had lower education and poverty ratio levels versus those with non‐CKD. Compared with individuals with CKD, participants without CKD had higher scores in LE8, physical activity, sleep health, body mass index, blood glucose, blood pressure, and blood lipids.
Table 1.
Baseline Characteristics of the Study Population*
| Characteristics | Overall | Non‐CKD | CKD | P value |
|---|---|---|---|---|
| Participant number | 24 960 | 20 523 | 4437 | … |
| Age, y, mean (SE) | 47.43 (0.26) | 45.28 (0.26) | 60.55 (0.36) | <0.0001 |
| Age strata, y, n (%) | ||||
| 20–39 | 8323 (35.98) | 7800 (39.44) | 523 (14.89) | <0.0001 |
| 40–59 | 8404 (38.08) | 7402 (40.06) | 1002 (26.04) | |
| ≥60 | 8233 (25.94) | 5321 (20.50) | 2912 (59.07) | |
| Sex, n (%) | ||||
| Male | 12 300 (48.92) | 10 186 (49.91) | 2114 (42.86) | <0.0001 |
| Female | 12 660 (51.08) | 10 337 (50.09) | 2323 (57.14) | |
| Race and ethnicity, n (%) | ||||
| Mexican American | 3689 (8.14) | 3128 (8.36) | 561 (6.84) | <0.0001 |
| Non‐Hispanic White | 10 925 (68.93) | 8756 (68.57) | 2169 (71.12) | |
| Non‐Hispanic Black | 5047 (10.17) | 4075 (9.94) | 972 (11.58) | |
| Other Hispanic | 2501 (5.42) | 2131 (5.60) | 370 (4.33) | |
| Other race/multiracial | 2798 (7.34) | 2433 (7.54) | 365 (6.13) | |
| Poverty ratio, n (%) | ||||
| ≤1.30 | 7903 (21.15) | 6379 (20.61) | 1524 (24.44) | <0.0001 |
| 1.31–1.85 | 3381 (10.55) | 2667 (9.97) | 714 (14.07) | |
| 1.86–3.50 | 6022 (24.99) | 4876 (24.61) | 1146 (27.29) | |
| >3.50 | 7654 (43.31) | 6601 (44.80) | 1053 (34.20) | |
| Education levels, n (%) | ||||
| Less than high school | 2317 (4.69) | 1704 (4.15) | 613 (7.96) | <0.0001 |
| High school | 9102 (32.91) | 7323 (32.05) | 1779 (38.14) | |
| More than high school | 13 541 (62.41) | 11 496 (63.81) | 2045 (53.90) | |
| Marital status, n (%) | ||||
| Coupled | 14 936 (63.88) | 12 506 (64.60) | 2430 (59.49) | <0.0001 |
| Single or separated | 10 024 (36.12) | 8017 (35.40) | 2007 (40.51) | |
| LE8 score, mean (SE) | 65.83 (0.28) | 66.99 (0.28) | 58.78 (0.41) | <0.0001 |
| HEI‐2015 diet score | 50.80 (0.23) | 50.71 (0.24) | 51.32 (0.33) | 0.0400 |
| Physical activity score | 49.92 (0.81) | 52.05 (0.82) | 36.94 (1.21) | <0.0001 |
| Nicotine exposure score | 71.64 (0.51) | 71.31 (0.52) | 73.61 (0.83) | 0.0020 |
| Sleep health score | 83.33 (0.30) | 83.62 (0.32) | 81.56 (0.49) | <0.0010 |
| Body mass index score | 59.99 (0.43) | 61.00 (0.43) | 53.79 (0.76) | <0.0001 |
| Blood glucose score | 77.63 (0.30) | 80.08 (0.29) | 62.67 (0.70) | <0.0001 |
| Blood pressure score | 68.39 (0.36) | 71.59 (0.38) | 48.95 (0.75) | <0.0001 |
| Blood lipids score | 64.99 (0.35) | 65.58 (0.37) | 61.39 (0.65) | <0.0001 |
| CVH†, n (%) | ||||
| Low (0–49) | 4680 (18.75) | 3198 (13.01) | 1482 (28.75) | <0.0001 |
| Moderate (50–79) | 16 591 (66.47) | 13 884 (66.56) | 2707 (62.38) | |
| High (80–100) | 3689 (14.78) | 3441 (20.43) | 248 (8.88) | |
CKD indicates chronic kidney disease; CVH, cardiovascular health; HEI, Healthy Eating Index; and LE8, Life's Essential 8.
Data are presented as weighted mean (SE) or unweighted frequencies (weighted percentages).
Low CVH was defined as an LE8 score of 0 to 49, moderate CVH of 50 to 79, and high CVH of 80 to 100.
LE8 Score and CKD
Participants with high CVH had a lower age‐adjusted prevalence of CKD compared with those with moderate CVH and low CVH (Figure 1). The fully adjusted multivariate logistic regression model revealed that the risk of CKD was significantly lower in the moderate CVH group and high CVH group compared with the low CVH group. The odds ratio (OR) for CKD was 0.79 (95% CI, 0.76–0.83) for each 10‐point increase in LE8 score (Table 2). The outcome of the multivariate‐adjusted restricted cubic spline regression analysis disclosed a nonlinear association between LE8 and CKD (nonlinear P<0.01; Figure 2A). The minimum threshold for favorable association was estimated to be 63.4 scores (OR, 1).
Figure 1. Age‐adjusted prevalence of CKD in different levels of LE8 scores.
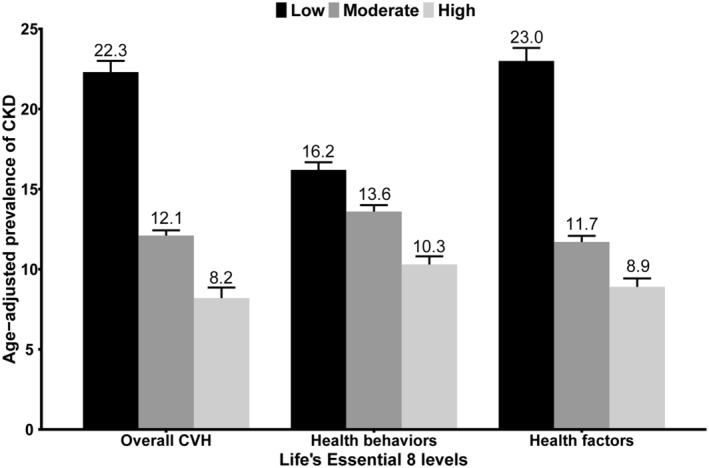
Numbers at the top of the bars represent the weighted percentage. Bar whiskers represent the SE. CKD indicates chronic kidney disease; and LE8, Life's Essential 8.
Table 2.
Association of the Life's Essential 8 Scores With Chronic Kidney Disease
| Univariable model | Multivariable model 1 | Multivariable model 2 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| LE8 score | ||||||
| Low (0–49) | 1.00 (Reference) | … | 1.00 (Reference) | … | 1.00 (Reference) | … |
| Moderate (50–79) | 0.42 (0.39–0.47) | <0.0001 | 0.49 (0.44–0.55) | <0.0001 | 0.52 (0.47–0.58) | <0.0001 |
| High (80–100) | 0.20 (0.17–0.23) | <0.0001 | 0.40 (0.33–0.48) | <0.0001 | 0.45 (0.37–0.55) | <0.0001 |
| Per 10‐point increase | 0.68 (0.66–0.71) | <0.0001 | 0.77 (0.74–0.80) | <0.0001 | 0.79 (0.76–0.83) | <0.0001 |
| Health behaviors score | ||||||
| Low (0–49) | 1.00 (Reference) | … | 1.00 (Reference) | … | 1.00 (Reference) | … |
| Moderate (50–79) | 0.89 (0.81–0.98) | 0.0214 | 0.81 (0.72–0.91) | <0.0010 | 0.88 (0.79–0.98) | 0.0260 |
| High (80–100) | 0.59 (0.52–0.67) | <0.0001 | 0.64 (0.54–0.75) | <0.0001 | 0.74 (0.63–0.87) | <0.0010 |
| Per 10‐point increase | 0.91 (0.89–0.93) | <0.0001 | 0.92 (0.89–0.94) | <0.0001 | 0.94 (0.92–0.97) | <0.0010 |
| Health factors score | ||||||
| Low (0–49) | 1.00 (Reference) | … | 1.00 (Reference) | … | 1.00 (Reference) | … |
| Moderate (50–79) | 0.40 (0.37–0.45) | <0.0001 | 0.48 (0.43–0.54) | <0.0001 | 0.49 (0.44–0.55) | <0.0001 |
| High (80–100) | 0.18 (0.16–0.21) | <0.0001 | 0.43 (0.37–0.50) | <0.0001 | 0.44 (0.37–0.52) | <0.0001 |
| Per 10‐point increase | 0.72 (0.70–0.73) | <0.0001 | 0.82 (0.79–0.84) | <0.0001 | 0.82 (0.80–0.85) | <0.0001 |
Multivariable model 1 was adjusted for age (as a continuous variable), sex, race and ethnicity, and obesity status.
Multivariable model 2 was additionally adjusted for poverty ratio (as a continuous variable), education levels, and marital status.
LE8 indicates Life's Essential 8; and OR, odds ratio.
Figure 2. The nonlinear associations between LE8 and its subscales with CKD.
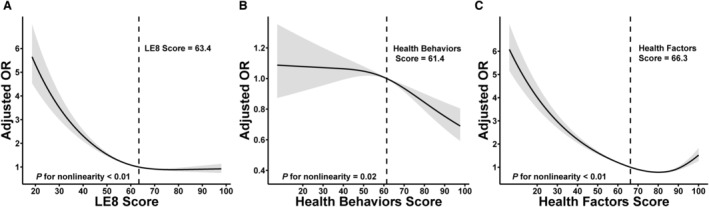
Dose–response relationships between LE 8 score (A), health behaviors score (B), health factors score (C), and CKD. ORs (solid lines) and 95% CIs (shaded areas) were adjusted for age (as a continuous variable), sex, race and ethnicity, poverty ratio (as a continuous variable), education levels, and marital status. Vertical dotted lines indicate the minimal threshold for the beneficial association with estimated OR of 1. CKD indicates chronic kidney disease; LE8, Life's Essential 8; and OR, odds ratio.
Health Behaviors and CKD
Participants with high health behavior had a lower age‐adjusted prevalence of CKD versus those with moderate and low health behavior (Figure 1). In the fully adjusted multivariate logistic regression model, the risk of CKD was significantly lower in the moderate and high health behavior groups compared with the low health behavior. The OR for CKD was 0.94 (95% CI, 0.92–0.97) for each 10‐point increase in health behavior score (Table 2). Multivariate adjusted restricted cubic spline results revealed a nonlinear association between health behavior scores and CKD (nonlinear P=0.02; Figure 2B). The minimum threshold for favorable association was estimated to be 61.4 scores (OR, 1).
Health Factors and CKD
Participants with high health factors had a lower age‐adjusted prevalence of CKD relative to those with moderate and low health factors (Figure 1). The fully adjusted multivariate logistic regression model revealed the risk of CKD was significantly lower in the moderate and high health factor groups compared with the low health factor. Additionally, the OR for CKD was 0.82 (95% CI, 0.80–0.85) for each 10‐point increase in health factor score (Table 2). The multivariate‐adjusted restricted cubic spline results indicated a nonlinear association between health factor scores and CKD (nonlinear P<0.01; Figure 2C). The minimum threshold for favorable association was evaluated at 66.3 scores (OR, 1). Trends in the nonlinear association between LE8 score and health factor score with CKD were similar.
Components of LE8 and CKD
In Table 3, the fully adjusted multivariate logistic regression model revealed significant negative associations between blood glucose score and blood pressure score of LE8 components with CKD. The risk of CKD was significantly lower in the moderate (no diabetes and fasting blood glucose 100–125 [or glycated hemoglobin 5.7–6.4]) and high (no history of diabetes and fasting blood glucose <100 [or glycated hemoglobin <5.7]) blood glucose score groups compared with the low (people with diabetes) blood glucose score groups. The OR for CKD was 0.88 (95% CI, 0.87–0.90) for each 10‐point increase in blood glucose score. Additionally, the risk of CKD was significantly lower in the moderate (120–139 or 80–90 mm Hg) and high (<120/<80 mm Hg) blood pressure score groups compared with the low (≥140 or ≥90 mm Hg) blood pressure score groups. The OR for CKD was 0.92 (95% CI, 0.0.91–0.94) for each 10‐point increase in blood pressure score.
Table 3.
Associations Between Components of LE8 and Chronic Kidney Disease
| Cases/participants | OR* (95% CI) | P value | |
|---|---|---|---|
| HEI‐2015 diet score | |||
| Low (0–49) | 2132/12 316 | 1.00 (Reference) | … |
| Moderate (50–79) | 2234/12 077 | 0.98 (0.89–1.07) | 0.5871 |
| High (80–100) | 71/567 | 0.68 (0.47–0.98) | 0.0394 |
| Per 10‐point increase | 4437/24 960 | 0.97 (0.94–1.00) | 0.0707 |
| Physical activity score | |||
| Low (0–49) | 3034/13 586 | 1.00 (Reference) | … |
| Moderate (50–79) | 154/1010 | 0.77 (0.58–1.03) | 0.0812 |
| High (80–100) | 1249/10 364 | 0.91 (0.81–1.03) | 0.1330 |
| Per 10‐point increase | 4437/24 960 | 0.99 (0.98–1.00) | 0.0733 |
| Nicotine exposure score | |||
| Low (0–49) | 834/5644 | 1.00 (Reference) | … |
| Moderate (50–79) | 1356/5462 | 1.07 (0.93–1.23) | 0.3108 |
| High (80–100) | 2247/13 854 | 1.02 (0.87–1.19) | 0.8118 |
| Per 10‐point increase | 4437/24 960 | 1.00 (0.99–1.02) | 0.7703 |
| Sleep health score | |||
| Low (0–49) | 991/4485 | 1.00 (Reference) | … |
| Moderate (50–79) | 829/5267 | 0.80 (0.69–0.93) | 0.0044 |
| High (80–100) | 2617/15 208 | 0.89 (0.79–1.00) | 0.0574 |
| Per 10‐point increase | 4437/24 960 | 0.99 (0.97–1.01) | 0.1925 |
| Body mass index score | |||
| Low (0–49) | 2025/9735 | 1.00 (Reference) | … |
| Moderate (50–79) | 1369/8153 | 0.81 (0.71–0.92) | 0.0015 |
| High (80–100) | 1043/7072 | 0.99 (0.87–1.14) | 0.9339 |
| Per 10‐point increase | 4437/24 960 | 0.98 (0.97–1.00) | 0.0725 |
| Blood glucose score | |||
| Low (0–49) | 1823/4719 | 1.00 (Reference) | … |
| Moderate (50–79) | 1406/8367 | 0.43 (0.37–0.49) | <0.0001 |
| High (80–100) | 1208/11 874 | 0.42 (0.37–0.48) | <0.0001 |
| Per 10‐point increase | 4437/24 960 | 0.88 (0.87–0.90) | <0.0001 |
| Blood pressure score | |||
| Low (0–49) | 2188/6650 | 1.00 (Reference) | … |
| Moderate (50–79) | 1064/7442 | 0.55 (0.48–0.63) | <0.0001 |
| High (80–100) | 1185/10 868 | 0.65 (0.57–0.74) | <0.0001 |
| Per 10‐point increase | 4437/24 960 | 0.92 (0.91–0.94) | <0.0001 |
| Blood lipids score | |||
| Low (0–49) | 1636/8332 | 1.00 (Reference) | … |
| Moderate (50–79) | 741/5730 | 0.90 (0.78–1.03) | 0.1364 |
| High (80–100) | 2060/10 898 | 1.17 (1.04–1.32) | 0.0120 |
| Per 10‐point increase | 4437/24 960 | 1.02 (1.00–1.03) | 0.0776 |
HEI indicates Healthy Eating Index; LE8, Life's Essential 8; and OR, odds ratio.
Adjusted for age (continuous), sex, race and ethnicity, education levels, poverty ratio (continuous), marital status, and the remaining 7 LE8 components.
Subgroup and Sensitivity Analysis
In all subgroups, LE8 scores were negatively associated with CKD (Figure 3). Significant interactions were found between LE8 and age, sex, and marital status with CKD (interaction P<0.05). The reverse relevance between LE8 score and CKD was more significant in middle‐aged participants, men, and coupled participants.
Figure 3. Subgroup analysis of the association of LE8 scores and the presence of CKD.
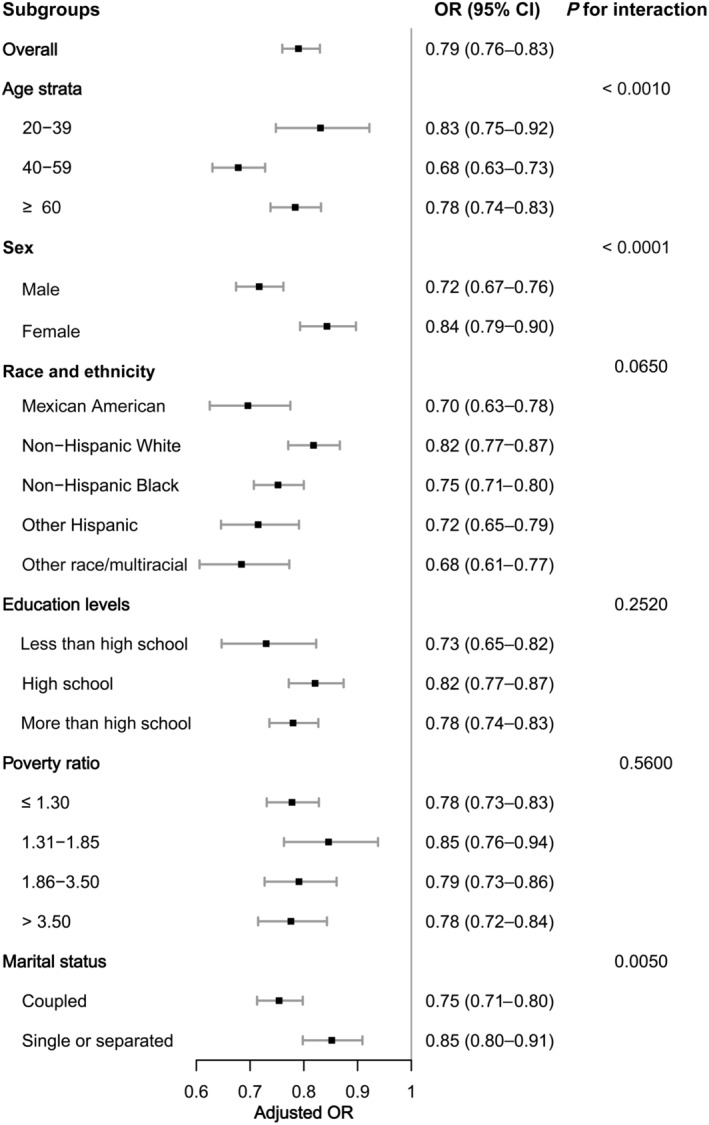
OR was calculated as per 10‐point increase in LE8 score. Each stratification was adjusted for age (as a continuous variable), sex, race and ethnicity, poverty ratio (as a continuous variable), education levels, and marital status. CKD indicates chronic kidney disease; LE8, Life's Essential 8; and OR, odds ratio.
Overall, the outcome of the sensitivity analysis was robust (Table 4). After excluding participants with histories of CVD from sensitivity analyses, the fully adjusted multivariable logistic regression models demonstrated that the results were consistent with the primary findings.
Table 4.
Sensitivity Analysis of the Association of the LE8 Scores With Chronic Kidney Disease
| Excluding participants with CVD history | |||
|---|---|---|---|
| Cases/participants | OR (95% CI) | P value | |
| LE8 score | |||
| Low (0–49) | 1482/4680 | 1.00 (Reference) | … |
| Moderate (50–79) | 2707/16 591 | 0.53 (0.48–0.60) | <0.0001 |
| High (80–100) | 248/3689 | 0.47 (0.38–0.58) | <0.0001 |
| Per 10‐point increase | 4437/24 960 | 0.81 (0.77–0.85) | <0.0001 |
| Health behaviors score | |||
| Low (0–49) | 1378/6477 | 1.00 (Reference) | … |
| Moderate (50–79) | 2401/12 852 | 0.96 (0.86–1.09) | 0.5520 |
| High (80–100) | 658/5631 | 0.84 (0.70–1.01) | 0.0572 |
| Per 10‐point increase | 4437/24 960 | 0.97 (0.94–1.00) | 0.0539 |
| Health factors score | |||
| Low (0–49) | 1801/5639 | 1.00 (Reference) | … |
| Moderate (50–79) | 2130/12 413 | 0.49 (0.44–0.55) | <0.0001 |
| High (80–100) | 506/6908 | 0.43 (0.36–0.50) | <0.0001 |
| Per 10‐point increase | 4437/24 960 | 0.82 (0.79–0.84) | <0.0001 |
ORs and 95% CIs were adjusted for age (as a continuous variable), sex, race and ethnicity, poverty ratio (as a continuous variable), education levels, and marital status.
CVD indicates cardiovascular disease; LE8, Life's Essential 8; and OR, odds ratio.
Discussion
This cross‐sectional study, which included a nationally representative sample of US adults, found that higher LE8 scores were significantly associated with a lower risk of CKD. Similar patterns were also identified in the associations of health behavior and health factor scores with CKD. Meanwhile, higher blood glucose and blood pressure scores were significantly associated with a lower risk of CKD. Subgroup analyses further revealed stronger negative associations between LE8 scores and CKD in middle‐aged, male, and coupled populations.
For now, this is the first study to evaluate the relationships of CVH defined by the LE8 metrics with CKD. The significant negative associations between LE8 score, particularly blood glucose and blood pressure scores within its components, and the risk of CKD, were consistent with previous research. A US‐based ARIC (Atherosclerosis Risk in Communities) cohort demonstrated that LS7 in AHA was negatively related to the risk of CKD.19 A prospective cohort study from the UK Biobank revealed a significant inverse correlation between prediabetes, type 2 diabetes, and the risk of CKD. 24 A review in 2023 indicated hypertension as a cardiovascular risk factor in CKD. 25 Additionally, extensive studies demonstrated that CKD was tightly linked to metabolic syndrome and lifestyle, 26 , 27 , 28 , 29 , 30 , 31 both of which were underlying health factors and indicators of healthy behaviors in LE8. During metabolic syndrome, increased secretion of proinflammatory factors by adipose tissue can contribute to the inflammatory response, leading to proteinuria and damaged kidney function. 32 Infiltration of inflammatory cells contributes to increased reactive oxygen species in kidney tissue. Reactive oxygen species may disturb renal tubular ion transport by affecting renal hemodynamics, thereby causing damage to the proximal tubule. 33 Numerous lipid droplets were found in the renal innate cells of patients with obesity. Lipid droplet deposition leads to renal cell energy depletion and ultimately endogenous renal cell apoptosis, leading to CKD. 34 This may explain the significant association between LE8 and CKD.
Our study also revealed significant nonlinear associations between the LE8 and its subscales with CKD, which have not been reported in previous studies. Previous research employed LS7 to assess CKD, and the definition of CVH for each LS7 component was divided into ideal, moderate, and poor CVH. This measurement could not be applied to evaluate dose–response effects. In contrast, we defined CVH with LE8, which adds significant evidence of a relationship between CVH and CKD. The ORs for LE8 scores and health factor scores associated with CKD declined acutely in the lower scope of the corresponding scores, leveled off, and then remained constant at the higher values. However, the association between health behavior scores and CKD was reversed, with ORs remaining constant in the lower scope of health behavior scores and declining rapidly in the higher scope. Saturation effects were identified in the associations of LE8 and health factors with CKD, but not in health behaviors, suggesting that stricter standards of health behavior may be preferable.
Furthermore, subgroup analysis demonstrated a strong negative association between LE8 and CKD in middle‐aged, male, and coupled participants. A Korean cohort study demonstrated that strict ideal CVH in midlife was related to lower CKD risk in old age, highlighting the importance of maintaining a healthy lifestyle in midlife. 17 Another population‐based study revealed that the epidemiology of CKD varies by sex, affecting more women than men, particularly concerning CKD stage G3 (estimated glomerular filtration rate [eGFR], 30‐59 mL/min/1.73m2). This may be the effect of the increased incidence of CKD in women through inappropriate use of equations for eGFR, 35 causing a stronger negative association between LE8 and CKD in male participants. Moreover, several studies have shown that marriage may mitigate disease progression by encouraging healthier lifestyles or providing social support or financial security, 36 , 37 , 38 , 39 , 40 which may explain the strong negative association between LE8 and CKD in participants with a spouse. These findings demonstrated that LE8 enhanced the quantitative approach to CVH and increased the sensitivity of the scores to differences between individuals and groups. Thus, differences in the underlying salutary value of CVH constituents need to be fully recognized, and population‐level methodologies are needed to improve CVH.
CKD poses the greatest burden to patients in terms of both economic cost and daily living. 41 Management of patients with CKD focuses on early diagnosis or prevention and attention to secondary processes leading to permanent nephron loss. 42 Healthy lifestyles are the foundation for mitigating CKD risks. 30 However, prior studies primarily focused on single factors for CKD, while comprehensive and integrated factors for patients with CKD were absent. 43 , 44 , 45 , 46 , 47 LE8 is a comprehensive, easy‐to‐use evaluation tool that promotes compliance with healthy behaviors and desirable healthy determinants in the clinical setting. Our outcomes broadened the scope of health effects relevant to the salutary effects of optimal CVH in CKD and CVD and demonstrated that insistence on optimal CVH may reduce the burden of CKD and other chronic diseases.
Based on a nationally representative sample of American adults, these outcomes were generalizable to a larger population. Furthermore, CVH and CKD were studied concerning the dose–response relationship, and the minimum threshold for the salutary association was identified. However, the analysis must also take into account several underlying limitations. First, the assessment of health behavior indicators was based on questionnaires, which were prone to estimating errors. Second, despite controlling for several potential confounders, the nature of the cross‐sectional study design prevented us from concluding the causality and temporality of CVH and CKD. The longitudinal and causal relationship between LE8 and CKD risk needs further study. Third, more pronounced results were observed for the moderate blood pressure score group than the optimal blood pressure score group in exploring the associations between components of LE8 and CKD. These may be due to potential confounders, and we intend to follow up with a large cohort study to obtain more precise evidence. Finally, only 1 measurement of serum creatinine and urine albuminuria was available, which may result in the misclassification of CKD status.
Conclusions
Scores on the LE8 and its subscales were negatively and nonlinearly related to the prevalence of CKD in this nationally representative sample of US adults. LE8 component should prioritize higher scores for blood glucose and blood pressure evaluations. Meanwhile, the intensity of the association between LE8 scores and CKD varied across the study population. These findings hint that LE8, as practical and salutary comprehensive indicators for improving kidney health, may be applied in the clinic to help patients, as well as the general population, identify CKD risk early and minimize the burden of CKD.
Sources of Funding
This study was supported by the Major Science and Technology projects of Henan Province (Grant No. 221100310100).
Disclosures
None.
Supporting information
Tables S1‐S2Figure S1
Y. Ren, Z. Cai, and C. Guo contributed equally and are co–first authors.
This manuscript was sent to Mahasin S. Mujahid, PhD, MS, FAHA, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030564
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Zaoqu Liu, Email: liuzaoqu@163.com.
Xinwei Han, Email: fcchanxw@zzu.edu.cn.
References
- 1. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–1252. doi: 10.1016/s0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- 2. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 4. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/s0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker‐Smith CM, Beaton AZ, Boehme AK, Buxton AE, et al. Heart disease and stroke statistics‐2023 update: a report from the American Heart Association. Circulation. 2023;147:e93–e621. doi: 10.1161/cir.0000000000001123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pugh D, Gallacher PJ, Dhaun N. Management of hypertension in chronic kidney disease. Drugs. 2019;79:365–379. doi: 10.1007/s40265-019-1064-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee HH, Lee H, Townsend RR, Kim DW, Park S, Kim HC. Cardiovascular implications of the 2021 KDIGO blood pressure guideline for adults with chronic kidney disease. J Am Coll Cardiol. 2022;79:1675–1686. doi: 10.1016/j.jacc.2022.02.040 [DOI] [PubMed] [Google Scholar]
- 8. Chen J, Budoff MJ, Reilly MP, Yang W, Rosas SE, Rahman M, Zhang X, Roy JA, Lustigova E, Nessel L, et al. Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol. 2017;2:635–643. doi: 10.1001/jamacardio.2017.0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsushita K, Ballew SH, Wang AY, Kalyesubula R, Schaeffner E, Agarwal R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol. 2022;18:696–707. doi: 10.1038/s41581-022-00616-6 [DOI] [PubMed] [Google Scholar]
- 10. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/circulationaha.109.192703 [DOI] [PubMed] [Google Scholar]
- 11. Lloyd‐Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, et al. Life's essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/cir.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/cir.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 13. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/cir.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 14. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/cir.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 15. Lloyd‐Jones DM, Ning H, Labarthe D, Brewer L, Sharma G, Rosamond W, Foraker RE, Black T, Grandner MA, Allen NB, et al. Status of cardiovascular health in US adults and children using the American Heart Association's new "Life's essential 8" metrics: prevalence estimates from the National Health and nutrition examination survey (NHANES), 2013 through 2018. Circulation. 2022;146:822–835. doi: 10.1161/circulationaha.122.060911 [DOI] [PubMed] [Google Scholar]
- 16. Wang X, Ma H, Li X, Heianza Y, Manson JE, Franco OH, Qi L. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in UK adults. JAMA Intern Med. 2023;183:340–349. doi: 10.1001/jamainternmed.2023.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cho SMJ, Jeon JY, Yoo TH, Lee HY, Lee YH, Kim HC. Ideal cardiovascular health duration and risk of chronic kidney disease and cardiovascular disease. Heart. 2022;108:523–528. doi: 10.1136/heartjnl-2021-320180 [DOI] [PubMed] [Google Scholar]
- 18. Ogunmoroti O, Allen NB, Cushman M, Michos ED, Rundek T, Rana JS, Blankstein R, Blumenthal RS, Blaha MJ, Veledar E, et al. Association between Life's simple 7 and noncardiovascular disease: the multi‐ethnic study of atherosclerosis. J Am Heart Assoc. 2016;5:e003954. doi: 10.1161/jaha.116.003954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rebholz CM, Anderson CA, Grams ME, Bazzano LA, Crews DC, Chang AR, Coresh J, Appel LJ. Relationship of the American Heart Association's impact goals (Life's simple 7) with risk of chronic kidney disease: results from the atherosclerosis risk in communities (ARIC) cohort study. J Am Heart Assoc. 2016;5:e003192. doi: 10.1161/jaha.116.003192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/s0140-6736(07)61602-x [DOI] [PubMed] [Google Scholar]
- 21. Alaimo K, Briefel RR, Frongillo EA Jr, Olson CM. Food insufficiency exists in the United States: results from the third National Health and nutrition examination survey (NHANES III). Am J Public Health. 1998;88:419–426. doi: 10.2105/ajph.88.3.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. KDIGO . Clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;2021(100):S1–s276. doi: 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 23. Krebs‐Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, Wilson MM, Reedy J. Update of the healthy eating index: HEI‐2015. J Acad Nutr Diet. 2018;118:1591–1602. doi: 10.1016/j.jand.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Honigberg MC, Zekavat SM, Pirruccello JP, Natarajan P, Vaduganathan M. Cardiovascular and kidney outcomes across the glycemic spectrum: insights from the UK biobank. J Am Coll Cardiol. 2021;78:453–464. doi: 10.1016/j.jacc.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burnier M, Damianaki A. Hypertension as cardiovascular risk factor in chronic kidney disease. Circ Res. 2023;132:1050–1063. doi: 10.1161/circresaha.122.321762 [DOI] [PubMed] [Google Scholar]
- 26. Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007 [DOI] [PubMed] [Google Scholar]
- 27. Locatelli F, Pozzoni P, Del Vecchio L. Renal manifestations in the metabolic syndrome. J Am Soc Nephrol. 2006;17:S81–S85. doi: 10.1681/asn.2005121332 [DOI] [PubMed] [Google Scholar]
- 28. Alexander MP, Patel TV, Farag YM, Florez A, Rennke HG, Singh AK. Kidney pathological changes in metabolic syndrome: a cross‐sectional study. Am J Kidney Dis. 2009;53:751–759. doi: 10.1053/j.ajkd.2009.01.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: a systematic review and meta‐analysis. Clin J Am Soc Nephrol. 2011;6:2364–2373. doi: 10.2215/cjn.02180311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kelly JT, Su G, Zhang L, Qin X, Marshall S, González‐Ortiz A, Clase CM, Campbell KL, Xu H, Carrero JJ. Modifiable lifestyle factors for primary prevention of CKD: a systematic review and meta‐analysis. J Am Soc Nephrol. 2021;32:239–253. doi: 10.1681/asn.2020030384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park S, Lee S, Kim Y, Lee Y, Kang MW, Kim K, Kim YC, Han SS, Lee H, Lee JP, et al. Short or long sleep duration and CKD: a Mendelian randomization study. J Am Soc Nephrol. 2020;31:2937–2947. doi: 10.1681/asn.2020050666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brennan E, Kantharidis P, Cooper ME, Godson C. Pro‐resolving lipid mediators: regulators of inflammation, metabolism and kidney function. Nat Rev Nephrol. 2021;17:725–739. doi: 10.1038/s41581-021-00454-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carlström M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev. 2015;95:405–511. doi: 10.1152/physrev.00042.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Z, Shrestha R, Yang X, Wu X, Jia J, Chiba H, Hui SP. Oxidative stress and lipid dysregulation in lipid droplets: a connection to chronic kidney disease revealed in human kidney cells. Antioxidants (Basel). 2022;11:1387. doi: 10.3390/antiox11071387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14:151–164. doi: 10.1038/nrneph.2017.181 [DOI] [PubMed] [Google Scholar]
- 36. Dupre ME, Beck AN, Meadows SO. Marital trajectories and mortality among US adults. Am J Epidemiol. 2009;170:546–555. doi: 10.1093/aje/kwp194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Waite LJ. Does marriage matter? Demography. 1995;32:483–507. doi: 10.2307/2061670 [DOI] [PubMed] [Google Scholar]
- 38. Joung IM, Stronks K, van de Mheen H, Mackenbach JP. Health behaviours explain part of the differences in self reported health associated with partner/marital status in the Netherlands. J Epidemiol Community Health. 1995;49:482–488. doi: 10.1136/jech.49.5.482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manzoli L, Villari PG, Pirone GM, Boccia A. Marital status and mortality in the elderly: a systematic review and meta‐analysis. Soc Sci Med. 2007;64:77–94. doi: 10.1016/j.socscimed.2006.08.031 [DOI] [PubMed] [Google Scholar]
- 40. Goldman N, Korenman S, Weinstein R. Marital status and health among the elderly. Soc Sci Med. 1995;40:1717–1730. doi: 10.1016/0277-9536(94)00281-w [DOI] [PubMed] [Google Scholar]
- 41. Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit‐Dahm KA, Zoungas S, Rossing P, Groop PH, Cooper ME. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018. doi: 10.1038/nrdp.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M, Massy Z, Wanner C, Anders HJ. Chronic kidney disease. Nat Rev Dis Primers. 2017;3:17088. doi: 10.1038/nrdp.2017.88 [DOI] [PubMed] [Google Scholar]
- 43. Kim H, Caulfield LE, Garcia‐Larsen V, Steffen LM, Grams ME, Coresh J, Rebholz CM. Plant‐based diets and incident CKD and kidney function. Clin J Am Soc Nephrol. 2019;14:682–691. doi: 10.2215/cjn.12391018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bach KE, Kelly JT, Palmer SC, Khalesi S, Strippoli GFM, Campbell KL. Healthy dietary patterns and incidence of CKD: a meta‐analysis of cohort studies. Clin J Am Soc Nephrol. 2019;14:1441–1449. doi: 10.2215/cjn.00530119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zelle DM, Klaassen G, van Adrichem E, Bakker SJ, Corpeleijn E, Navis G. Physical inactivity: a risk factor and target for intervention in renal care. Nat Rev Nephrol. 2017;13:152–168. doi: 10.1038/nrneph.2016.187 [DOI] [PubMed] [Google Scholar]
- 46. Jhee JH, Joo YS, Kee YK, Jung SY, Park S, Yoon CY, Han SH, Yoo TH, Kang SW, Park JT. Secondhand smoke and CKD. Clin J Am Soc Nephrol. 2019;14:515–522. doi: 10.2215/cjn.09540818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhu P, Herrington WG, Haynes R, Emberson J, Landray MJ, Sudlow CLM, Woodward M, Baigent C, Lewington S, Staplin N. Conventional and genetic evidence on the association between adiposity and CKD. J Am Soc Nephrol. 2021;32:127–137. doi: 10.1681/asn.2020050679 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S2Figure S1


