Key Points
Question
Does twice-annual mass azithromycin distribution prevent all-cause childhood mortality among children in Burkina Faso aged 1 to 59 months in the setting of seasonal malaria chemoprevention distribution?
Findings
In this randomized trial of twice-yearly mass azithromycin distribution, 498 deaths were recorded over 60 592 person-years (8.2 deaths/1000 person-years) in the azithromycin group compared with 588 deaths over 58 547 person-years (10.0 deaths/1000 person-years) in the placebo group. The difference was not statistically significant.
Meaning
Communities with mass azithromycin distribution had lower child mortality than controls, although the difference was not statistically significant. The study may have been underpowered to detect a clinically relevant difference.
Abstract
Importance
Repeated mass distribution of azithromycin has been shown to reduce childhood mortality by 14% in sub-Saharan Africa. However, the estimated effect varied by location, suggesting that the intervention may not be effective in different geographical areas, time periods, or conditions.
Objective
To evaluate the efficacy of twice-yearly azithromycin to reduce mortality in children in the presence of seasonal malaria chemoprevention.
Design, Setting, and Participants
This cluster randomized placebo-controlled trial evaluating the efficacy of single-dose azithromycin for prevention of all-cause childhood mortality included 341 communities in the Nouna district in rural northwestern Burkina Faso. Participants were children aged 1 to 59 months living in the study communities.
Interventions
Communities were randomized in a 1:1 ratio to receive oral azithromycin or placebo distribution. Children aged 1 to 59 months were offered single-dose treatment twice yearly for 3 years (6 distributions) from August 2019 to February 2023.
Main Outcomes and Measures
The primary outcome was all-cause childhood mortality, measured during a twice-yearly enumerative census.
Results
A total of 34 399 children (mean [SD] age, 25.2 [18] months) in the azithromycin group and 33 847 children (mean [SD] age, 25.6 [18] months) in the placebo group were included. A mean (SD) of 90.1% (16.0%) of the censused children received the scheduled study drug in the azithromycin group and 89.8% (17.1%) received the scheduled study drug in the placebo group. In the azithromycin group, 498 deaths were recorded over 60 592 person-years (8.2 deaths/1000 person-years). In the placebo group, 588 deaths were recorded over 58 547 person-years (10.0 deaths/1000 person-years). The incidence rate ratio for mortality was 0.82 (95% CI, 0.67-1.02; P = .07) in the azithromycin group compared with the placebo group. The incidence rate ratio was 0.99 (95% CI, 0.72-1.36) in those aged 1 to 11 months, 0.92 (95% CI, 0.67-1.27) in those aged 12 to 23 months, and 0.73 (95% CI, 0.57-0.94) in those aged 24 to 59 months.
Conclusions and Relevance
Mortality in children (aged 1-59 months) was lower with biannual mass azithromycin distribution in a setting in which seasonal malaria chemoprevention was also being distributed, but the difference was not statistically significant. The study may have been underpowered to detect a clinically relevant difference.
Trial Registration
ClinicalTrials.gov Identifier: NCT03676764
This cluster randomized placebo-controlled trial examines the efficacy of single-dose azithromycin for prevention of all-cause childhood mortality in rural Burkina Faso.
Introduction
Despite major reductions in child mortality worldwide, mortality rates remain well above Sustainable Development Goals in some regions of sub-Saharan Africa.1 The World Health Organization (WHO) has acknowledged the need for simple, feasible, and cost-effective interventions to reduce child mortality in low- and middle-income countries. The MORDOR (Macrolides Oraux pour Réduire les Décès avec un Oeil sur la Résistance) trial found that twice-yearly distribution of azithromycin reduced all-cause mortality in children aged 1 to 59 months by 14% in sub-Saharan Africa, but varied from 3% to 18% in the 3 study countries.2 WHO conditionally recommended that azithromycin distribution could be considered in settings with high child mortality, although encouraged further research before making any stronger recommendations due to the risk of selection for antimicrobial resistance.3 WHO identified several major research gaps, including evaluation of mass drug administration with azithromycin for prevention of child mortality in additional geographic areas in sub-Saharan Africa, including in the presence of seasonal malaria chemoprevention (SMC).3
The most common causes of childhood mortality in settings with high mortality are infectious, including malaria, pneumonia, and diarrhea. Although the exact mechanism of azithromycin for reducing mortality is unclear, results of the MORDOR study showed a reduction in mortality due to malaria, dysentery, and pneumonia in children who received azithromycin compared with placebo.4 However, the MORDOR study was done in the absence of SMC, which consists of monthly mass drug administration of sulfadoxine-pyramethamine and amodiaquine to children aged 3 to 59 months during the high malaria transmission season. SMC not only targets malaria, but sulfadoxine could, in theory, also treat bacterial infectious diseases in a similar manner as azithromycin.5 A previous randomized trial in Burkina Faso and Mali failed to demonstrate a benefit of oral azithromycin on mortality when provided with SMC, which raised questions about whether azithromycin could continue to be effective in areas in which SMC is distributed.6
The Community Health With Azithromycin Treatment (CHAT) trial was a cluster randomized clinical trial of twice-yearly mass azithromycin distribution compared with placebo in Nouna, Burkina Faso. Nouna has high rates of childhood vaccine coverage and SMC implementation and universal access to health care for children younger than 5 years.7 It was hypothesized that, in this setting, communities receiving twice-yearly mass azithromycin distribution would have lower mortality rates compared with those receiving placebo.
Methods
Study Overview
The current study was a 1:1 cluster randomized trial comparing twice-yearly azithromycin distribution to children aged 1 to 59 months with matching placebo for all-cause mortality (NCT03676764). Complete methods for the trial have been previously reported and are shown in the trial protocol (Supplement 1).8 The trial was reviewed and approved by the institutional review boards at the University of California, San Francisco, the Comité National d’Ethique pour la Recherche, and the Comité Technique d’Examen des Demandes d’Autorisation d’Essais Cliniques in Ouagadougou, Burkina Faso. Written informed consent was obtained from the caregiver of each participant.
Study Setting
This study was conducted in the Nouna district, located in northwestern Burkina Faso bordering Mali.6 The Nouna district experiences heavy seasonal rainfall from June through September, with malarial deaths subsequently peaking from August to November. Each year, 4 monthly rounds of SMC with sulfadoxine-pyremethamine and amodiaquine were distributed to children aged 3 to 59 months from July through October.9 The Nouna district experienced escalating geopolitical insecurity over the study period, and some communities originally included in the study became inaccessible due to security concerns. These communities either ceased to participate or were able to reenter the study during a later census. This was anticipated in the study design, and no important changes were made to the methods after commencement.
Eligibility Criteria
A census of all communities in the Nouna district was conducted prior to the study. Nouna Town, the capital of Kossi Province, was excluded from the study because it is urban and has presumably lower mortality rates. Communities with a census population of 2000 or more were split into multiple randomization units, such that each study cluster had a population of less than 2000 individuals. Children were eligible for treatment as part of the study if they were aged between 1 and 59 months at the time of the census (ie, younger than 5 years), weighed at least 3800 g, had appropriate consent from their caregiver, and had no known allergies to macrolides per caregiver report. To assess vital status at each subsequent phase, children up to age 65 months were included in the census, but those aged 60 months or older were not offered treatment.
Census
A door-to-door enumerative census was conducted twice yearly as part of the study. The study team visited each community and visited each household in the community to conduct the census. A list was maintained of households in which no one was home, and the study team returned to the community up to 2 times to ensure each household was visited. During the census, the head of each household was interviewed as to how many children younger than 5 years were currently living in the household. The guardian-reported age and sex of each child was recorded. Vital status was assessed via guardian interview. For households that had been previously censused as part of the study, a list was available to the study enumerator so they could update the vital status for each eligible child. Enumerators could add children to the census who aged in or moved to the study area and were eligible or marked children as having moved or of unknown status. All enumerators were masked to the treatment assignment of the community.
Randomization and Intervention
Communities were randomized in a 1:1 ratio to receive twice-yearly distribution of a single oral dose of azithromycin (20 mg/kg suspension) or equivalent volume of matching placebo (Pfizer) (Figure 1). Randomization was stratified by whether communities fell within an existing Health and Demographic Surveillance Site (HDSS) and were selected for additional morbidity monitoring; of 341 communities originally mapped, 48 were in the HDSS.7 Communities in the HDSS have undergone surveillance since 1992, and the infrastructure built as part of the HDSS may itself result in improved child health outcomes. Additional morbidity monitoring will be reported separately. Children aged 1 to 11 months were weighed using a hanging infant scale to facilitate weight-based dosing of azithromycin or equivalent volume of placebo. Children aged 12 to 59 months were measured using a height stick to approximate the weight-based dose.10 Infants aged 5 to 12 weeks were also eligible for an individually randomized trial of single-dose azithromycin compared with placebo for prevention of infant mortality; approximately half of the infants enrolled in that trial would have received a single dose of azithromycin. All doses of study medications were administered by a trained study staff member and were directly observed. Six rounds of census and treatment occurred during the study: August 2019 to January 2020, February to July 2020, August 2020 to January 2021, February to July 2021, August 2021 to January 2022, and February to July 2022. The final study census occurred from August 2022 to February 2023.
Figure 1. Cluster and Participant Flow in a Study of Mass Azithromycin Distribution.
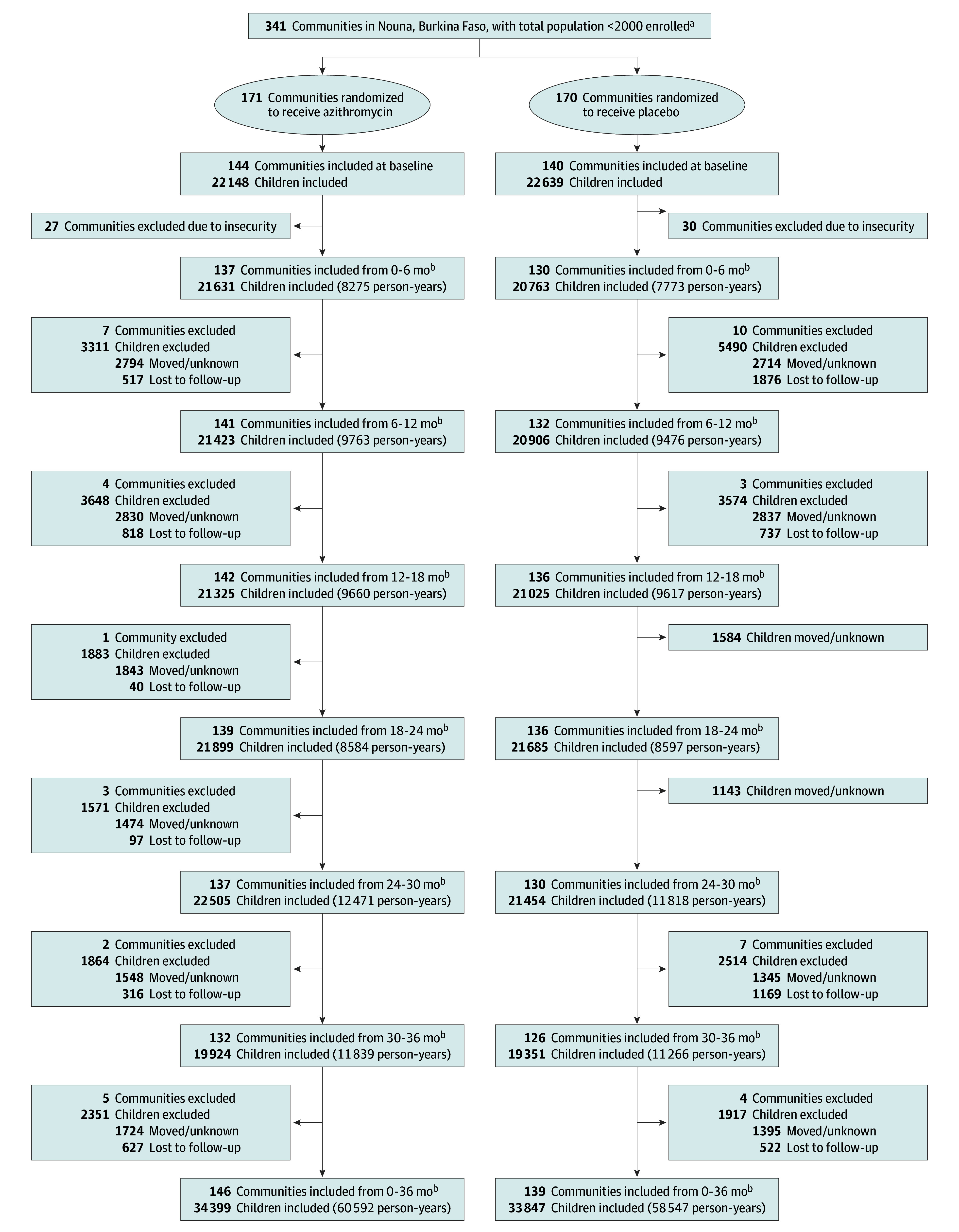
aAll communities in the Nouna district in Burkina Faso were eligible for inclusion and all were randomized. Randomization was stratified by whether the community was in an existing Health and Demographic Surveillance Site and selected for morbidity monitoring (n = 48) or not (n = 293).
bMonth ranges of follow-up indicate the census period of the study: August 2019 to January 2020, February to July 2020, August 2020 to January 2021, February to July 2021, August 2021 to January 2022, and February to July 2022. The final study census occurred from August 2022 to February 2023.
Masking and Randomization Concealment
Azithromycin and placebo were identical in composition, with exception of the active ingredient, and were similar in taste, smell, and appearance. Each bottle of study medication was labeled with a study-specific label. All labels were identical except for a letter (eg, “A,” “B,” “C”) that corresponded to either azithromycin or placebo. Four letters each were assigned to azithromycin and placebo. Each community was randomly assigned to 1 of the 8 study letters, and the community was treated with study medication labeled with that letter, facilitating both masking and randomization concealment.
Primary Outcome
The prespecified primary outcome was all-cause mortality rate at the cluster level determined by the twice-yearly census. A death counted toward the primary outcome if a child was recorded as alive and living in the household at one census and died prior to the next census, approximately 6 months later. Person-time for the denominator was calculated by summing the person-time for each intercensal period. Children who were alive during both censuses contributed the full amount of person-time for that intercensal period. Children who moved or had an unknown vital status did not contribute person-time to the intercensal period. Children who died between census periods were assigned half of the person-time between census periods. A cluster that was not censused, eg, due to security, could reenter the study at a later census. However, outcomes and person-time at risk during the gap would not be included in the primary outcome. By design, we did not attempt to follow up children who moved during the study.
Subgroup Analyses
A series of prespecified subgroup analyses were conducted to assess heterogeneity of effects, including by age group (1-11 months, 12-23 months, and 24-59 months) and by study year.
Adverse Events
During the first treatment round, an adverse event survey was conducted among caregivers of infants aged 1 to 5 months for active case detection of adverse events in a subset of 48 communities. Approximately 2 weeks after the treatment dose was administered, a study staff member interviewed the caregiver of each treated infant to determine the frequency of vomiting, nausea, diarrhea, abdominal pain, constipation, skin rash, and hemorrhoids. During the informed consent process, each caregiver was instructed that should their child experience a serious adverse event, including hospitalization, they should inform a community health worker who would then directly call study staff to deploy an adverse event team investigation.
Trial Oversight
The trial was overseen by an independent data and safety monitoring committee consisting of experts in biostatistics, clinical trials, pediatrics, mass drug administration, and bioethics. The committee met once annually during the conduct of the trial to review the study’s progress and interim data and reviewed quarterly data reports in aggregate.
Sample Size
The sample size of the trial was determined based on the primary outcome of all-cause childhood mortality. During the initial planning phase of the trial, 341 trial clusters were identified, and thus the trial’s sample size was fixed at 341. Assuming a mean community size of 1000 people, and that 16.7% of the population would be in the age range of 1 to 59 months, a base rate for mortality of 20 per 1000 person-years based on Institute for Health Metrics and Evaluation estimates and a coefficient of variation for cluster-level mortality rates of 0.34, as observed in the Niger site of the MORDOR trial, and loss to follow-up of 10%, a sample size of 341 clusters would provide approximately 80% power to detect a 13.7% relative reduction in mortality in the age group of 1 to 59 months.1,10 The observed coefficient of variation for cluster-level mortality rates was 0.55.
Interim Analysis
A single interim analysis was prespecified after 1 year of data collection (approximately one-third of total person-time). The interim analysis was conducted at an α of .001 for efficacy (Haybittle-Peto) following the primary analysis approach (described below). At that time, a prespecified interim analysis for futility was also conducted, providing stopping guidance should conditional power fall below 20% to detect a 30% relative reduction in the mortality incidence rate. No interim stopping guidelines were met and the data and safety monitoring committee recommended continuation without modifications to the trial protocol.
Statistical Methods
The prespecified primary analysis compared incidence rates in the 2 groups using a permutation test based on negative binomial regression (Supplement 2). Negative binomial regression was conducted allowing for a different shape parameter by group and permuted cluster-level treatment assignments (10 000 permutations).11 To account for the stratified randomization, the permutation test conditionally permuted treatment within the 2 strata (HDSS and non-HDSS) following the original randomization. Overall and stratum-specific incidence rates were estimated and incidence rate ratios (IRRs) and incidence rate differences (IRDs), along with their 95% CIs, were estimated using a nonparametric estimator and bootstrap that resampled clusters with replacement (10 000 replicates). Prespecified assessments of additive effect modification by age category and time period were done by including interaction terms between treatment assignment and effect modifiers in linear binomial models fit to child phase-level data with clustered robust standard errors. All analyses were conducted in R version 4.2 (R Foundation for Statistical Computing) and a 2-sided P value less than .05 was considered statistically significant.
Results
A total of 341 communities were randomized to receive twice-yearly azithromycin (171 communities) or twice-yearly placebo (170 communities) (Figure 1). Due to security concerns, only 284 were able to enter the first census, although this possibility had been anticipated in the statistical analysis plan (Table 1). The number of communities and children lost to follow-up due to security concerns was similar between treatment groups (Figure 1 and Figure 2). A total of 68 246 children (mean [SD] age, 25.4 [18] months) contributed 119 139 person-years at risk to the mortality monitoring. A mean (SD) of 90.1% (16.0%) of the censused children received the scheduled study drug in the azithromycin group and 89.8% (17.1%) received the scheduled study drug in the in the placebo group (eTable 1 in Supplement 3). The main reason a child did not receive treatment was because they were absent during a study visit, although 65 treatments were withheld due to reported allergy and 221 due to not meeting the weight threshold. Anecdotally, these allergies may have been reactions to previous SMC rather than azithromycin. The percentage of children whose census status was recorded as “moved” or “unknown” did not differ significantly between the azithromycin vs placebo groups (moved: 10.6% vs 9.6% [P = .48]; unknown: 0.3% vs 0.2% [P = .66]) (eTable 2 in Supplement 3).
Table 1. Baseline Characteristics of Study Communities Measured in the Baseline Census.
| Characteristic | Azithromycin | Placebo |
|---|---|---|
| No. (%) of communitiesa | 144 | 140 |
| No. (%) of HDSS communitiesb | 23 (16.0) | 18 (12.9) |
| No. of children | 22 148 | 22 639 |
| No. (%) of children in HDSS | 3295 (14.9) | 2599 (11.5) |
| No. of children per community, median (IQR) | 146 (85-210) | 148 (96-226) |
| Child’s sex, No. (%) | ||
| Female | 10 883 (49.1) | 11 249 (49.7) |
| Male | 11 265 (50.9) | 11 390 (50.3) |
| Age group, No. (%) | ||
| 1-11 mo | 3606 (16.3) | 3580 (15.8) |
| 12-23 mo | 4575 (20.7) | 4709 (20.8) |
| 24-59 mo | 13 967 (63.1) | 14 350 (63.4) |
| Mid-upper arm circumference, median (IQR), cm | 14.6 (14.0-15.5) | 14.5 (3.8-15.3) |
Abbreviation: HDSS, health and demographic surveillance site.
Number of communities measured at the baseline census; 27 communities in the azithromycin group and 30 communities in the placebo group were not measured in the baseline census due to security concerns.
Randomization was stratified by whether the study community fell within an existing HDSS and were selected for additional morbidity monitoring. The HDSS has been conducting population-based surveillance and monitoring since 1992 and a subset of the trial’s communities were part of the HDSS.
Figure 2. Map of Study Clusters by Treatment and Number of Completed Census Visits.
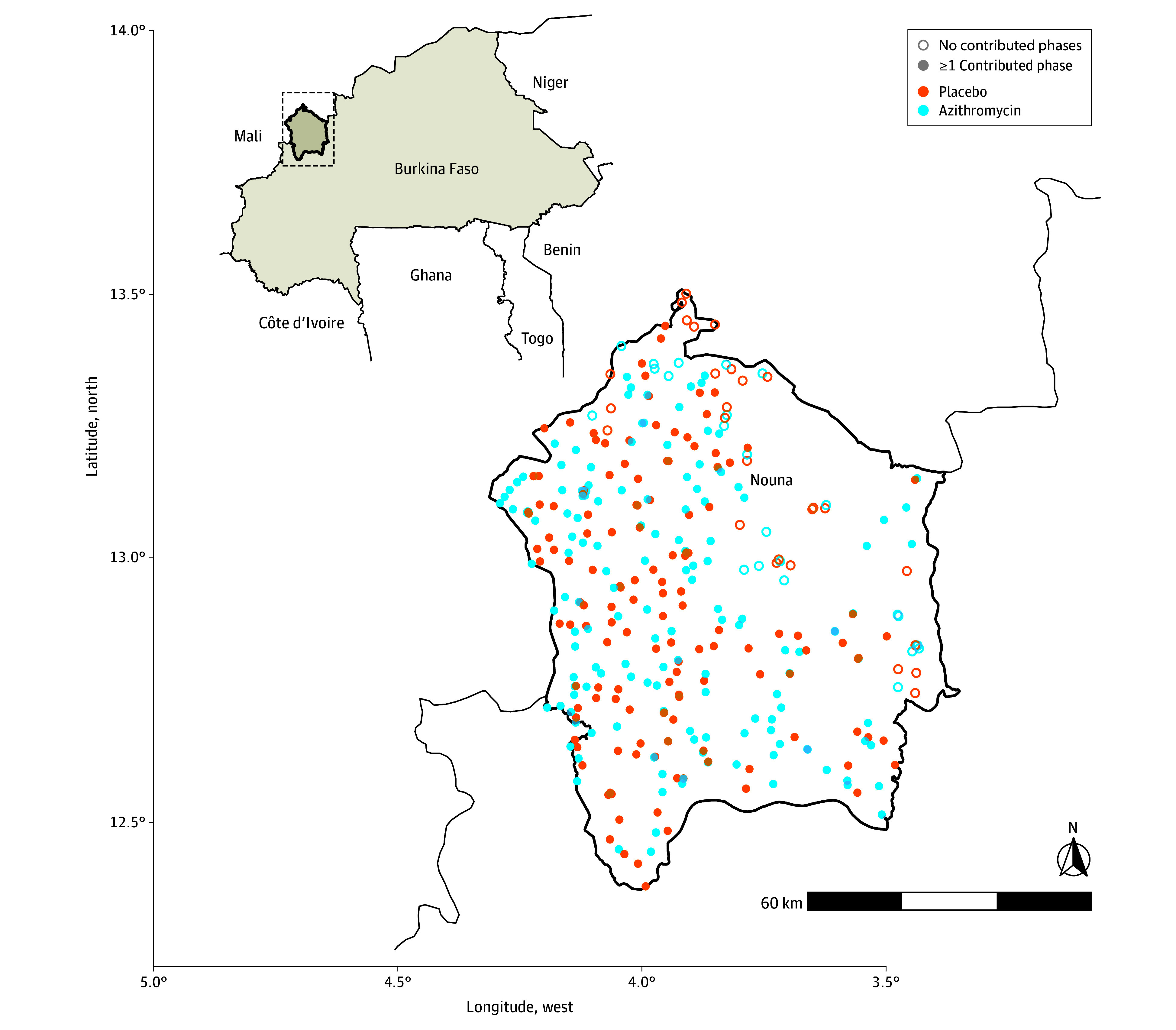
In azithromycin-treated communities, 498 deaths were recorded over 60 592 person-years (8.2 deaths/1000 person-years). In placebo-treated communities, 588 deaths were recorded over 58 547 person-years (10.0 deaths/1000 person-years). The IRR for mortality was 0.82 (95% CI, 0.67-1.02; P = .07) in the azithromycin group compared with the placebo group (IRR <1 indicating a lower mortality rate in the azithromycin group) (Figure 3). A nonprespecified sensitivity analysis that adjusted for covariates predictive of mortality led to nearly identical results (eTable 3 in Supplement 3).
Figure 3. All-Cause Mortality Rates by Treatment Group.
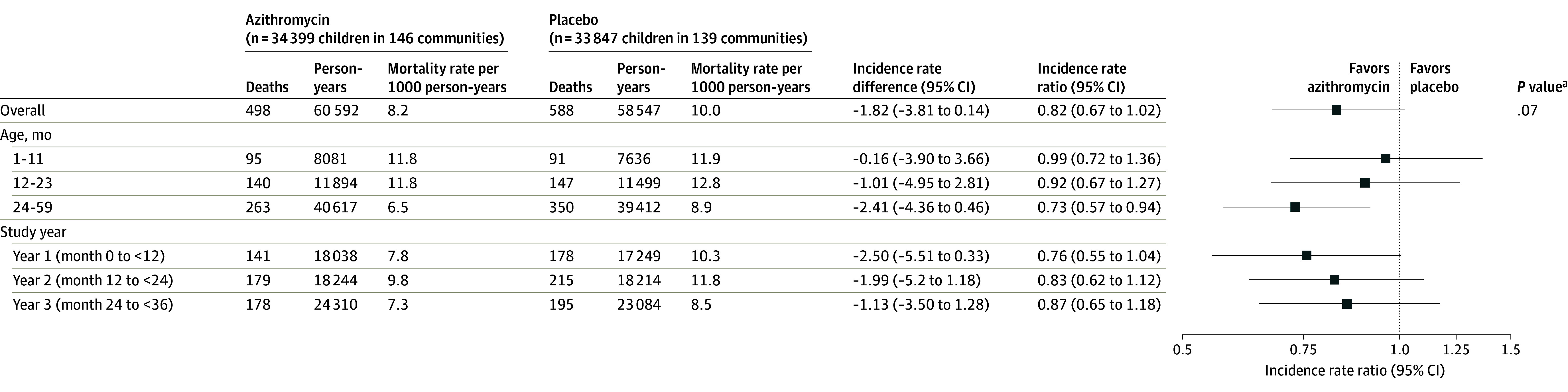
aPermutation P value with 10 000 replicates, using the log(IRR) between groups as the test statistic.
Prespecified subgroup analyses included mortality rate by age group and study year. The IRR was 0.99 (95% CI, 0.72-1.36) in those aged 1 to 11 months, 0.92 (95% CI, 0.67-1.27) in those aged 12 to 23 months, and 0.73 (95% CI, 0.57-0.94) in those aged 24 to 59 months (Figure 3). The IRR was 0.76 (95% CI, 0.55-1.04) in the first year of the study, 0.83 (95% CI, 0.62-1.12) in the second year, and 0.87 (95% CI, 0.65-1.18) in the third year (Figure 3).
No nonmortality serious adverse events were reported. The most common adverse events were gastrointestinal and included diarrhea, vomiting, and constipation (eTable 4 in Supplement 3).
Discussion
The current study done in the Nouna district in Burkina Faso demonstrated an 18% reduction in mortality following mass azithromycin distribution among children aged 1 to 59 months in a setting in which SMC was distributed and where mortality was lower compared with a previous study, although the study was likely underpowered for the primary outcome. Although the trial was large, mortality was less common than anticipated in sample size calculations, and 95% CIs narrowly overlapped the null.
Previous evidence supporting distribution of azithromycin for prevention of childhood mortality was in large part driven by the Niger site of the MORDOR trial, which also reported an 18% reduction in mortality.2 Some experts have questioned the generalizability of the findings across different settings, mortality rates, and cointerventions.12,13 Although methods in the current trial were similar to those in the MORDOR trial, the setting and conditions were different. SMC was provided to communities for all 3 years of the current trial, whereas SMC was not distributed in communities that participated in the MORDOR trial. The childhood mortality rate during the current trial was less than half of that observed in the Niger site in the MORDOR trial.3,10 SMC may have contributed to these lower mortality rates, reducing absolute differences compared with the Niger site in the MORDOR study.14 However, despite differences in cointerventions, including SMC and mortality rates, the effect of azithromycin in the current study was similar to that at the Niger site of the MORDOR trial.
The greatest reduction in mortality seen in this study was in those aged 2 to 4 years, with little difference in mortality observed in those aged 1 to 11 months. This contrasts with the MORDOR trial, in which the largest effect was seen in those aged 1 to 11 months.2 Analyses for age subgroups were prespecified but not well powered, thus this discrepancy may be due to chance. Another explanation is that infants in the study district were offered a single dose of oral azithromycin during early infancy in the concurrent CHATON trial.8,15 In this trial, children attending primary health care facilities during the first 5 to 12 weeks of life for a healthy child visit or recruited in their communities for public health outreach were randomized in a 1:1 ratio to receive a single oral dose of azithromycin or placebo. Thus, approximately half of the infants in both groups of the current trial had received an individual dose of azithromycin, perhaps lessening the difference between the 2 current trial groups for that age group. As a result, the younger subgroup may have been underpowered relative to the older subgroup.
Unlike mass community distributions, targeting azithromycin to individuals or households has not been shown to reduce mortality. When azithromycin was provided with SMC to children aged 3 to 59 months at the household level, mortality was not significantly different than with SMC alone.6 When azithromycin was provided at primary health care facilities to neonates aged 8 to 28, no significant reduction in mortality was observed.16 When provided to children with acute watery diarrhea or at hospital discharge, no significant benefit was found.17,18 Azithromycin given intrapartum reduced maternal sepsis and death, but had no discernable effect on neonatal mortality.19,20 Simultaneous community-wide distribution may reduce the community load of pathogens, rather than treating individual infections. Models suggest that simultaneous distribution offers greater antibiotic pressure than the same number of treatments taken sporadically throughout the year.21 Perhaps most importantly, the door-to-door distribution of mass campaigns may reach more vulnerable children less likely to visit health care facilities.
Mass periodic distribution of azithromycin clearly selects for macrolide-resistant bacteria.22 Increased resistance in Streptococcus pneumoniae and Escherichia coli has been well documented following mass azithromycin distribution for trachoma.23 The prevalence of macrolide resistance appears to decline after mass distributions are discontinued.24 Current distributions for childhood mortality are targeted to preschool children, as in the current trial, or infants (under conditional guidelines from WHO)—approximately one-sixth or one-thirtieth of the number of doses given in a trachoma distribution, respectively. Nevertheless, macrolide resistance increases in the nasopharynx and the gut within 2 years.25 The harm from resistant infections may be offset by the benefit of fewer deaths overall. Azithromycin remained efficacious for prevention of mortality over time in the MORDOR trial.26 Future studies will evaluate posttreatment selection for resistance in this trial and other ongoing trials.
Limitations
This study had many of the limitations inherent in a large simple trial.27 First, the size of the trial made it difficult to collect detailed information on each individual child. Deaths were determined by consecutive censuses. Children who were born after one census and died before the next census 6 months later did not contribute to either deaths or person-time at risk for the primary outcome. However, these children did not receive study treatment because treatment was provided at the same time as census and thus would not have added additional information to the study. Second, by design, no effort was made to follow up children after they moved, and death rates may have differed among children who moved or had an unknown census status. Approximately 10% of children moved during the study period; however, there was no evidence that migration was differential by group. Undercounting of deaths is unlikely to be differential by group due to the masked placebo and thus may have biased results toward the null.
Third, security concerns prevented a number of communities from undergoing even their first census and treatment. Although this was anticipated in the analysis plan, it did lower the power of the trial. Fourth, for logistical reasons, it was not possible to geographically separate clusters in the trial. If the mechanism of action of azithromycin is via reduction in pathogen transmission at the community level, it is possible that spillover effects could have benefitted children in the placebo group, biasing effects toward the null. This may have been exacerbated in larger communities that were split into multiple randomization units.
Fifth, although azithromycin was not routinely available outside of the trial, erythromycin is available in local pharmacies and children may have taken erythromycin. Sixth, although larger communities were included in the present study, the town of Nouna was excluded given its anticipated lower baseline mortality rate because it is an urban center. This may limit generalizability of the trial to rural settings. Seventh, this study was not able to evaluate the mechanism through which azithromycin reduces childhood mortality.
Conclusions
Mortality in children (age 1-59 months) was lower with biannual mass azithromycin distribution in a setting where SMC was also being distributed. The difference was not statistically significant, although the study may have been underpowered to detect a clinically relevant difference.
Educational Objective: To identify the key insights or developments described in this article.
-
Azithromycin has previously been demonstrated to reduce mortality in settings with high childhood mortality rates. What does this current study of mass azithromycin distribution add?
Prior work was conducted in the absence of seasonal malaria chemoprevention, leaving it unclear whether azithromycin added benefit.
The largest prior study (The MORDOR trial) was limited to a single country while this work expanded the effort to multiple countries across Africa and so allows for more meaningful generalization.
The World Health Organization requested this confirmatory study prior to issuing any recommendation for azithromycin distribution.
-
What were the intervention and placebo for this trial?
Community based distribution of 20 mg/kg azithromycin suspension twice yearly versus distribution of matching placebo.
Community based distribution of a single dose annual (250 mg) azithromycin tablet versus no distribution.
Home provision of weekly 20 mg/kg azithromycin suspension versus monthly 20 mg/kg azithromycin suspension.
-
What was the trial result for the primary outcome of all-cause child mortality?
In a setting with distribution of seasonal malaria prevention, there was no mortality reduction with the addition of azithromycin.
Severe medication side effects eliminated any potential benefit of azithromycin therapy.
Treated communities demonstrated an 18% reduction in mortality although confidence intervals overlapped the null and the study may have been underpowered.
Trial protocol
Statistical analysis plan
eResults
Data sharing statement
References
- 1.Golding N, Burstein R, Longbottom J, et al. Mapping under-5 and neonatal mortality in Africa, 2000-15: a baseline analysis for the Sustainable Development Goals. Lancet. 2017;390(10108):2171-2182. doi: 10.1016/S0140-6736(17)31758-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keenan JD, Bailey RL, West SK, et al. ; MORDOR Study Group . Azithromycin to reduce childhood mortality in sub-Saharan Africa. N Engl J Med. 2018;378(17):1583-1592. doi: 10.1056/NEJMoa1715474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Guideline on Mass Drug Administration of Azithromycin to Children Under Five Years of Age to Promote Child Survival. World Health Organization ; 2020. [PubMed] [Google Scholar]
- 4.Keenan JD, Arzika AM, Maliki R, et al. ; MORDOR-Niger Study Group . Cause-specific mortality of children younger than 5 years in communities receiving biannual mass azithromycin treatment in Niger: verbal autopsy results from a cluster-randomised controlled trial. Lancet Glob Health. 2020;8(2):e288-e295. doi: 10.1016/S2214-109X(19)30540-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capan M, Mombo-Ngoma G, Makristathis A, Ramharter M. Anti-bacterial activity of intermittent preventive treatment of malaria in pregnancy: comparative in vitro study of sulphadoxine-pyrimethamine, mefloquine, and azithromycin. Malar J. 2010;9:303. doi: 10.1186/1475-2875-9-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandramohan D, Dicko A, Zongo I, et al. Effect of adding azithromycin to seasonal malaria chemoprevention. N Engl J Med. 2019;380(23):2197-2206. doi: 10.1056/NEJMoa1811400 [DOI] [PubMed] [Google Scholar]
- 7.Sié A, Louis VR, Gbangou A, et al. The Health and Demographic Surveillance System (HDSS) in Nouna, Burkina Faso, 1993-2007. Glob Health Action. 2010;3:5284. doi: 10.3402/gha.v3i0.5284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sié A, Ouattara M, Bountogo M, et al. ; Étude CHAT Study Group . A double-masked placebo-controlled trial of azithromycin to prevent child mortality in Burkina Faso, West Africa: Community Health with Azithromycin Trial (CHAT) study protocol. Trials. 2019;20(1):675. doi: 10.1186/s13063-019-3855-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Cola MA, Sawadogo B, Richardson S, et al. Impact of seasonal malaria chemoprevention on prevalence of malaria infection in malaria indicator surveys in Burkina Faso and Nigeria. BMJ Glob Health. 2022;7(5):7. doi: 10.1136/bmjgh-2021-008021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basilion EV, Kilima PM, Mecaskey JW. Simplification and improvement of height-based azithromycin treatment for paediatric trachoma. Trans R Soc Trop Med Hyg. 2005;99(1):6-12. doi: 10.1016/j.trstmh.2004.01.014 [DOI] [PubMed] [Google Scholar]
- 11.Greene W. Functional forms for the negative binomial model for count data. Econ Lett. 2008;99:585-590. doi: 10.1016/j.econlet.2007.10.015 [DOI] [Google Scholar]
- 12.Oron AP, Burstein R, Mercer LD, et al. Effect modification by baseline mortality in the MORDOR azithromycin trial. Am J Trop Med Hyg. 2020;103(3):1295-1300. doi: 10.4269/ajtmh.18-1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutler T, Jannat-Khah D, Evans A. Azithromycin and childhood mortality in Africa. N Engl J Med. 2018;379(14):1382-1384. doi: 10.1056/NEJMc1808346 [DOI] [PubMed] [Google Scholar]
- 14.Issiaka D, Barry A, Traore T, et al. Impact of seasonal malaria chemoprevention on hospital admissions and mortality in children under 5 years of age in Ouelessebougou, Mali. Malar J. 2020;19(1):103. doi: 10.1186/s12936-020-03175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sié A, Ouattara M, Bountogo M, et al. Azithromycin during Routine well-infant visits to prevent death. N Engl J Med. 2024;390(3):221-229. doi: 10.1056/NEJMoa2309495 [DOI] [PubMed] [Google Scholar]
- 16.Oldenburg CE, Sie A, Bountogo M, et al. Neonatal azithromycin administration for prevention of infant mortality. NEJM Evid. Published online March 17, 2022. doi: 10.1056/EVIDoa2100054 [DOI] [PMC free article] [PubMed]
- 17.Ahmed T, Chisti MJ, Rahman MW, et al. ; Antibiotics for Children With Diarrhea (ABCD) Study Group . Effect of 3 days of oral azithromycin on young children with acute diarrhea in low-resource settings: a randomized clinical trial. JAMA Netw Open. 2021;4(12):e2136726. doi: 10.1001/jamanetworkopen.2021.36726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavlinac PB, Singa BO, Tickell KD, et al. Azithromycin for the prevention of rehospitalisation and death among Kenyan children being discharged from hospital: a double-blind, placebo-controlled, randomised controlled trial. Lancet Glob Health. 2021;9(11):e1569-e1578. doi: 10.1016/S2214-109X(21)00347-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tita ATN, Carlo WA, McClure EM, et al. ; A-PLUS Trial Group . Azithromycin to prevent sepsis or death in women planning a vaginal birth. N Engl J Med. 2023;388(13):1161-1170. doi: 10.1056/NEJMoa2212111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roca A, Camara B, Bognini JD, et al. ; PregnAnZI-2 Working Group . Effect of intrapartum azithromycin vs placebo on neonatal sepsis and death: a randomized clinical trial. JAMA. 2023;329(9):716-724. doi: 10.1001/jama.2022.24388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porco TC, Gao D, Scott JC, et al. When does overuse of antibiotics become a tragedy of the commons? PLoS One. 2012;7(12):e46505. doi: 10.1371/journal.pone.0046505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolfe RJ, Shaikh H, Tillekeratne LG. Mass drug administration of antibacterials: weighing the evidence regarding benefits and risks. Infect Dis Poverty. 2022;11(1):77. doi: 10.1186/s40249-022-00998-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Brien K, Emerson P, Hooper PJ, Dennis EG, Keenan JD, Lietman TM, Oldenburg CE. Antimicrobial resistance following mass azithromycin distribution for trachoma: a systematic review. Lancet Infect Dis. 2019;19(1):e14-e25. doi: 10.1016/S1473-3099(18)30444-4 [DOI] [PubMed] [Google Scholar]
- 24.Haug S, Lakew T, Habtemariam G, et al. The decline of pneumococcal resistance after cessation of mass antibiotic distributions for trachoma. Clin Infect Dis. 2010;51(5):571-574. doi: 10.1086/655697 [DOI] [PubMed] [Google Scholar]
- 25.Doan T, Arzika AM, Hinterwirth A, et al. ; MORDOR Study Group . Macrolide resistance in MORDOR I: a cluster-randomized trial in Niger. N Engl J Med. 2019;380(23):2271-2273. doi: 10.1056/NEJMc1901535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keenan JD, Arzika AM, Maliki R, et al. Longer-term assessment of azithromycin for reducing childhood mortality in Africa. N Engl J Med. 2019;380(23):2207-2214. doi: 10.1056/NEJMoa1817213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yusuf S, Collins R, Peto R. Why do we need some large, simple randomized trials? Stat Med. 1984;3(4):409-422. doi: 10.1002/sim.4780030421 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eResults
Data sharing statement


