Summary
Background
Gonorrhoea is a highly prevalent sexually transmitted infection and an urgent public health concern because of increasing antibiotic resistance in Neisseria gonorrhoeae. Only ceftriaxone remains as the recommended treatment in the USA. With the prospect of new anti-gonococcal antibiotics being approved, we aimed to evaluate how to deploy a new drug to maximise its clinically useful lifespan.
Methods
We used a compartmental model of gonorrhoea transmission in a US population of men who have sex with men (MSM) to compare strategies for introducing a new antibiotic for gonorrhoea treatment. The MSM population was stratified into three sexual activity groups (low, intermediate, and high) characterised by annual rates of partner change. The four introduction strategies tested were: (1) random 50–50 allocation, where each treatment-seeking infected individual had a 50% probability of receiving either drug A (current drug; a ceftriaxone-like antibiotic) or drug B (a new antibiotic), effective at time 0; (2) combination therapy of both the current drug and the new antibiotic; (3) reserve strategy, by which the new antibiotic was held in reserve until the current therapy reached a 5% threshold prevalence of resistance; and (4) gradual switch, or the gradual introduction of the new drug until random 50–50 allocation was reached. The primary outcome of interest was the time until 5% prevalence of resistance to each of the drugs (the new drug and the current ceftriaxone-like antibiotic); sensitivity of the primary outcome to the properties of the new antibiotic, specifically the probability of resistance emergence after treatment and the fitness costs of resistance, was explored. Secondary outcomes included the time to a 1% resistance threshold for each drug, as well as population-level prevalence, mean and range annual incidence, and the cumulative number of incident gonococcal infections.
Findings
Under baseline model conditions, a 5% prevalence of resistance to each of drugs A and B was reached within 13·9 years with the reserve strategy, 18·2 years with the gradual switch strategy, 19·2 years with the random 50–50 allocation strategy, and 19·9 years with the combination therapy strategy. The reserve strategy was consistently inferior for mitigating antibiotic resistance under the parameter space explored and was increasingly outperformed by the other strategies as the probability of de novo resistance emergence decreased and as the fitness costs associated with resistance increased. Combination therapy tended to prolong the development of antibiotic resistance and minimise the number of annual gonococcal infections (under baseline model conditions, mean number of incident infections per year 178 641 [range 177 998–181 731] with combination therapy, 180 084 [178 011–184 405] with the reserve strategy).
Interpretation
Our study argues for rapid introduction of new anti-gonococcal antibiotics, recognising that the feasibility of each strategy must incorporate cost, safety, and other practical concerns. The analyses should be revisited once robust estimates of key parameters—ie, the likelihood of emergence of resistance and fitness costs of resistance for the new antibiotic—are available.
Funding
US Centers for Disease Control and Prevention, National Institute of Allergy and Infectious Diseases.
Introduction
Increasing antibiotic resistance in Neisseria gonorrhoeae poses an urgent clinical and public health threat. Only one antibiotic—ceftriaxone, an extended spectrum cephalosporin—remains recommended by the US Centers for Disease Control and Prevention guidelines for empirical treatment of gonorrhoea in the USA,1 underscoring the need to develop new antibiotics to treat this highly prevalent sexually transmitted infection. Two promising, first-in-class candidates currently in phase 3 clinical trials are zoliflodacin and gepotidacin. In phase 2 trials, both demonstrated cure rates of 96% for urogenital infection; cure rates for pharyngeal infection, which has been historically harder to cure, were lower.2,3 Given the scarcity of tools remaining for gonorrhoea treatment, it is imperative to deploy new antibiotics in a way that prolongs their clinical effectiveness.
The question of how best to use multiple effective antibiotics with the aim of minimising population-level resistance has been explored in mathematical modelling studies considering a range of strategies: (1) random allocation of multiple drugs, (2) combination therapy, (3) holding in reserve a second-line drug until prevalence of resistance to the first-line drug reaches a defined threshold, (4) cycling treatment, and (5) targeted (directed) treatment, where a second-line drug is given only to patients who are non-responsive to the first.4-11 One pervasive finding across these studies is that antibiotic cycling, or the rotation of the antibiotic used as first-line therapy according to a set schedule, is inferior for mitigating antibiotic resistance; however, among alternative approaches the optimal strategy varies by study setting and pathogen of interest. Although one study focused specifically on gonorrhoea,11 none explored how and when to optimally introduce a new antibiotic.
Historically, US guidelines for gonorrhoea treatment have followed several strategies. Multiple options enabled providers to choose antibiotics during the 1990s. Increasing resistance then led to combination therapy of ceftriaxone plus azithromycin; however, rising azithromycin resistance in 2020 prompted a return to single-drug treatment with ceftriaxone.12 The question of how to best allocate multiple antibiotics will once again become relevant if zoliflodacin, gepotidacin, or another candidate gains US Food and Drug Administration (FDA) approval. Therefore, this study aimed to compare the effect of introduction strategies for a novel antibiotic on resistance of N gonorrhoeae in a US population of men who have sex with men (MSM) using a mathematical model of gonorrhoea transmission. We focused on MSM as approximately a third of US cases occur within this population13 and, because of the historic appearance of resistance to antibiotics such as ciprofloxacin within this population,14 gonorrhoea transmission models characterising US MSM have been well researched.15-17
Methods
Model overview
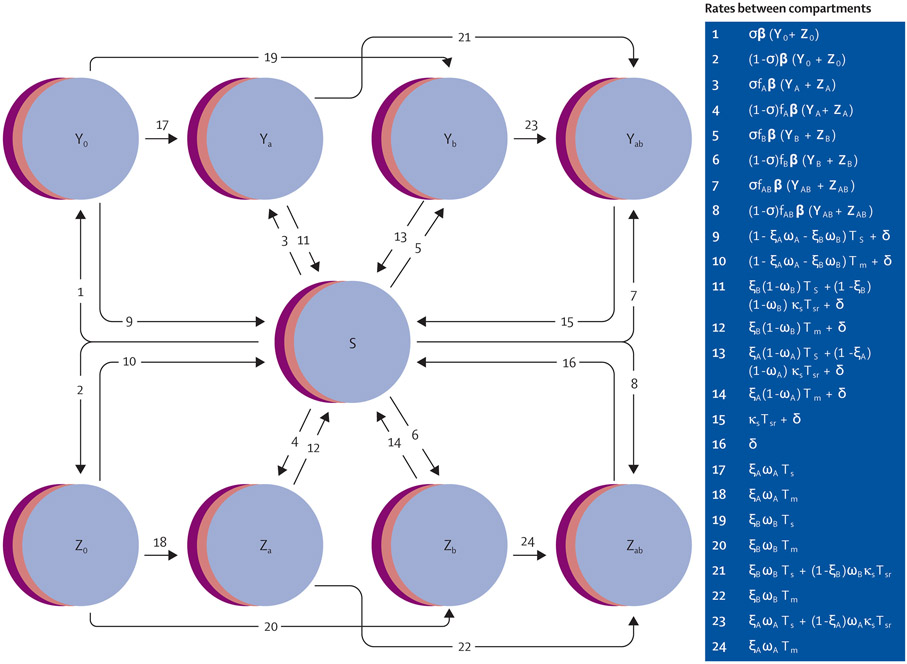
We modified the susceptible–infectious–susceptible deterministic compartmental gonorrhoea transmission model described in a previous study (figure 1, appendix pp 7-10).17 Briefly, our model consisted of an MSM population stratified into three sexual activity groups (low, intermediate, and high) characterised by annual rates of partner change. Sexual activity group was fixed for each individual, but individuals from different groups interacted with mixing parameter ε. Individuals aged into and out of the sexually active population at rate ρ, contributing for 20 years on average. Infected individuals could recover spontaneously or through antibiotic treatment. For those who sought treatment, one could receive drug A (ceftriaxone-like antibiotic) or drug B (new antibiotic), or both. Infections were stratified by symptomatic (Y) versus asymptomatic (Z) status and by resistance profile, where each infection could be resistant to drug A, drug B, neither, or both. We use the term individual only to help conceptualise the movement of this population between model compartments. No ethical approval was required for this modelling study.
Figure 1: Schematic of gonorrhoea transmission model.
Infections are further stratified by resistance profile, where 0=susceptible, a=resistant to drug A, b=resistant to drug B, and ab=resistant to both drugs. Overlapping discs for all compartments represent the model’s stratification into three sexual activity groups: low, intermediate, and high. Arrows depict rates between compartments and would be multiplied by the compartment from which they flow to generate the model’s set of differential equations (appendix, pp 8-9). Individuals can also enter and exit the population at rate ρ (arrows not shown). Rates shown here apply to random 50–50 allocation, reserve, and gradual switch strategies; rates for combination treatment vary slightly and are shown in the appendix (p 9). A transition from a drug-susceptible infection (Y0, Z0) to a dual resistant infection (YAB, ZAB) is only possible under combination therapy (arrows not shown). Definitions of all parameters used in rate equations are in table 1. S=susceptible. Y=symptomatic infection. Z=asymptomatic Infection.
Model parameterisation
Model parameters were determined from the scientific literature or model calibration using maximum likelihood estimations (table 1). We calibrated our model to a beta distribution [Beta(α=59·4, β=1919·2)] with mean 3·0% gonorrhoea prevalence (variance 1·47 × 10−5) at equilibrium, based on recent estimates in MSM.13,21,22 Model simulations for calibration were run for a drug A-only model over 2 years and parameterised using the R package bbmle.23
Table 1:
Model parameters
| Value | Domain | Source(s) | |
|---|---|---|---|
| Model calibration target | |||
| Gonorrhoea prevalence at start (calibration target mean) | 0·03 (ie, 3·0%) | (0,1) | .. |
| Model population, sexual behaviour parameters | |||
| N, population size | 106 | (0,¥) | Assumption |
| n, relative size of sexual activity groups | .. | .. | Tuite et al, 201717 |
| Low | n1=0·3 | (0,1) | .. |
| Intermediate | n2=0·6 | (0,1) | .. |
| High | n3=0·1 | (0,1) | .. |
| θ, rate of partner change per sexual activity group (per year) | .. | .. | Model fitting; Tuite et al, 201717 |
| Low (θ1) | 1*1·22 | (0,¥) | .. |
| Intermediate (θ2) | 5*1·22 | (0,¥) | .. |
| High (θ3) | 20*1·22 | (0,¥) | .. |
| ε, mixing parameter | 0·24 | (0,1) | Model fitting; Tuite et al, 201717 |
| Proportion of cases drug A (ceftriaxone-like) resistant at start | 0·0001 | (0,1) | CDC GISP 2020;12 Tuite et al, 201717 |
| Proportion of cases drug B (new drug) resistant at start | 0 | (0,1) | Assumption |
| ρ, model entry or exit rate (per year) | 1/20 | (0,¥) | Tuite et al, 201717 |
| Gonorrhoea natural history parameters | |||
| σ, proportion of incident infections that are symptomatic | 0·60 | (0,1) | Model fitting; Tuite et al, 201717 |
| b, transmission probability per partnership | 0·46 | (0,1) | Model fitting; Fingerhuth et al, 2016;15 Tuite et al, 2017;17 Tuite et al, 201818 |
| δ, natural recovery rate from infection (per year) | 1/0·462 | (0,¥) | Model fitting; Tuite et al,2017;17 Vegvari et al, 202019 |
| Treatment parameters | |||
| Ts, treatment rate if initial treatment success, symptomatic infection (per year) | 1/0·031 | (0,¥) | Model fitting; Tuite et al, 2017;17 Tuite et al, 201818 |
| Tsr, treatment rate if initial treatment failure (requiring retreatment), symptomatic infection (per year) | Ts/3 | (0,¥) | Model fitting; Tuite et al, 201717 |
| Tm, screening rate, asymptomatic infection (per year) | 0·40 | (0,¥) | Model fitting; Tuite et al, 2017;17 Tuite et al 2018;18 Hui et al, 201320 |
| ξ, probability of receiving drug upon initial treatment | |||
| Assumption | |||
| Drug A (ξA) | Strategy dependent | (0,1) | .. |
| Drug B (ξB) | Strategy dependent | (0,1) | .. |
| ω, probability of emergence of resistance upon treatment | |||
| Drug A (ωA) | 10−8 | (0,1) | Tuite et al, 2017;17 Vegvari et al, 202019 |
| Drug B (ωB) | 10−4 (10−10–10−2) | (0,1) | Assumption (range) |
| Drugs A and B (ωAB) | ωA*ωB | (0,1) | Assumption |
| f, relative fitness of resistant bacteria, compared to susceptible | |||
| A resistant (fA) | 0·98 | (0,1) | Tuite et al, 201717 |
| B resistant (fB) | 0·95 (0·80–1) | (0,1) | Assumption (range) |
| Dual A and B resistance (fAB) | fA*fB | (0,1) | Assumption |
| ks, proportion receiving retreatment if initial treatment failure, symptomatic infection | 0·90 | (0,1) | Tuite et al, 201717 |
Parameters determined through model fitting might also cite previous literature sources, which were used to inform starting values for the maximum likelihood estimation procedure. CDC GISP=US Centers for Disease Control and Prevention Gonococcal Isolate Surveillance Project. ¥=infinity.
Antimicrobial susceptibility surveillance data from the Gonococcal Isolate Surveillance Project informed a conservative estimate of prevalence of resistance to the ceftriaxone-like drug (0·01%) at time 0.12 Relevant properties of ceftriaxone, including the probability of de novo resistance (ωA) and the fitness cost associated with resistance (1–fA), were inferred from previous studies.17 We used the term drug A to emphasise that we were modelling a ceftriaxone-like drug, as its parameters are only informed estimates. All gonococcal infections were presumed susceptible to the novel antibiotic, drug B, at time 0. Baseline properties of drug B, including the probability of emergence of de novo resistance upon treatment (ωB) and the associated fitness cost (1–fB), were partly informed by the few data points from phase 2 clinical trials for gepotidacin and zoliflodacin. The baseline probability of de novo resistance for drug B was greater than for drug A given gepotidacin’s trial results suggesting resistance upon treatment, observed in two of 69 patients, is more common than with ceftriaxone.3 The baseline relative fitness of strains resistant to drug B was lower than for drug A, in keeping with reports that gyrB mutations associated with zoliflodacin resistance incur a substantial fitness cost.24 However, absolute parameter estimates were assumed and widely explored in the sensitivity analysis, partly to present results useful for any newly approved antibiotic. We assumed resistance mechanisms to drugs A and B were independent, as both zoliflodacin and gepotidacin are first-in-class candidates (of spiropyrimidinetriones and triazaacenaphthylenes, respectively) that target gyrase and topoisomerase, whereas the third-generation cephalosporin ceftriaxone targets penicillin-binding proteins.2,3
Antibiotic introduction strategies
We compared four different strategies for the deployment of drug B into the population. (1) Random 50–50 allocation: each treatment-seeking infected individual has a 50% probability of receiving either drug A or drug B, effective at time 0. (2) Combination therapy: each treatment-seeking infected individual receives both drug A and drug B, effective at time 0. (3) Reserve strategy: each treatment-seeking infected individual receives drug A until a 5% prevalence of resistance is reached in the population, at which point drug B is introduced with a sigmoid growth function until it is used for 100% of presenting cases. (4) Gradual switch: each treatment-seeking infected individual receives drug A at time 0, but drug B is gradually introduced into the population until complete random 50–50 allocation is reached within 9·5 years (midpoint 4 years).
A threshold of 5% resistance prevalence was used to trigger the switch from drug A to B under the reserve strategy, as this constitutes WHO’s threshold for changing treatment recommendations.25 We incorporated a gradual switch strategy into our analysis to depict a more realistic introduction scenario for a first-in-class antibiotic, assuming factors such as clinician hesitancy, drug supply, and distribution issues might delay uptake. For both the reserve and gradual switch strategies, the probabilities of receiving drugs A and B (ξA, ξB) for treatment-seeking individuals were not constant but updated throughout the model using sigmoid functions (appendix p 2). Antibiotic cycling was not considered in this analysis because of the aforementioned evidence against its benefit.
Of note, the above scenarios describe only the allocation of an individual’s initial course of treatment. Symptomatic individuals prescribed a drug that was ineffective against their infection could seek retreatment at rate Tsr with probability ks, at which point they received the alternative antibiotic, incorporating a targeted element into all approaches. An exception was assumed for symptomatic individuals with dual-resistant infection; these cases were retreated with a last-resort antibiotic external to our model for which we assumed complete efficacy and did not monitor resistance trends.
Model implementation
The base model was initialised with the equilibrium prevalence of gonorrhoea achieved via calibration (3·0%) and simulated over 40 years using the R package deSolve version 1.34.26 For the primary outcome of interest, we compared the time until each of drugs A and B lost clinical effectiveness for empiric use, defined by reaching a 5% prevalence of resistance among gonococcal infections. We defined this time to loss outcome (TL) by the maximum of (1) the time to 5% resistance to drug A and (2) the time to 5% resistance to drug B. Secondary outcomes explored include the time to a 1% resistance threshold for each drug, as well as population-level prevalence, mean and range annual incidence, and the cumulative number of incident gonococcal infections.
Because baseline parameters describing the properties of the novel drug B were assumed, we performed sensitivity analyses to compare strategies across a large parameter space for both the probability of resistance emergence upon treatment with drug B (ωB), and the relative fitness of infections resistant to drug B (fB). Since parameters are not known with certainty for drug A (ceftriaxone-like antibiotic), we reran the sensitivity analysis over 100 years exploring drug B’s properties under two alternate scenarios for drug A: (1) fitness cost for resistant strains (1–fA) increased to 0·10, and (2) the probability of de novo resistance (ωA) increased to 10−4. Finally, to broadly assess the effect of model parameterisation, we recalibrated the model and assessed the primary time-to-loss outcome under two alternative targets: 1·5% and 6·0% mean gonorrhoea prevalence at baseline. All code needed to run the model and produce numeric output and figures is available online.
Role of the funding source
The US Centers for Disease Control and Prevention contributed to study design and writing of the report. The National Institute of Allergy and Infectious Diseases had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
The transmission model projected that continued monotherapy with drug A (a ceftriaxone-like antibiotic) for gonorrhoea treatment would result in a 5% prevalence of resistance warranting new empirical treatment guidelines within 6·5 years in our population (table 2; appendix p 3). Assuming introduction of the novel antibiotic B, with baseline parameters for its rate of de-novo resistance emergence and associated fitness cost, the time to loss (ie, a 5% resistance threshold) of drug A ranged from 6·5 to 19·9 years depending on the introduction strategy, whereas drug B’s clinical effectiveness ranged between 13·9 and 19·9 years (table 2; appendix p 3).
Table 2:
Time to predefined 1% and 5% resistance thresholds by antibiotic introduction strategy under baseline model conditions
| Time to 1% resistance threshold (years) |
Time to 5% resistance threshold (years) |
End of drugs’ lifespan (TL) |
Prevalence of gonorrhoea at TL |
Mean number of incident infections per year, t <TL (range) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Drug A | Drug B | Drugs A and B |
Drug A | Drug B | Drugs A and B |
||||
| Random 50–50 allocation | 15·0 | 16·3 | 17·2 | 18·7 | 19·2 | 19·5 | 19·2 | 3·16% | 179 023 (178 005–182 998) |
| Combination therapy | 17·7 | 17·7 | 17·7 | 19·9 | 19·9 | 19·9 | 19·9 | 3·14% | 178 641 (177 998–181 731) |
| Reserve strategy | 4·7 | 11·8 | 14·6 | 6·5 | 13·9 | 17·9 | 13·9 | 3·13% | 180 084 (178 011–184 405) |
| Gradual switch to 50–50 | 7·9 | 15·6 | 15·8 | 13·6 | 18·2 | 18·2 | 18·2 | 3·36% | 180 983 (178 011–191 703) |
| Monotherapy, drug A | 4·7 | NA | NA | 6·5 | NA | NA | 6·5 | 3·15% | 179 220 (178 011–182 179) |
TL=time in years until both drugs A and B hit their 5% resistance thresholds, warranting new treatment recommendations. The range is bounded by the absolute minimum and maximum annual results. NA=not applicable.
Combination therapy maximised the time until 5% resistance was met for each of the drugs (TL=19·9 years); random 50–50 allocation was a close alternative, reducing the time until the drugs were lost to TL=19·2 years (table 2; appendix p 3). By contrast, the reserve strategy led to a faster build-up of infections resistant to drug A; a 5% prevalence of resistance to drug A was present within 6·5 years. This facilitated an earlier emergence of dual resistance and resulted in the shortest combined empirical lifespan of the drugs for the reserve strategy (T=13·9 years) relative to alternative approaches.
Combination therapy also minimised the mean annual number of incident gonococcal infections up until TL (table 2). Except for the reserve strategy, every strategy involving the introduction of drug B saw dual resistant strains rise to comprise more than 99% of infections after the treatments were lost given sufficient time and no alternative antibiotics; this led to the model reequilibrating at a gonorrhoea prevalence approximately three times that at baseline (appendix p 3). Under the reserve strategy, because the overlap of drug A and drug B’s use was minimised, strains resistant to drug B only took over following TL. However, if combination therapy was enacted after the loss of drug B at 13·9 years, dual resistant strains quickly rose to make up more than 99% of infections (appendix p 3). Although the reserve strategy minimised time to loss TL, it in turn maximised the incidence of gonococcal infection over time (appendix p 4).
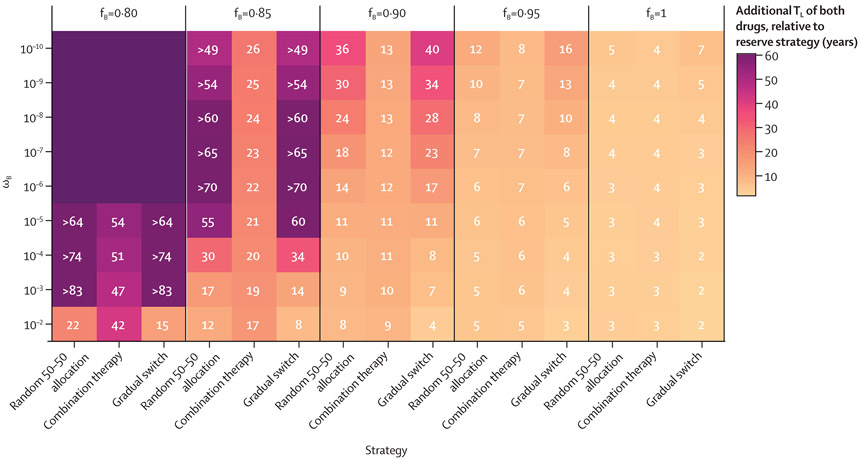
Model simulations were then run for 100 years over a larger, two-dimensional parameter space for the properties of new drug B. The probability of emergence of resistance after treatment with drug B (ωB) varied from 10−2 to 10−10, and the fitness cost associated with resistance (1–fB) varied from 0 to 0·20. Assuming baseline parameters for drug A, keeping the novel antibiotic B in reserve until drug A’s failure was not favourable under any scenario as this strategy minimised the time until each of the drugs were lost for empiric use (TL; figure 2). The combined lifespan of the drugs under the reserve strategy lagged behind alternative approaches by 2–3 years if resistance to drug B was likely to emerge (ωB≥10−4) and spread (fB=1). Altering drug B’s characteristics to make the emergence and spread of resistance more unlikely resulted in the reserve strategy performing worse by greater margins compared with the alternatives (figure 2).
Figure 2: Additional TL (in years) of each of drugs A (ceftriaxone-like) and B (new antibiotic) by strategy relative to that of the reserve strategy (TL strategy–TL reserve), based on properties of a new antibiotic B.
Strategies (x-axis) were compared over a range of plausible parameter values for ωB (y-axis) and fB (vertical facets). These properties were held constant for drug A: ωA=0·98 and fA=10−8. The model run time was extended to 100 years because some parameter sets increased the lifespan of available antibiotics to more than 40 years for all strategies. If the lifespan of the drugs extended beyond 100 years, that strategy’s results are shown either in relative terms for comparison or with an unlabelled dark purple tile, if no strategies on the x-axis had a defined TL under that parameter set for drug B. fB=fitness of drug B resistant strains relative to susceptible bacteria. TL=time in years until both drugs A and B hit their 5% resistance thresholds, warranting new treatment recommendations. ωB=probability of emergence of resistance upon treatment with drug B.
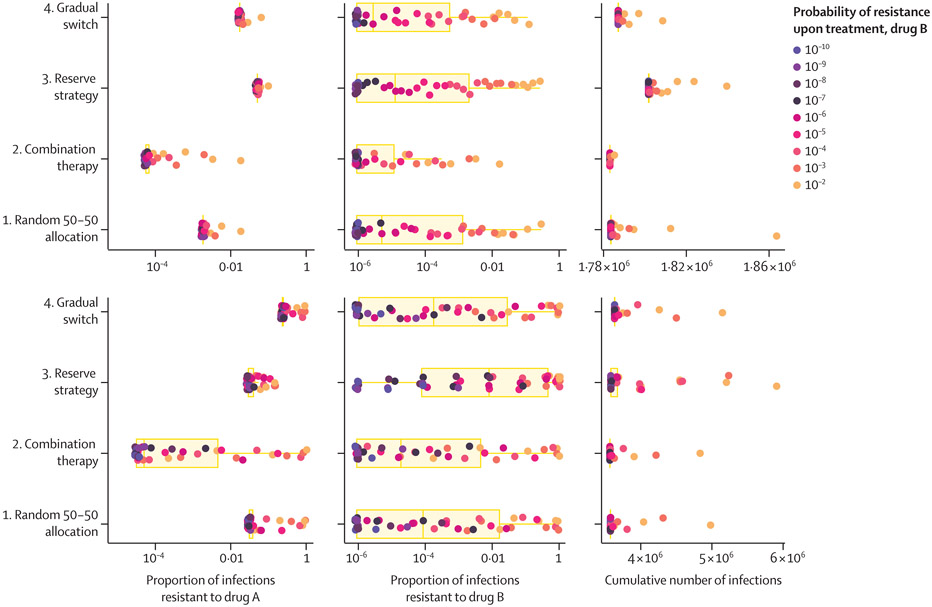
We then explored the within-strategy and between-strategy distributions of the prevalence of infections resistant to drug A and drug B, and the cumulative number of infections at two points in time (figure 3) for the same 45 potential combinations of parameters for drug B. The median proportion of infections resistant to drug A ranged from 0·006% (range 0·006–1·82) with combination therapy to 5·08% (5·08–10·0) with the reserve strategy at 10 years, and from 0·005% (range 0·003–98·7) with combination therapy to 23·4% (23·4–98·2) with the gradual switch strategy at 20 years. For drug B, the median proportion of resistant infections was 0·001% or less at 10 years for all strategies but ranged from 0·002% (range 0·000–98·7) with combination therapy to 0·80% (0·000–99·9) with the reserve strategy at 20 years.
Figure 3: Distribution of antibiotic resistance to drugs A and B and the cumulative number of infections at years 10 and 20, by introduction strategy.
Each point represents the outcome from a different model run over one of 45 parameter sets for the relative fitness of bacteria resistant to drug B (fB =0·80–1) and the probability of resistance upon treatment (ωB=10−10−10−2). Points are coloured by drug B’s probability of resistance upon treatment. Boxplots summarise the distribution of these outcomes across the 45 model runs by introduction strategy. These properties were held constant at fA=0·98 and ωA =10−8 for drug A.
To account for uncertainty in the parameter estimates for ceftriaxone, we evaluated two scenarios: (1) fitness cost for resistant strains (1–fA) increased to 0·10, and (2) probability of de novo resistance (ωA) increased to 10−4. Although absolute estimates varied, the additional time to loss of each of drugs A and B (TL) relative to that of the reserve strategy remained positive across all introduction strategies and drug B parameterisations (appendix p 5). The time by which other strategies extended the empirical use of drugs A and B relative to the reserve strategy generally increased in magnitude as both the probability of resistance emergence and the relative fitness of resistant strains decreased.
Finally, we recalibrated the model to achieve a target of 1·5% and 6·0% mean gonorrhoea prevalence at baseline (half and two times our initial assumption, respectively) and evaluated the primary time-to-loss outcome for each introduction strategy under this new model parameterisation (appendix p 6). Relative results were consistent with those previously observed.
Discussion
This study showed that in a model of gonorrhoea transmission in a population representative of US MSM, among strategies to introduce a new antibiotic with the aim of slowing the spread of resistance, reserving the novel antibiotic until substantial resistance to the current first-line drug has arisen is inferior to strategies that introduce the novel antibiotic earlier. The effect of introducing the novel drug once it becomes available varies depending on its parameters; as resistance to drug B becomes less likely to emerge and more costly, the reserve strategy is increasingly outperformed by alternative strategies in terms of extending the clinical effectiveness of the available antibiotics.
In concordance with previous studies, no single introduction strategy for a new antibiotic targeting N gonorrhoeae proves robustly optimal in the long term.4,6,9,10 Under baseline assumptions, combination therapy maximised the clinical utility of the antibiotics and minimised average annual infections, with random 50–50 allocation a close second. Across all parameters explored for drug B, combination therapy also minimised cumulative infections at 10 and 20 years on average. Although the eventual emergence of dual resistant gonorrhoea strains made combination therapy comparable to other strategies long term, its benefits were seen most starkly in the short term, as it delayed emergence of resistant gonococcal strains. As the probability of resistance after drug B treatment decreased, other strategies became more favourable than combination therapy in maximising the drugs’ combined lifespan. This observation indicates that if resistance acquisition is already unlikely, combination therapy loses some advantage. For example, if FDA approval precludes antibiotic candidates with more than 10−7 probability of resistance emergence, the random 50–50 allocation and gradual switch approaches are consistently similar or superior to combination therapy.
Substantial between-strategy variation was found in the proportion of infections resistant to drug A at 10 and 20 years, despite drug A’s fixed parameters. This finding suggests that the introduction strategy used for drug B has consequences on resistance trends to drug A.
Our analyses focused on the time to loss of the drugs for empirical treatment (TL). Interpretation of results after TL warrants caution. For example, the reserve strategy might initially appear attractive (appendix p 3), as it precludes the takeover of dual resistant strains. After the loss of drug B at 13·9 years, combination therapy could be enacted to successfully treat cases resistant to drug B only, but only for a short time until dual resistance takes over. Short-term model outcomes are also more relevant than long-term projections, as new tools for gonorrhoea management and prevention (eg, rapid antimicrobial susceptibility diagnostics and vaccines) in development might affect N gonorrhoeae’s dynamics. Of note, the reserve strategy lengthened the projected time between the loss of drugs A and B; although not inherently clinically relevant, having this lag might be more palatable to medical providers than losing both drugs in rapid succession.
Other factors beyond those explored here inform how to best deploy a new antibiotic. The cost of a novel therapeutic is a major factor in its use, as are safety, tolerability, allergy, mode and duration of administration, and drug-interaction concerns. Our analysis is best interpreted as an exploration of the ideal antibiotic introduction strategy for deterring resistance in N gonorrhoeae assuming that other practical concerns are not prohibitive (and keeping in mind key model limitations). For example, through exploring combination therapy, we assumed that this option is clinically safe and effective—ie, that drugs A and B have compatible pharmacokinetics, no antagonistic effects, and using a therapeutic dose of both in combination is non-toxic. The feasibility and effectiveness of combination therapy relies on these assumptions. A disparity in the pharmacokinetics of ceftriaxone and azithromycin is one hypothesis for why this combination therapy failed—azithromycin’s long elimination half-life compared with ceftriaxone could leave patients exposed to only a sub-inhibitory azithromycin concentration, an environment selective for resistance given recurrent gonococcal infection.27 Once cost and safety concerns are incorporated, random 50–50 allocation might be the most competitive choice as it proved only slightly less effective than combination therapy in reducing the burden of infection and resistance, on average, but is likely to minimise cost and risk of adverse events relative to combination therapy.
We were further limited in our study by ignoring bystander selection, or selection for resistance caused by gonorrhoea-infected individuals receiving antibiotics for other indications. The relative importance of bystander compared to direct selection for resistance in N gonorrhoeae is still unclear, and further research is needed to clarify its extent.27 Ceftriaxone is used to treat a variety of bacterial infections; evidence that bystander selection is a major driver of N gonorrhoeae resistance would support restricting use of a novel antibiotic to gonorrhoea indications. We also did not consider the possible importation of drug resistant strains to this US population from other countries with differing treatment policies.
Many of the model parameters describing gonorrhoea’s natural history and transmission are impossible to measure empirically, so we estimated these values with maximum likelihood estimation, an optimisation procedure that here used a single equilibrium prevalence target for model calibration. Prevalence estimates—of gonorrhoea as well as ceftriaxone-resistant strains—are potentially underestimated because of undetected asymptomatic infections. Further, estimating multiple parameters using a single prevalence estimate for calibration suggests that individual parameters would vary under alternative calibration targets or procedures.
Baseline assumptions for the characteristics of drug B were based on few phase 2 trial data for zoliflodacin and gepotidacin. Because of parameter uncertainty and our aim to present results applicable for any novel drug, we varied these parameters widely in sensitivity analysis. We also assumed no resistance to the novel antibiotic existed before its introduction, which might not be accurate. Mutations in gyrB associated with zoliflodacin resistance were found at a 0% prevalence in a large N gonorrhoeae genome database, but mutations associated with gepotidacin resistance appeared in isolates resistant to tetracycline, ciprofloxacin, and penicillin, suggesting that gepotidacin resistance might be more easily acquired in a subset of infections.28,29 Absolute estimates of the time to 5% resistance prevalence for both drugs should therefore be interpreted with caution and not extrapolated; results are best suited for relative interpretation within our population of interest, consistent with our aim of comparing the various introduction strategies.
It is key to acknowledge that our model focuses specifically on the MSM population; however, trends in gonorrhoea resistance in MSM in the USA have historically been forewarnings for resistance trends in the general population.13,14 The model does not differentiate infections by anatomical site, which might be relevant given the drug B candidates’ variable efficacy in curing non-urogenital infection.2,3 We also assumed uniform screening and treatment-seeking behaviour across MSM, by symptom status.
Overall, the steady accumulation of antibiotic resistance in N gonorrhoeae and rising gonorrhoea rates underscore the importance of optimising all tools that prevent and treat infection. The results here suggest reserve strategies are least optimal and favour combination therapy, although it will be important to revisit these conclusions with updated estimates of the rates of emergence of resistance and fitness cost of resistance, as well as with the effect of additional interventions as they become available.
Supplementary Material
Research in context.
Evidence before this study
We searched Google Scholar for modelling studies published between database inception and December, 2022 that compared antibiotic introduction strategies with the purpose of mitigating antibiotic resistance, using one or a combination of key search terms (ie, “antibiotic introduction”, “treatment protocol”, “antibiotic/antimicrobial resistance”, “gonorrhea”) plus “modeling”, with no language restrictions. We found several mathematical modelling studies that compared allocation strategies for multiple antibiotics, most of which focused on hospital-acquired infections. However, few studies had explored this question for sexually transmitted infections, and none focused on the optimal introduction approach for a novel and first-in-class antibiotic.
Added value of this study
Our modelling study focused specifically on comparing introduction strategies for a novel, first-in-class antibiotic for gonorrhoea treatment within the existing ceftriaxone monotherapy landscape. It provides evidence that is pertinent to treatment protocol development once an antibiotic candidate for gonorrhoea treatment gains regulatory approval.
Implications of all the available evidence
Reserving or restricting a novel antibiotic until substantial resistance has arisen to the current first-line drug might not be the optimal strategy for slowing the emergence and spread of resistance. In our exploratory model, combination therapy was the optimal strategy for delaying antibiotic resistance in most scenarios.
Acknowledgments
This work was supported by the US Centers for Disease Control and Prevention, the National Center for HIV, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement (grant to JAS, https://www.cdc.gov/nchhstp/neema), the National Institute of Allergy and Infectious Diseases (https://www.niaid.nih.gov/). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention. We thank Ashleigh Tuite (Department of Epidemiology, University of Toronto Dalla Lana School of Public Health, Toronto, ON, Canada) and colleagues for their deterministic compartmental gonorrhoea transmission model in MSM17 which was adapted for this research study.
Declaration of interests
YHG reports funding for this work from the US Centers for Disease Control and Prevention (CDC; contract 200-2016-91779) and from the National Institute of Allergy and Infectious Diseases (NIAID; grants R01AI132606 and R01AI153521), as well as consulting fees from GlaxoSmithKline outside the scope of the current work. MMR and JAS report funding support from the CDC and the National Center for HIV, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement (5NU38PS004651RY) for the current work. MMR also receives funding from WHO, the Harvard University Center for AIDS Research Developmental Grant, and Harvard Data Science Exploratory Award all outside the scope of the current work. RY reports R01AI153351 grant funding from NIAID for the current work. TLG and ER declare no competing interests.
Contributor Information
Emily Reichert, Department of Immunology and Infectious Diseases, Harvard TH Chan School of Public Health, Boston, MA, USA.
Reza Yaesoubi, Department of Health Policy and Management, Yale School of Public Health, New Haven, CT, USA.
Minttu M Rönn, Department of Global Health and Population, Harvard TH Chan School of Public Health, Boston, MA, USA.
Thomas L Gift, Division of STD Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Joshua A Salomon, Department of Health Policy, Stanford University School of Medicine, Stanford, CA, USA.
Yonatan H Grad, Department of Immunology and Infectious Diseases, Harvard TH Chan School of Public Health, Boston, MA, USA.
Data sharing
All code needed to simulate the data, run the model and analysis, and produce numeric output and figures are available at https://github.com/emreichert13/gc-antibioticintro.
References
- 1.US Centers for Disease Control and Prevention. Sexually transmitted infections treatment guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm (accessed Feb 14, 2023).
- 2.Taylor SN, Marrazzo J, Batteiger BE, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogenital gonorrhea. N Engl J Med 2018; 379: 1835–45. [DOI] [PubMed] [Google Scholar]
- 3.Taylor SN, Morris DH, Avery AK, et al. Gepotidacin for the treatment of uncomplicated urogenital gonorrhea: a phase 2, randomized, dose-ranging, single-oral dose evaluation. Clin Infect Dis 2018; 67: 504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonhoeffer S, Lipsitch M, Levin BR. Evaluating treatment protocols to prevent antibiotic resistance. Proc Natl Acad Sci USA 1997; 94: 12106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergstrom CT, Lo M, Lipsitch M. Ecological theory suggests that antimicrobial cycling will not reduce antimicrobial resistance in hospitals. Proc Natl Acad Sci USA 2004; 101: 13285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YC, Lipsitch M. Upgrading antibiotic use within a class: tradeoff between resistance and treatment success. Proc Natl Acad Sci USA 2006; 103: 9655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haber M, Levin BR, Kramarz P. Antibiotic control of antibiotic resistance in hospitals: a simulation study. BMC Infect Dis 2010; 10: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joyner ML, Manning CC, Canter BN. Modeling the effects of introducing a new antibiotic in a hospital setting: a case study. Math Biosci Eng 2012; 9: 601–25. [DOI] [PubMed] [Google Scholar]
- 9.Obolski U, Stein GY, Hadany L. Antibiotic restriction might facilitate the emergence of multi-drug resistance. PLoS Comput Biol 2015; 11: e1004340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tepekule B, Uecker H, Derungs I, Frenoy A, Bonhoeffer S. Modeling antibiotic treatment in hospitals: a systematic approach shows benefits of combination therapy over cycling, mixing, and mono-drug therapies. PLoS Comput Biol 2017; 13: e1005745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiridou M, Soetens LC, Koedijk FDH, van der Sande MAB, Wallinga J. Public health measures to control the spread of antimicrobial resistance in Neisseria gonorrhoeae in men who have sex with men. Epidemiol Infect 2015; 143: 1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020: Gonococcal Isolate Surveillance Project (GISP) profiles. https://www.cdc.gov/std/statistics/gisp-profiles/default.htm (accessed Feb 14, 2023).
- 13.US Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020. https://www.cdc.gov/std/statistics/2020/overview.htm (accessed Feb 14, 2023).
- 14.US Centers for Disease Control and Prevention. Increases in fluoroquinolone-resistant Neisseria gonorrhoeae among men who have sex with men—United States, 2003, and revised recommendations for gonorrhea treatment, 2004. MMWR Morb Mortal Wkly Rep 2004; 53: 335–38. [PubMed] [Google Scholar]
- 15.Fingerhuth SM, Bonhoeffer S, Low N, Althaus CL. Antibiotic-resistant Neisseria gonorrhoeae spread faster with more treatment, not more sexual partners. PLoS Pathog 2016; 12: e1005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fingerhuth SM, Low N, Bonhoeffer S, Althaus CL. Detection of antibiotic resistance is essential for gonorrhoea point-of-care testing: a mathematical modelling study. BMC Med 2017; 15: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuite AR, Gift TL, Chesson HW, Hsu K, Salomon JA, Grad YH. Impact of rapid susceptibility testing and antibiotic selection strategy on the emergence and spread of antibiotic resistance in gonorrhea. J Infect Dis 2017; 216: 1141–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuite AR, Rönn MM, Wolf EE, et al. Estimated impact of screening on gonorrhea epidemiology in the United States: insights from a mathematical model. Sex Transm Dis 2018; 45: 713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vegvari C, Grad YH, White PJ, et al. Using rapid point-of-care tests to inform antibiotic choice to mitigate drug resistance in gonorrhoea. Eurosurveillance 2020; 25: 1900210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hui BB, Wilson DP, Ward JS, et al. The potential impact of new generation molecular point-of-care tests on gonorrhoea and chlamydia in a setting of high endemic prevalence. Sex Health 2013; 10: 348–56. [DOI] [PubMed] [Google Scholar]
- 21.Johnson Jones ML, Chapin-Bardales J, Bizune D, et al. Extragenital chlamydia and gonorrhea among community venue-attending men who have sex with men—five cities, United States, 2017. MMWR Morb Mortal Wkly Rep 2019; 68: 321–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grov C, Cain D, Rendina HJ, Ventuneac A, Parsons JT. Characteristics associated with urethral and rectal gonorrhea and chlamydia diagnoses in a US national sample of gay and bisexual men: results from the one thousand strong panel. Sex Transm Dis 2016; 43: 165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolker B, R Development Core Team. bbmle: tools for general maximum likelihood estimation. R package version 1.0.25. https://CRAN.R-project.org/package=bbmle (accessed June 1, 2022). [Google Scholar]
- 24.Jacobsson S, Golparian D, Oxelbark J, et al. Pharmacodynamic evaluation of zoliflodacin treatment of Neisseria gonorrhoeae strains with amino acid substitutions in the zoliflodacin target GyrB using a dynamic hollow fiber infection model. Front Pharmacol 2022; 13: 874176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tapsall J, Anti-Infective Drug Resistance Surveillance and Containment Team. Antimicrobial resistance in Neisseria gonorrhoeae. World Health Organization; 2001, report no: WHO/CDS/CSR/DRS/2001.3. https://apps.who.int/iris/handle/10665/66963 (accessed Feb 14, 2023). [Google Scholar]
- 26.Soetaert K, Petzoldt T, Setzer R. Solving differential equations in R: Package deSolve. J Stat Softw 2010; 33: 1–25.20808728 [Google Scholar]
- 27.Olesen SW, Grad YH. Deciphering the impact of bystander selection for antibiotic resistance in Neisseria gonorrhoeae. J Infect Dis 2020; 221: 1033–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adamson PC, Lin EY, Ha SM, Klausner JD. Using a public database of Neisseria gonorrhoeae genomes to detect mutations associated with zoliflodacin resistance. J Antimicrob Chemother 2021; 76: 2847–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scangarella-Oman NE, Hossain M, Dixon PB, et al. Microbiological analysis from a phase 2 randomized study in adults evaluating single oral doses of gepotidacin in the treatment of uncomplicated urogenital gonorrhea caused by Neisseria gonorrhoeae. Antimicrob Agents Chemother 2018; 62: e01221–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All code needed to simulate the data, run the model and analysis, and produce numeric output and figures are available at https://github.com/emreichert13/gc-antibioticintro.