Abstract

E(hmds)(bqfam) (E = Ge (1a), Sn (1b); hmds = N(SiMe3)2, bqfam = N,N′-bis(quinol-8-yl)formamidinate), which are amidinatotetrylenes equipped with quinol-8-yl fragments on the amidinate N atoms, have been synthesized from the formamidine Hbqfam and Ge(hmds)2 or SnCl(hmds). Both 1a and 1b are fluxional in solution at room temperature, as the E atom oscillates from being attached to the two amidinate N atoms to being chelated by an amidinate N atom and its closest quinolyl N atom (both situations are similarly stable according to density functional theory calculations). The hmds group of 1a and 1b is still reactive and the deprotonation of another equivalent of Hbqfam can be achieved, allowing the formation of the homoleptic derivatives E(bqfam)2 (E = Ge, Sn). The reactions of 1a and 1b with [AuCl(tht)] (tht = tetrahydrothiophene), [PdCl2(MeCN)2], [PtCl2(cod)] (cod = cycloocta-1,5-diene), [Ru3(CO)12] and [Co2(CO)8] have been investigated. The gold(I) complexes [AuCl{κE-E(hmds)(bqfam)}] (E = Ge, Sn) have a monodentate κE-tetrylene ligand and display fluxional behavior in solution the same as that of 1a and 1b. However, the palladium(II) and platinum(II) complexes [MCl{κ3E,N,N′-ECl(hmds)(bqfam)}] (M = Pd, Pt; E = Ge, Sn) contain a κ3E,N,N′-chloridotetryl ligand that arises from the insertion of the tetrylene E atom into an M–Cl bond and the coordination of an amidinate N atom and its closest quinolyl N atom to the metal center. Finally, the binuclear ruthenium(0) and cobalt(0) complexes [Ru2{μE-κ3E,N,N′-E(hmds)(bqfam)}(CO)6] and [Co2{μE-κ3E,N,N′-E(hmds)(bqfam)}(μ-CO)(CO)4] (E = Ge, Sn) have a related κ3E,N,N′-tetrylene ligand that bridges two metal atoms through the E atom. For the κ3E,N,N′-metal complexes, the quinolyl fragment not attached to the metal is pendant in all the germanium compounds but, for the tin derivatives, is attached to (in the Pd and Pt complexes) or may interact with (in the Ru2 and Co2 complexes) the tin atom.
Short abstract
Amidinatotetrylenes donor functionalized on both nitrogen atoms have been prepared for the first time from N,N′-bis(quinol-8-yl)formamidine and Ge{N(SiMe3)2}2 or SnCl{N(SiMe3)2}. The resulting germylene and stannylene, decorated with two quinol-8-yl arms on both sides of the amidinato fragment, have led to metal complexes equipped with an unexpected pincer κ3E,N,N′-ligand configuration (E = Ge, Sn). Curiously, one of the two quinolyl fragments is pendant for the germanium compounds, but interacting with the tetrel atom for the tin derivatives.
1. Introduction
Heavier carbene analogues (silylenes, germylenes, stannylenes, and plumbylenes), also known as heavier tetrylenes (HTs),1 are increasingly utilized as ligands in coordination chemistry and homogeneous catalysis.2,3 Among the currently known HTs, those stabilized by amidinate fragments (amidinatotetrylenes, ATs) are playing a predominant role, since many of their metal complexes,4,5 more frequently those fitted with polydentate-AT variants,5 have shown remarkable activities in a wide variety of catalytic transformations.
In this regard, the metal complexes that are equipped with polydentate ATs have been generally6 prepared from metal-free ATs (generically E(R1NC(R2)NR1)X; E = heavier tetrel atom, X = anionic group; see Figure 1) functionalized with at least one additional donor group (D). Figure 1 shows the currently known types of metal-free potentially polydentate ATs,7−34 which formally result from (a) attaching a donor fragment to the E atom (X position; type I),7−17 (b) connecting with a linker, which can also have additional donor or prone to undergo metalation groups, two ATs through their E atoms (X position; type II),18−28 through the amidinate central C atoms (R2 position; type III)29−32 or through one of the two amidinate N atoms (R1 position; type IV),31 and (c) attaching a donor fragment to one of the N atoms (R1 position; type V).33,34 By far, types I and II, whose syntheses normally imply an easy Cl replacement with an appropriate lithiated group on well-known chlorido-ATs,35 are the most explored donor-functionalized ATs, having led to a great variety of metal complexes fitted with κ2E,D-,36−39 κ2E,E-,40,41 κ3E,D,E-,42 κ3E,C,E-43 ligands, many of them with catalytic applications.5 On the other hand, the functionalization pathways that lead to types III, IV, and V, which imply modifications on the amidinate skeleton before the AT synthesis, have been comparatively much less studied. In fact, only a few of these systems, particularly of type V, have been involved (as ligands) in coordination chemistry. They have so far led to complexes featuring κ2Ge,P-,33 κ3E,N,P-,34 and κ4E,N,P,P-33,34 ligands (E = Ge, Sn).
Figure 1.
Types of currently known metal-free potentially polydentate ATs.
This work, prompted by the current lack of studies on the coordination chemistry of polydentate-ATs nondirectly donor functionalized on the tetrel atom (types III–V), adds an unexplored configuration to the field, describing the synthesis, characterization, and some coordination chemistry of E(hmds)(bqfam) (E = Ge (1a), Sn (1b); see Figure 2, right). These compounds are, as far as we are aware, the first N,N′-bis(donor-functionalized)ATs. Germylene 1a and stannylene 1b are potentially tridentate ligands featuring an AT in the central position. Such a type of tridentate ligands has only been recently reported by our group for the PEP bis(amidinato)tetrylenes E(bzamP)2 (E = Ge, Sn; HbzamP = N-isopropyl-N′-diphenylphosphanylethyl)benzamidine; see Figure 2, left),34 which are inscribed in the also rare type V category.
Figure 2.

Currently known, including this work, potentially tridentate ligands featuring an AT fragment in the central position.
2. Results and Discussion
The reactions of Hbqfam44 with E(hmds)2 (E= Ge, Sn) in a 1:1 ratio at room temperature led to different results depending on the nature of the E atom (Scheme 1, central pathway). For E= Ge, the deprotonation of the NH of Hbqfam by one of the hmds groups of Ge(hmds)2 led to Ge(hmds)(bqfam) (1a) (94% isolated yield). However, for E = Sn, the hmds group of Sn(hmds)(bqfam) (1b), which is presumably initially formed, can also deprotonate unreacted Hbqfam, leading to the bis(amidinato)stannylene Sn(bqfam)2 (2b) as the only observed AT reaction product (mixed with Hhmds and unreacted Sn(hmds)2). Stannylene 2b was later rationally prepared (97% isolated yield) by a 2:1 reaction of Hbqfam and Sn(hmds)2 (Scheme 1, right pathway). These results show the higher basicity of the Sn-bonded hmds group (compared to that of the Ge analogue) and also indicate that the deprotonation of Hbqfam by Sn(hmds)(bqfam) (1b) is faster than that by Sn(hmds)2. Additionally, it has to be considered that tin, larger and more acidic than germanium, can accommodate easily two bqfam fragments. Heteroleptic stannylene 1b was later successfully prepared (83% isolated yield) following a two steps procedure, first deprotonating Hbqfam with Li(hmds) followed by a reaction with chloridostannylene SnCl(hmds) (Scheme 1, left pathway). This method could also be used for the preparation of 1a, albeit in lower yield than that attained using the reaction of Hbqfam with Ge(hmds)2 (77% vs 94% for the latter method). Aiming at synthesizing a germanium analogue of 2b, namely, Ge(bqfam)2 (2a), a 2:1 reaction of Hbqfam and Ge(hmds)2 was carried out, leading to the formation of the expected product as major species, but it could not be satisfactorily isolated in a pure form (Scheme 1, right pathway).
Scheme 1. Reactions Leading to Compounds 1a, 1b, 2a, and 2b.

The 1H NMR spectra of 1a and 1b in C6D6 are very similar, showing only six quinoline signals (each one integrating for 2 H) in addition to a highly deshielded signal corresponding to the central H atom of the formamidinate fragment (δ 9.61 (1a) and 9.95 (1b) ppm) and an intense (18 H) and sharp singlet corresponding to the hmds group (δ 0.37 (1a) and 0.36 (1b) ppm). These data (and also the corresponding 13C{1H} NMR spectra) are in agreement with the structure depicted for 1a and 1b in Scheme 1, in which the amidinate fragment is chelating the E atom (4-membered ENCN ring). Note that the great majority of the known metal-free ATs2f,4,5 show this chelating arrangement for the amidinate fragment (only bis(amidinato)tetrylenes have shown in certain cases that one of the two amidinato moieties is not chelating the E atom).13
The solid-state structure of stannylene 1b was established by single-crystal X-ray diffraction (SCXRD) (Figure 3). Interestingly, in contrast with the suggestion of the nuclear magnetic resonance (NMR) spectra, in the solid state, the tin atom is not chelated by the amidinate fragment, but it is attached to an amidinate N atom (Sn1–N2 2.174(1) Å) and to its closest quinolyl N atom (Sn1–N1 2.381(1) Å), forming a 5-membered SnNCCN ring. The Sn–N bond distances, including Sn–N5 (2.122(1) Å), are similar to those previously found for related pyridyl–amide-stabilized hmds stannylenes.45 While the remaining quinoline group is clearly pendant, the remaining amidinato N atom (N3) is weakly interacting with the tin atom because the Sn1···N3 distance (2.869(1) Å) is clearly shorter than the sum of the vdW radii of both elements (3.72 Å).46 The different C10–N2 and C10–N3 distances (the latter is ca. 0.06 Å shorter) reflect the iminic character of the pendant amidinate N3 atom.
Figure 3.

SCXRD molecular structure of 1b [only one of the two positions in which one of the SiMe3 groups (Si1) is disordered is shown; 50% displacement ellipsoids; H atoms omitted for clarity]. Selected interatomic distances (Å) and angles (°): Sn1···N3 2.869(1), Sn1–N1 2.381(1), Sn1–N2 2.174(1), Sn1–N5 2.122(1) N2–C10 1.352(2), N3–C10 1.297(2); N3–C10–N2 116.4(1), N5–Sn1–N2 101.09(5), N5–Sn1–N1 93.05(5), N2–Sn1–N1 70.19(5).
The asymmetric solid-state structure of 1b is not maintained in solution (according to its NMR spectra). Therefore, a fluxional process that equilibrates both quinoline fragments, possibly also operating for germylene 1a, might be operating in solution (Figure 4, top). In order to gain further insights into these processes, we modeled by density functional theory (DFT) calculations (Figure 4, bottom) the oscillation of the E atom of 1a and 1b from being attached to both amidinate N atoms (1r4E) to being chelated by an amidinate N atom and its closest quinolyl N atom (1r5E). For both tetrels, the computed Gibbs energy differences between the κN-amidinate 1r5E and the κ2N,N′-amidinate 1r4E are so small (≈ 2 kcal mol–1) that can be considered unsignificant (DFT energy calculations are affected by the used functional and atomic basis sets).47 The interconversion of 1r5E to 1r4E (Figure 4) is an elementary process for E = Sn but has two steps for E = Ge (through intermediate I1–Ge). Regarding the stannylene, the κN-amidinate 1r5Sn (Sn1–N1 2.506, Sn1–N2 2.190 Å) evolves to 1r4Sn via an easily accessible transition state (TS1) in a process that implies, in addition to the κ2N,N′-amidinate chelation, the rotation of the pendant quinolyl group to render a quasi-planar macrocycle where the amidinate and quinolyl N atoms are bonded (Sn1–N2 2.308, Sn1–N3 2.337 Å) or interacting (Sn1–N1 2.886, Sn1–N4 2.967 Å), respectively, with the tin atom. For the germylene, the 1r5Ge to 1r4Ge interconversion is analogous to that of the stannylene, but an intermediate (I1–Ge) was found in this case. Interestingly, the Ge–N distances involving the quinolyl N atoms of 1r4Ge are in the same range (Ge1–N1 = 2.877, Ge1–N4 = 2.965 Å) as those corresponding to 1r4Sn, indicating that, possibly reflecting the larger acidity and higher tendency of tin to attain larger coordination numbers, these weak E–N(quinolyl) interactions on 1r4E are stronger for tin. This fact is possibly related with 1r4E being, differently from the germylene, the most stable configuration for the stannylene. For E= Ge, the process has also a very low energy barrier (ΔG(TS2-Ge) = 4.1 (E = Ge) kcal mol–1), which is in agreement with a variable temperature 1H NMR study carried out with 1a (see Figure S2), which showed that while the Ge–N(hmds) bond rotation is impeded at very low temperatures (the sharp singlet observed at room temperature for the two SiMe3 groups of hmds splits into too broad resonances), only one set of signals is observed for the two quinolyl fragments all the way down to −90 °C.
Figure 4.
Dynamic behavior found for 1a and 1b in solution (top) and DFT-calculated (wB97xd/SDD(Ge,Sn)/cc-pVDZ) energy profile for the κN-amidinate (1r5E) to κ2N,N′-amidinate (1r4E) interconversion for E= Ge, Sn. For clarity, the optimized structures of the transition states (TS) are not shown here but are shown in Figure S17. Gibbs energies (CPCM-toluene) are given in kcal mol–1. Interatomic distances are given in Å.
The structure of the bis(amidinato)stannylene Sn(bqfam)2 (2b) could not be unambiguously determined by SCXRD; however, its NMR data (1H and 13C in CD2Cl2) indicate that in solution, the four quinolyl fragments are equivalent. These data are in agreement with the structure depicted for 2b in Scheme 1, where both amidinate fragments are chelating the Sn atom, however, considering the fluxional processes described above for 1a and 1b and having in mind the known tendency of bis(amidinato)-ATs to exhibit one amidinate fragment not chelating the E atom, many different isomeric structures, quickly exchanging in solution, are possible. Although the bis(amidinato)germylene Ge(bqfam)2 (2a) could not be isolated in a pure form, it is possibly the major product of the 2:1 reaction of Hbqfam and Ge(hmds)2 because the 1H NMR spectrum of the crude reaction outcome (see Figure S4) shows, in addition to the signals of other unidentified minor species, a set of signals very similar to that observed for stannylene 2b.
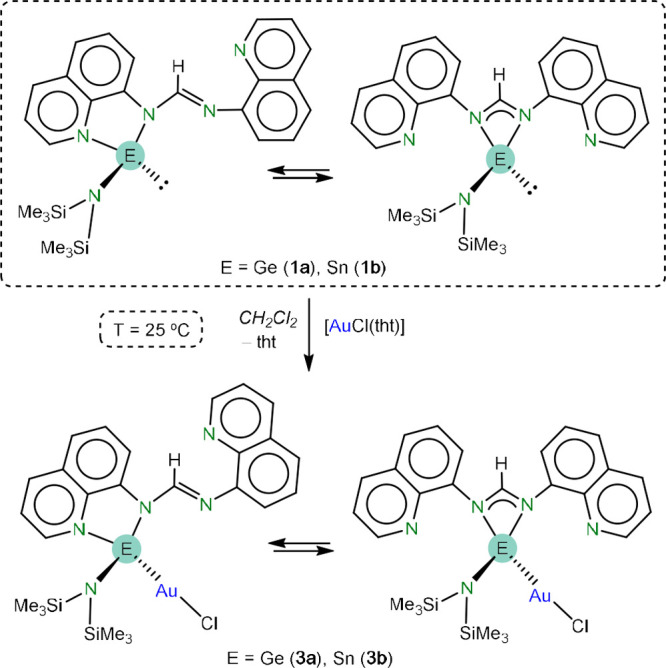
Considering that 1a and 1b represent a novel type of donor-functionalized ATs, we thought studying their reactivity toward transition metal complexes would be of interest. Their room temperature reactions with [AuCl(tht)] (tht = tetrahydrothiophene) in CH2Cl2 quickly led, upon tht replacement, to [AuCl{κ1E-E(hmds)(bqfam)} (E = Ge (3a), Sn (3b); Scheme 2) as the major reaction products (NMR analysis of crude reaction outcomes). Both complexes decomposed after standing in solution for long periods, with the formation of dark insoluble solids. Reasonably pure samples were obtained upon cooling to −20 °C saturated hexane/CH2Cl2 solutions of the complexes, which were isolated in yields of 84% (3a) and 46% (3b) as red and yellow crystals, respectively.
Scheme 2. Syntheses of Compounds 3a and 3b and Their Dynamic Behavior in Solution.
The SCXRD structure of germylene derivative 3a (Figure 5) shows a linear gold(I) complex (Cl1–Au1–Ge1 177.81(5)°) featuring a monodentate κGe-germylene. Regarding the ligand conformation, it strongly resembles that of the free stannylene 1b (Figure 3), showing also: (i) that the tetrel atom is not chelated by the amidinate fragment but attached to a amidinate N atom (Ge1–N2 1.905(5) Å) and to its closest quinolyl N atom (Ge1–N1 2.025(6) Å), forming, in this case, a 5-membered GeNCCN ring, (ii) that the remaining amidinate and quinolyl N atoms (N3 and N4) are pendant (the Ge1···N3 distance of 2.963(5) Å is also clearly shorter than the sum of the vdW radii of both elements, which is 3.66 Å),46 and (iii) that the C10–N2 and C10–N3 distances (the latter is ca. 0.06 Å shorter than the former) also reflect the iminic character of the pendant amidinate N3 atom. The Au–Ge bond distance (2.3280(8) Å) is very similar to those reported for other crystallographically characterized gold complexes equipped with neutral tricoordinated germylenes.48
Figure 5.

SCXRD molecular structure of 3a (30% displacement ellipsoids; H atoms omitted for clarity). Selected interatomic distances (Å) and angles (deg): Au1–Ge1 2.3280(8), Au1–Cl1 2.306(2), Ge1···N3 2.963(5), Ge1–N1 2.025(6), Ge1–N2 1.905(5), Ge1–N5 1.841(5); N2–C10 1.364(9), N3–C10 1.283(8); Cl1–Au1–Ge1 177.81(5), N3–C10–N2 118.0(6), N5–Ge1–N2 113.7(2), N5–Ge1–N1 102.9(2), N2–Ge1–N1 81.6(2), N5–Ge1–Au1 121.2(2), N2–Ge1–Au1 117.3(2), N1–Ge1–Au1 111.4(2).
The NMR data of 3a and 3b in CD2Cl2 are very similar to each other and also to those described above for 1a and 1b. For example, their 1H NMR spectra, in addition to the signals of the formamidinate central H atom (δ 9.74 (3a) and 10.11 (3b) ppm) and the hmds group (δ 0.24 (3a) and 0.12 (3b) ppm), show equivalent quinoline groups even at −90 °C, as evidenced by a variable temperature 1H NMR study carried out with 3a (Figure S7). Again, the asymmetric solid-state structure of 3a is not maintained in solution. Therefore, a fluxional process, similar to that described for 1a and 1b, is possibly also operating for 3a and, in extension, for the analogous stannylene complex 3b (Scheme 2).
The simple monodentate κE-coordination found for 1a and 1b in gold complexes 3a and 3b changed drastically when other metals were used. In particular, the reactions of 1a and 1b with [PdCl2(MeCN)2] and [PtCl2(cod)] at room temperature led to the pincer-type derivatives [MCl{κ3E,N,N′-ECl(hmds)(bqfam)}] (E = Ge: M = Pd (4a), Pt (5a); E = Sn: M = Pd (4b), Pt (5b)), which were isolated in moderate (46% for 5b) to excellent yields (>84%) (Scheme 3).
Scheme 3. Syntheses of Compounds 4a, 4b, 5a, and 5b.

Figure 6 shows the SCXRD molecular structures of 4a and 4b. The structure of platinum complex 5a was also established by SCXRD and is analogous to that of 4a (see Figure S18). In addition to a roughly square planar metal coordination, the structures show a tridentate κ3E,N,N′-chloridotetryl ligand that arises from the insertion of the tetrylene E atom into an M–Cl bond and the additional coordination of an amidinate N atom (N3) and its closest quinolyl N atom (N4) to the metal center. Curiously, the quinolyl fragment not attached to the metal is pendant in the germanium complex 4a but attached to tin in 4b (Sn1–N1 2.314(1) Å; note that the Sn–N(quinolyl) distance in the free ligand 1b is 2.381(3) Å). This difference (tetracoordinate germanium vs pentacoordinate tin) can be again attributed to the greater acidity of tin and its higher tendency to attain higher coordination numbers compared to those of germanium. The two quinolyl fragments are in an approximate gauche disposition in 4a (the dihedral angle between the planes defined by the quinolyl planar rings is 58.31°) and in a perfect syn arrangement for 4b, in such a way that the bqfam fragment is engaged in forming three fused roughly coplanar 5-membered rings. A similar μ–κ4N4-arrangement has been previously found for bqfam (or related N,N′-donor-functionalized amidinate ligands) in homometallic binuclear complexes of zinc, copper, palladium, platinum, etc.49 Within the amidinate fragment, the C10–N3 bond distances are only 0.02–0.03 Å shorter than the C10–N2 ones for both complexes, reflecting a higher degree of delocalization of the N=C double bond than that observed for the SCRXD characterized free ligand 1b and gold complex 3a, which feature pendant iminic N atoms.
Figure 6.

SCXRD molecular structures of 4a (top) and 4b (bottom) (30% displacement ellipsoids, H atoms omitted for clarity). Selected interatomic distances (Å) and angles (deg): 4a: Pd1–Ge1 2.2839(5), Pd1–Cl2 2.298(1), Pd1–N3 2.004(3), Pd1–N4 2.106(3), Ge1–N2 1.971(3), Ge1–N5 1.820(4), Ge1–Cl1 2.211(1), N2–C10 1.339(5), N3–C10 1.320(5); N3–Pd1–N4 80.8(1), N3–Pd1–Ge1 84.78(9), N4–Pd1–Ge1 165.5(1), N3–Pd1–Cl2 176.1(1), N4–Pd1–Cl2 101.9(1), Ge1–Pd1–Cl2 92.56(3), N3–C10–N2 121.6(4), N5–Ge1–N2 109.3(1), N5–Ge1–Cl1 106.1(1), N2–Ge1–Cl1 98.5(1), N5 Ge1 Pd1 129.9(1), N2–Ge1–Pd1 95.8(1), Cl1–Ge1–Pd1 112.39(3). 4b: Pd1–Sn1 2.4936(3), Pd1–Cl2 2.2967(9), Pd1–N3 2.016(3), Pd1–N4 2.124(3), Sn1–N1 2.314(3), Sn1–N2 2.248(3), Sn1–N5 2.107(3), Sn1–Cl1 2.431(1), N2–C10 1.336(5), N3–C10 1.305(5); N3–Pd1–N4 80.8(1), N3–Pd1–Sn1 89.64(9), N4–Pd1–Sn1 170.39(9), N3–Pd–Cl2 179.1(1), N4–Pd1–Cl2 98.82(9), Sn1–Pd1–Cl2 92.72(3), N3–C10–N2 122.4(3), N5–Sn1–N2 122.4(1), N5–Sn1–N1 85.6(1), N2–Sn1–N1 69.9(1), N5–Sn1–Cl1 116.40(9), N2–Sn1–Cl1 108.55(9), N1–Sn1–Cl1 81.76(9), N5 Sn1 Pd1 112.99(9), N2–Sn1–Pd1 85.44(8), N1–Sn1–Pd1 155.34(8), Cl1–Sn1–Pd1 105.97(3).
The NMR data of the analogous germyl complexes 4a and 5a in CD2Cl2 are very similar to each other and, differently to what was observed for the free ligands 1a and 1b and the gold complexes 3a and 3b, they show inequivalent quinoline groups, in agreement with their molecular structures. For example, their 13C{1H} NMR spectra show, in addition to the formamidinate central CH group (δ 157.1 (4a) and 157.8 (5a) ppm) and the hmds group (δ 5.3 (4a) and 5.4 (5a) ppm), 18 different quinoline signals. The NMR data of the stannyl complexes 4b and 5b are also very similar to each other, therefore indicating that both compounds are structurally analogous and show, as expected, inequivalent quinoline groups. Curiously, different from the Ge-quinolyl-pendant compounds 4a and 5a, the Sn-quinolyl-attached derivatives 4b and 5b showed very low solubility in dichloromethane (see experimental section), also rendering diluted solutions even in THF-d8. This might explain the higher degree of hydrolysis (Hhmds detection) observed for the tin complexes in their NMR spectra.
Finally, the coordination chemistry study was extended to polynuclear complexes. The reactions of 1a,b with 0.66 equiv of [Ru3(CO)12] or 1 equiv of [Co2(CO)8] at 60 °C led to the bimetallic derivatives [M2{μE-κ3E,N,N′-E(hmds)(bqfam)}(μ-CO)x(CO)y] (M = Ru; x = 0; y = 6: E = Ge (6a), Sn (6b). M = Co; x = 1; y = 4: E = Ge (7a), Sn (7b)), which were isolated in very good yields (80–90%) (Scheme 4). Note that the reactions of 1a,b with [Ru3(CO)12] in a 1:1 ratio led to the same products, leaving some unreacted [Ru3(CO)12]. All complexes feature a tetrylene-bridging ligand that is additionally coordinated to one of the metals by one amidinate N atom and its closest quinolyl N atom, forming a μE-κ3E,N,N′-ligand, being the quinolyl fragment not attached to the metal, pendant for the germanium complexes 6a and 7a, and possibly weakly interacting with the tin atom for 6b and 7b. The coordination spheres of the two metals are completed by carbonyl ligands (six terminal for the ruthenium derivatives and four terminal and one bridging for the cobalt complexes).
Scheme 4. Syntheses of Compounds 6a, 6b, 7a, and 7b.

The structures of these bimetallic complexes are proposed based on the following: (i) the molecular structure of the Ru2Ge derivative 6a could be unambiguously determined by SCXRD (Figure 7, top), showing the aforementioned ligand coordination and a pendant quinolyl fragment, (ii) the DFT-optimized structure of the Ru2Sn complex 6b (Figure 7, bottom), which is analogous to that of 6a, shows a much shorter E···N(quinolyl) distance, (iii) the pincer-type κ3E,N,N′-ligand coordination mode exhibited by the ligands is essentially identical to that observed in the tetryl-palladium and -platinum complexes 4a,b and 5a,b, which also feature tetra- and penta-coordinated germanium and tin atoms, respectively, (iv) the ring opening of the EN2C four-membered ring of nondonor-functionalized amidinatogermylenes to produce related M2Ge (M = Ru, Co) carbonyl complexes has been previously described by our group.50,51 In particular, the compounds [Ru2{μGe-κ2Ge,N-GeX(iPrNC(Ph)NiPr)}(CO)7] (X = hmds, tBu)50 and [Co2{μGe-κ2Ge,N-Ge(hmds)(iPrNC(Ph)iPr)}(μ-CO)(CO)5]51 could be prepared in reactions of the corresponding germylenes with [Ru3(CO)12] or [Co2(CO)8], which differ with 6a,b and 7a,b in the number of carbonyl ligands (they have one more), since they are fitted with bidentate μGe-κ2Ge,N-ligands.
Figure 7.

SCXRD molecular structure of 6a (top; 30% displacement ellipsoids) and the DFT-optimized structure of 6b (bottom). H atoms been omitted for clarity. Selected interatomic distances (Å) and angles (deg): 6a: Ru1–Ge1 2.3898(3), Ru1–Ru2 2.9628(3), Ru1–N3 2.109(2), Ru1–N4 2.176(2), Ge1–N2 1.992(2), Ge1···N1 3.600(2), Ge1–N5 1.862(2), Ge1–Ru2 2.5063(3), N2–C10 1.330(3), N3–C10 1.320(3); N3–C10–N2 121.3(2), N5–Ge1–N2 102.6(1) N5–Ge1–Ru2 132.02(7), N2–Ge1–Ru2 111.38(6), N5–Ge1–Ru1 135.15(7), N2–Ge1–Ru1 95.91(6), Ru1–Ge1–Ru2 74.43(1) 6b: Ru1–Sn1 2.560, Ru1–Ru2 3.130, Ru1–N3 2.152, Ru1–N4 2.202, Sn1–N2 2.209, Sn1···N1 3.044, Sn1–N5 2.024, Sn1–Ru2 2.662, N2–C10 1.324, N3–C10 1.320; N3–C10–N2 123.11, N5–Sn1–N2 100.65, N5–Sn1–Ru2 140.28, N2–Sn1–Ru2 109.14, N5Sn1Ru1 133.47, N2–Sn1–Ru1 89.34, Ru1–Sn1–Ru2 73.63.
The geometrical parameters (Ru–Ru, Ru–Ge, Ge–N and Ru–N bond distances) of the SCXRD molecular structure of 6a (Figure 7, top) are very similar to those previously reported for the aforementioned related Ru2Ge complexes.50 Within the amidinate fragment, the C–N bond distances differ by only 0.01 Å, reflecting a high degree of delocalization of the N=C bond. The geometrical parameters (Ru–Ru, Ru–Sn, Sn–N, and Ru–N bond distances) of the DFT-optimized structure of 6b (Figure 7, bottom), compared to those of the SCXRD structure of 6a (Figure 7, top), reflect the larger size of the tin atom (note that de DFT-optimized structure of 6a, Figure S19, is closely related to its solid state structure). However, as previously mentioned, the distance between the N atom (N1) of the quinolyl fragment not attached to the metal and the tetrel atom is much shorter for tin (E···N1 = 3.600(2) (6a), 3.044 (6b) Å). While this Sn···N1 distance is clearly shorter than the sum of the vdW radii of both elements,46 indicating the existence of a weak interaction, it is much larger than the Sn–N(quinolyl) bond distances found in the free ligand 1b (2.381(1) Å) or in the stannyl-palladium complex 4b (Sn1–N1 2.314(3) Å), where the quinolyl N atom is attached to tin.
The NMR data of all complexes are in agreement with the structures unambiguously established (6a) or proposed (6b, 7a, and 7b), since they show inequivalent quinoline groups (18 different quinoline signals can be found in the 13C{1H} NMR spectra). Differently to that observed for the tetryl-palladium and -platinum complexes, at room temperature, the E–N(hmds) bond rotation is absent (for 6a and 7a,b) or impeded (for 6b), since two different signals (broad for 6b) are observed for the SiMe3 groups of hmds. This reflects the higher steric hindrance exerted by the M(CO)4 unit (M = Ru, Co) compared with that of the Cl atom attached to the tetrel atom in the corresponding complexes. A small (6a, 7a,b) or high (6b) degree of hydrolysis (Hhmds detection), even in carefully dried CD2Cl2, was observed. Similarly to the tetryl-palladium and -platinum complexes, the tin derivatives 6b and 7b showed lower solubility than the germanium analogues 6a and 7a, which seems to be related to their main structural difference (short vs long E···N(quinolyl) distances, respectively).
Emphasizing the novelty of the metal complexes described (M ≠ Au), a search in the Cambridge Crystallographic database52 showed that, while κ3E,N2-ligands (E = group-14 atom in no specific position within the ligand scaffold) are very well represented for E = Si,53 very few examples are known for the heavier group-14 elements; in particular, they are only known for E = Sn (tungsten complexes featuring κ3N2,Sn organotin-functionalized bis(pyrazol-1-yl)methane ligands).54
The coordination chemistry of bis(amidinato)stannylene Sn(bqfam)2 (2b) was not investigated in detail since an initial assessment resulted in discouraging results. For example, the reaction of [PdCl2(MeCN)2] with 2b led to the formation of an intractable, very insoluble solid that could not be identified. The large amount of donor groups available in 2b, which facilitates intermolecular interactions making the formation of different aggregates highly possible, may be behind this result.
3. Conclusions
In this work, we have prepared and characterized the compounds E(hmds)(bqfam) (E = Ge (1a), Sn (1b)), which are, as far as we are aware, the first N,N′-bis(donor)-functionalized amidinatotetrylenes, adding a novel type to this important family of ligands. The unique features of 1a and 1b, equipped with two quinol-8-yl arms on both sides of the amidinate fragment, allow the tetrel atom to easily oscillate in solution, in a fluxional process, from κ2N,N′-amidinate to being chelated by an amidinate N atom and its closest quinolyl N atom, the latter being the preferred arrangement in the solid state (SCXRD structure of 1b). This feature contrasts with the great majority of the metal-free ATs known,2f,4,5 which lack donor-functionalization on their N atoms and exhibit a closed κ2N,N′-amidinate arrangement.
The coordination chemistry of 1a and 1b (we designed them looking for κ3N,E,N′-ligands) reported in this work has shown that (i) a monodentate κE-coordination is possible despite all the donor groups available (complexes 3a and 3b), (ii) a pincer tridentate κ3E,N,N′-coordination, different from the expected κ3N,E,N′-one, is the preferred behavior (complexes 4a,b–7a,b), as a consequence of the facile involvement of one of the amidinate N atoms in the coordination process, and, (iii) for the pincer metal complexes, one of the two quinolyl fragments is pendant for the germanium compounds but attached or interacting with the tetrel atom for the tin derivatives. This fact highlights the great versatility of HTs since a simple modification of the tetrel atom in isostructural ligands can lead to substantial structural changes.
Acknowledgments
This work has been supported by research grants obtained from Agencia Estatal de Investigación (PID2019-104652GB-I00 and RED2022-134287-T). The authors also acknowledge the technical support provided by Servicios Científico-Técnicos de la Universidad de Oviedo.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.3c04135.
The authors declare no competing financial interest.
Supplementary Material
References
- For some reviews on general chemistry of HTs (they might include coordination chemistry), see:; a Zhang Y.; Wu L.; Wang H. Application of N-heterocyclic silylenes in low-valent group 13, 14 and 15 chemistry. Coord. Chem. Rev. 2023, 477, 214942–214961. 10.1016/j.ccr.2022.214942. [DOI] [Google Scholar]; b Yao S.; Saddington A.; Xiong Y.; Driess M. Chelating Bis-silylenes As Powerful Ligands To Enable Unusual Low-Valent Main-Group Element Functions. Acc. Chem. Res. 2023, 56, 475–488. 10.1021/acs.accounts.2c00763. [DOI] [PubMed] [Google Scholar]; c Wang L.; Li Y.; Li Z.; Kira M. Isolable Silylenes and their Diverse Reactivity. Coord. Chem. Rev. 2022, 457, 214413–214431. 10.1016/j.ccr.2022.214413. [DOI] [Google Scholar]; d Se N.; Khan S. Heavier Tetrylenes as Single Site Catalysts. Chem.—Asian J. 2021, 16, 705–719. 10.1002/asia.202100038. [DOI] [PubMed] [Google Scholar]; e Dasgupta R.; Khan S. N-Heterocyclic Germylenes and Stannylenes: Synthesis, Reactivity and Catalytic Application in a Nutshell. Adv. Organomet. Chem. 2020, 74, 105–152. 10.1016/bs.adomc.2020.04.001. [DOI] [Google Scholar]; f Fujimori S.; Inoue S. Small Molecule Activation by Two-Coordinate Acyclic Silylenes. Eur. J. Inorg. Chem. 2020, 3131–3142. 10.1002/ejic.202000479. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Khan S.; Roesky H. W. Carbene-Stabilized Exceptional Silicon Halides. Chem.—Eur. J. 2019, 25, 1636–1648. 10.1002/chem.201801672. [DOI] [PubMed] [Google Scholar]; h Hadlington T.; Driess M.; Jones C. Low-Valent Group 14 Element Hydride Chemistry: Towards Catalysis. Chem. Soc. Rev. 2018, 47, 4176–4197. 10.1039/C7CS00649G. [DOI] [PubMed] [Google Scholar]; i Rivard E. Group 14 Inorganic Hydrocarbon Analogues. Chem. Soc. Rev. 2016, 45, 989–1003. 10.1039/C5CS00365B. [DOI] [PubMed] [Google Scholar]; j Marschner C. Silylated Group 14 Ylenes: An Emerging Class of Reactive Compounds. Eur. J. Inorg. Chem. 2015, 2015, 3805–3820. 10.1002/ejic.201500495. [DOI] [Google Scholar]; k Prabusankar G.; Sathyanarayana A.; Suresh P.; Babu C. N.; Srinivas K.; Metla B. P. R. N-Heterocyclic Carbene Supported Heavier Group 14 Elements: Recent Progress and Challenges. Coord. Chem. Rev. 2014, 269, 96–133. 10.1016/j.ccr.2014.01.036. [DOI] [Google Scholar]; l Izod K. Heavier group 14 complexes with anionic P-donor ligands Coord. Chem. Rev. 2013, 257, 924–945. 10.1016/j.ccr.2013.01.004. [DOI] [Google Scholar]; m Xiong Y.; Yao S.; Driess M. Chemical Tricks To Stabilize Silanones and Their Heavier Homologues with E = O Bonds (E = Si–Pb): From Elusive Species to Isolable Building Blocks. Angew. Chem., Int. Ed. 2013, 52, 4302–4311. 10.1002/anie.201209766. [DOI] [PubMed] [Google Scholar]; n Asay M.; Jones C.; Driess M. N-Heterocyclic Carbene Analogues with Low-Valent Group 13 and Group 14 Elements: Syntheses, Structures, and Reactivities of a New Generation of Multitalented Ligands. Chem. Rev. 2011, 111, 354–396. 10.1021/cr100216y. [DOI] [PubMed] [Google Scholar]; o Mandal S. K.; Roesky H. W. Interstellar molecules: guides for new chemistry. Chem. Commun. 2010, 46, 6016–6041. 10.1039/c0cc01003k. [DOI] [PubMed] [Google Scholar]; p Mizuhata Y.; Sasamori T.; Tokitoh N. Stable Heavier Carbene Analogues. Chem. Rev. 2009, 109, 3479–2511. 10.1021/cr900093s. [DOI] [PubMed] [Google Scholar]
- For some reviews more focused on the coordination chemistry of HTs, see:; a Cabeza J. A.; García-Álvarez P. Tetrelanes versus Tetrylenes as Precursors to Transition Metal Complexes Featuring Tridentate PEP Tetryl Ligands (E=Si, Ge, Sn). Chem. Eur. J. 2023, 29, e202203096. 10.1002/chem.202381861. [DOI] [PubMed] [Google Scholar]; b Lee V. L. Schrock-Type Silylidenes and Germylidenes Found Among the Silylene and Germylene Complexes of the Early and Mid-Transition Metals. Eur. J. Inorg. Chem. 2022, 2022, e202200175 10.1002/ejic.202200175. [DOI] [Google Scholar]; c Somerville R. J.; Campos J. Cooperativity in Transition Metal Tetrylene Complexes. Eur. J. Inorg. Chem. 2021, 2021, 3488–3498. 10.1002/ejic.202100460. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Gosh M.; Khan S. N-Heterocyclic silylenes in coinage metal chemistry: an account of recent advances. Dalton. Trans. 2021, 50, 10674–10688. 10.1039/D1DT01955D. [DOI] [PubMed] [Google Scholar]; e Tacke R.; Ribbeck T. Bis(amidinato)- and bis(guanidinato)- silylenes and silylenes with one sterically demanding amidinato or guanidinato ligand: synthesis and reactivity. Dalton Trans. 2017, 46, 13628–13659. 10.1039/C7DT01297G. [DOI] [PubMed] [Google Scholar]; f Álvarez-Rodríguez L.; Cabeza J. A.; García-Álvarez P.; Polo D. The transition-metal chemistry of amidinatosilylenes, -germylenes and -stannylenes. Coord. Chem. Rev. 2015, 300, 1–28. 10.1016/j.ccr.2015.04.008. [DOI] [Google Scholar]; g Baumgartner J.; Marschner C. Coordination of Non-Stabilized Germylenes, Stannylenes, and Plumbylenes to Transition Metals. Rev. Inorg. Chem. 2014, 34, 119–152. 10.1515/revic-2013-0014. [DOI] [Google Scholar]; h Blom B.; Stoelzel M.; Driess M. New Vistas in N-Heterocyclic Silylene (NHSi) Transition-Metal Coordination Chemistry: Syntheses, Structures and Reactivity towards Activation of Small Molecules. Chem.—Eur. J. 2013, 19, 40–62. 10.1002/chem.201203072. [DOI] [PubMed] [Google Scholar]; i Waterman R.; Hayes P. G.; Tilley T. D. Development and Chemical Reactivity of Transition-Metal Silylene Complexes. Acc. Chem. Res. 2007, 40, 712–719. 10.1021/ar700028b. [DOI] [PubMed] [Google Scholar]; j Lappert M. F.; Rowe R. S. The Role of Group 14 Element Carbene Analogues in Transition Metal Chemistry. Coord. Chem. Rev. 1990, 100, 267–292. 10.1016/0010-8545(90)85012-H. [DOI] [Google Scholar]
- For some reviews more focused on HT transition-metal complexes in catalysis, see:; a Cabeza J. A.; García-Álvarez P. Cyclometallation of Heavier Tetrylenes: Reported Complexes and Applications in Catalysis. Eur. J. Inorg. Chem. 2021, 2021, 3315–3326. 10.1002/ejic.202100430. [DOI] [Google Scholar]; b Zhou Y.-P.; Driess M. Isolable Silylene Ligands Can Boost Efficiencies and Selectivities in Metal-Mediated Catalysis. Angew. Chem., Int. Ed. 2019, 58, 3715–3728. 10.1002/anie.201811088. [DOI] [PubMed] [Google Scholar]; c Raoufmoghaddam S.; Zhou Y.-P.; Wang Y.; Driess M. N-heterocyclic silylenes as powerful steering ligands in catalysis. J. Organomet. Chem. 2017, 829, 2–10. 10.1016/j.jorganchem.2016.07.014. [DOI] [Google Scholar]; d Blom B.; Gallego D.; Driess M. N-heterocyclic silylene complexes in catalysis: new frontiers in an emerging field. Inorg. Chem. Front. 2014, 1, 134–148. 10.1039/C3QI00079F. [DOI] [Google Scholar]
- For examples of catalytic transformations promoted by metal-complexes equipped with monodentate-ATs, see:; a Fan Q.; Du X.; Yang W.; Li Q.; Huang W.; Sun H.; Hinz A.; Li X. Effects of silylene ligands on the performance of carbonyl hydrosilylation catalyzed by cobalt phosphine complexes. Dalton Trans. 2023, 52, 6712–6721. 10.1039/D3DT00372H. [DOI] [PubMed] [Google Scholar]; b Hossain J.; Sai J. S.; Srinu T.; Parameswaran P.; Khan S. NHSi/NHGe-Supported Copper Halide and Pseudohalide Complexes: Synthesis and Application. Organometallics 2022, 41, 3706–3717. 10.1021/acs.organomet.2c00480. [DOI] [Google Scholar]; c Parvin N.; Hossain J.; George A.; Parameswaran P.; Khan S. N-heterocyclic silylene stabilized monocordinated copper(I)–arene cationic complexes and their application in click chemistry. Chem. Commun. 2020, 56, 273–276. 10.1039/C9CC09115G. [DOI] [PubMed] [Google Scholar]; d Parvin N.; Mishra B.; George A.; Neralkar M.; Hossain J.; Parameswaran P.; Hotha S.; Khan S. N-Heterocyclic silylene/germylene ligands in Au(I) catalysis. Chem. Commun. 2020, 56, 7625–7628. 10.1039/D0CC03156A. [DOI] [PubMed] [Google Scholar]; e Paesch A. N.; Kreyenschmidt A.-K.; Herbst-Irmer R.; Stalke D. Side-Arm Functionalized Silylene Copper(I) Complexes in Catalysis. Inorg. Chem. 2019, 58, 7000–7009. 10.1021/acs.inorgchem.9b00629. [DOI] [PubMed] [Google Scholar]; f Qi X.; Sun H.; Li X.; Fuhr O.; Fenske D. Synthesis and catalytic activity of N-heterocyclic silylene (NHSi) cobalt hydride for Kumada coupling reactions. Dalton Trans. 2018, 47, 2581–2588. 10.1039/C7DT04155A. [DOI] [PubMed] [Google Scholar]; g Álvarez-Rodríguez L.; Cabeza J. A.; García-Álvarez P.; Pérez-Carreño E. Ruthenium Carbene Complexes Analogous to Grubbs-I Catalysts Featuring Germylenes as Ancillary Ligands. Organometallics 2018, 37, 3399–3406. 10.1021/acs.organomet.7b00905. [DOI] [Google Scholar]; h Khoo S. S.; Jiajia C.; Yang M.-C.; Shan Y.-L.; Su M.-D.; So C.-W. Synthesis of a Dimeric Base-Stabilized Cobaltosilylene Complex for Catalytic C–H Bond Functionalization and C–C Bond Formation. Chem.—Eur. J. 2018, 24, 14329–14334. 10.1002/chem.201803410. [DOI] [PubMed] [Google Scholar]; i Álvarez-Rodríguez L.; Cabeza J. A.; Fernández-Colinas J. M.; García-Álvarez P.; Polo D. Amidinatogermylene Metal Complexes as Homogeneous Catalysts in Alcoholic Media. Organometallics 2016, 35, 2516–2523. 10.1021/acs.organomet.6b00426. [DOI] [Google Scholar]; j Blom B.; Enthaler S.; Inoue S.; Irran E.; Driess M. Electron-Rich N-Heterocyclic Silylene (NHSi)–Iron Complexes: Synthesis, Structures, and Catalytic Ability of an Isolable Hydridosilylene–Iron Complex. J. Am. Chem. Soc. 2013, 135, 6703–6803. 10.1021/ja402480v. [DOI] [PubMed] [Google Scholar]
- For examples of catalytic transformations promoted by metal complexes equipped with polydentate-ATs, see:; a Jia H.; Du S.; Xu C.; Mo Z. Hydrogenation of Olefins Catalyzed by a Cobalt(I) Hydride Complex with N-Heterocyclic Silylene. Eur. J. Inorg. Chem. 2023, 26, e202300086 10.1002/ejic.202300086. [DOI] [Google Scholar]; b Roque J. B.; Pabst T. P.; Chirik P. J. C(sp2)–H Activation with Bis(silylene)pyridine Cobalt(III) Complexes: Catalytic Hydrogen Isotope Exchange of Sterically Hindered C–H Bonds. ACS Catal. 2022, 12, 8877–8885. 10.1021/acscatal.2c02429. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Lücke M.-P.; Yao S.; Driess M. Boosting homogeneous chemoselective hydrogenation of olefins mediated by a bis(silylenyl)terphenyl-nickel(0) pre-catalyst. Chem. Sci. 2021, 12, 2909–2915. 10.1039/D0SC06471H. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Sun X.; Simler T.; Kraetschmer F.; Roesky P. W. Thermally Stable Rare-Earth Metal Complexes Supported by Chelating Silylene Ligands. Organometallics 2021, 40, 2100–2107. 10.1021/acs.organomet.1c00238. [DOI] [Google Scholar]; e Li S.; Wang Y.; Yang W.; Li K.; Sun H.; Li X.; Fuhr O.; Fenske D. N2 Silylation Catalyzed by a Bis(silylene)-Based [SiCSi] Pincer Hydrido Iron(II) Dinitrogen Complex. Organometallics 2020, 39, 757–766. 10.1021/acs.organomet.0c00025. [DOI] [Google Scholar]; f Arevalo R.; Pabst T. P.; Chirik P. J. C(sp2)–H Borylation of Heterocycles by Well-Defined Bis(silylene)pyridine Cobalt(III) Precatalysts: Pincer Modification, C(sp2)–H Activation, and Catalytically Relevant Intermediates. Organometallics 2020, 39, 2763–2773. 10.1021/acs.organomet.0c00382. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Qi X.; Zheng T.; Zhou J.; Dong Y.; Zuo X.; Li X.; Sun H.; Fuhr O.; Fenske D. Synthesis and Catalytic Activity of Iron Hydride Ligated with Bidentate N-Heterocyclic Silylenes for Hydroboration of Carbonyl Compounds. Organometallics 2019, 38, 268–277. 10.1021/acs.organomet.8b00700. [DOI] [Google Scholar]; h Zhou Y.-P.; Mo Z.; Luecke M.-P.; Driess M. Stereoselective Transfer Semi-Hydrogenation of Alkynes to E-Olefins with N-Heterocyclic Silylene–Manganese Catalysts. Chem.—Eur. J. 2018, 24, 4780–4784. 10.1002/chem.201705745. [DOI] [PubMed] [Google Scholar]; i Bai Y.; Zhanga J.; Cui C. An arene-tethered silylene ligand enabling reversible dinitrogen binding to iron and catalytic silylation. Chem. Commun. 2018, 54, 8124–8127. 10.1039/C8CC03734E. [DOI] [PubMed] [Google Scholar]; j Mo Z.; Kostenko A.; Zhou Y.-P.; Yao S.; Driess M. Chelate Silylene–Silyl Ligand Can Boost Rhodium-Catalyzed C–H Bond Functionalization Reactions. Chem.—Eur. J. 2018, 24, 14608–14612. 10.1002/chem.201803089. [DOI] [PubMed] [Google Scholar]; k Cabeza J. A.; García-Álvarez P.; González-Álvarez L. Facile cyclometallation of a mesitylsilylene: synthesis and preliminary catalytic activity of iridium(III) and iridium(V) iridasilacyclopentenes. Chem. Commun. 2017, 53, 10275–10278. 10.1039/C7CC04832G. [DOI] [PubMed] [Google Scholar]; l Schmidt M.; Blom B.; Szilvási T.; Schomacker R.; Driess M. Improving the Catalytic Activity in the Rhodium-Mediated Hydroformylation of Styrene by a Bis(N-heterocyclic silylene) Ligand. Eur. J. Inorg. Chem. 2017, 2017, 1284–1291. 10.1002/ejic.201700148. [DOI] [Google Scholar]; m Luecke M. P.; Porwai D.; Kostenko A.; Zhou Y.-P.; Yao S.; Keck M.; Limberg C.; Oestreich M.; Driess M. Bis(silylenyl)-substituted ferrocene-stabilized η6-arene iron(0) complexes: synthesis, structure and catalytic application. Dalton Trans. 2017, 46, 16412–16418. 10.1039/C7DT03301J. [DOI] [PubMed] [Google Scholar]; n Ren H.; Zhou Y.-P.; Bai Y.; Cui C.; Driess M. Cobalt-Catalyzed Regioselective Borylation of Arenes: N-Heterocyclic Silylene as an Electron Donor in the Metal-Mediated Activation of C–H Bonds. Chem.—Eur. J. 2017, 23, 5663–5667. 10.1002/chem.201605937. [DOI] [PubMed] [Google Scholar]; o Wang Y.; Kostenko A.; Yao S.; Driess M. Divalent Silicon-Assisted Activation of Dihydrogen in a Bis(N-heterocyclic silylene)xanthene Nickel(0) Complex for Efficient Catalytic Hydrogenation of Olefins. J. Am. Chem. Soc. 2017, 139, 13499–13506. 10.1021/jacs.7b07167. [DOI] [PubMed] [Google Scholar]; p Zhou Y.-P.; Raoufmoghaddam S.; Szilvási T.; Driess M. A Bis(silylene)-Substituted ortho-Carborane as a Superior Ligand in the Nickel-Catalyzed Amination of Arenes. Angew. Chem., Int. Ed. 2016, 55, 12868–12872. 10.1002/anie.201606979. [DOI] [PubMed] [Google Scholar]; q Metsänen T. T.; Gallego D.; Szilvási T.; Driess M.; Oestreich M. Peripheral mechanism of a carbonyl hydrosilylation catalysed by an SiNSi iron pincer complex. Chem. Sci. 2015, 6, 7143–7149. 10.1039/C5SC02855H. [DOI] [PMC free article] [PubMed] [Google Scholar]; r Gallego D.; Inoue S.; Blom B.; Driess M. Highly Electron-Rich Pincer-Type Iron Complexes Bearing Innocent Bis(metallylene)pyridine Ligands: Syntheses, Structures, and Catalytic Activity. Organometallics 2014, 33, 6885–6897. 10.1021/om500966t. [DOI] [Google Scholar]; s Gallego D.; Bruck A.; Irran E.; Meier F.; Kaupp M.; Driess M.; Hartwig J. F. From Bis(silylene) and Bis(germylene) Pincer-Type Nickel(II) Complexes to Isolable Intermediates of the Nickel-Catalyzed Sonogashira Cross-Coupling Reaction. J. Am. Chem. Soc. 2013, 135, 15617–15626. 10.1021/ja408137t. [DOI] [PubMed] [Google Scholar]; t Someya C. I.; Haberberger M.; Wang W.; Enthalter S.; Inoue S. Application of a Bis(silylene) Nickel Complex as Precatalyst in C–C Bond Formation Reactions. Chem. Lett. 2013, 42, 286–288. 10.1246/cl.2013.286. [DOI] [Google Scholar]; u Wang W.; Inoue S.; Enthaler S.; Driess M. Bis(silylenyl)- and Bis(germylenyl)-Substituted Ferrocenes: Synthesis, Structure, and Catalytic Applications of Bidentate Silicon(II)–Cobalt Complexes. Angew. Chem., Int. Ed. 2012, 51, 6167–6171. 10.1002/anie.201202175. [DOI] [PubMed] [Google Scholar]; v Brück A.; Gallego D.; Wang W.; Irran E.; Driess M.; Hartwig J. F. Pushing the σ-Donor Strength in Iridium Pincer Complexes: Bis(silylene) and Bis(germylene) Ligands Are Stronger Donors than Bis(phosphorus(III)) Ligands. Angew. Chem., Int. Ed. 2012, 51, 11478–11482. 10.1002/anie.201205570. [DOI] [PubMed] [Google Scholar]; w Ahuja H.; Kaur H.; Arevalo R. Chemoselective C(sp)–H borylation of terminal alkynes catalyzed by a bis(N-heterocyclicsilylene) manganese complex. Inorg. Chem. Front. 2023, 10, 6067–6076. 10.1039/D3QI01033C. [DOI] [Google Scholar]
- There are examples of metal complexes featuring polydentate ATs synthesized using potentially monodentate ATs that, for example, underwent, in addition to the AT tetrel atom coordination, a cyclometalation or arene coordination of an appropriate pendant group. See, for example:; a Cabeza J. A.; Fernández-Colinas J. M.; García-Álvarez P.; González-Álvarez L.; Pérez-Carreño E. Reactivity of Amidinatosilylenes and Amidinatogermylenes with [PtMe2(η4-cod)]: cis- versus trans-[PtMe2L2] Complexes and Cyclometalation Reactions. Organometallics 2020, 39, 2026–2036. 10.1021/acs.organomet.0c00188. [DOI] [Google Scholar]; b Cabeza J. A.; Fernández-Colinas J. M.; García-Álvarez P.; González-Álvarez L.; Pérez-Carreño E. Mesityl(amidinato)tetrylenes as ligands in iridium(i) and iridium(iii) complexes: silicon versus germanium and simple κ1-coordination versus cyclometallation. Dalton Trans. 2019, 48, 10996–11003. 10.1039/C9DT01853K. [DOI] [PubMed] [Google Scholar]; (c) Refs. (5i,5j,5k).
- Sarish S. P.; Sen S. S.; Roesky H. W.; Objartel I.; Stalke D. Elegant approach to spacer arranged silagermylene and bis(germylene) compounds. Chem. Commun. 2011, 47, 7206–7208. 10.1039/c1cc12205c. [DOI] [PubMed] [Google Scholar]
- a Zhong M.; Wei J.; Zhang W.-X.; Xi Z. Synthesis and Reactivity of Side-Arm Phosphine Functionalized Amidinatosilylene- and Amidinatogermylene-Supported Nickel(0) Complexes. Organometallics 2021, 40, 310–313. 10.1021/acs.organomet.0c00770. [DOI] [Google Scholar]; b Parvin N.; Pai S.; Rojisha V. C.; De S.; Parameswaran P.; Khan S. Comparing Nucleophilicity of Heavier Heteroleptic Amidinato-Amido Tetrelylenes: An Experimental and Theoretical Study. ChemistrySelect 2016, 1, 1991–1995. 10.1002/slct.201600656. [DOI] [Google Scholar]; c Khan S.; Pal S.; Kathewad N.; Purushothaman I.; De S.; Parameswaran P. Stepwise isolation of an unprecedented silylene supported dinuclear gold(i) cation with aurophilic interaction. Chem. Commun. 2016, 52, 3880–3882. 10.1039/C6CC00597G. [DOI] [PubMed] [Google Scholar]
- Lentz N.; Mallet-Ladeira S.; Baceiredo A.; Kato T.; Madec D. Germylene–sulfoxide as a potential hemilabile ligand: application in coordination chemistry. Dalton Trans. 2018, 47, 15751–15756. 10.1039/C8DT03669A. [DOI] [PubMed] [Google Scholar]
- Nazish M.; Siddiqui M. M.; Sarkar S. K.; Münch A.; Legendre C. M.; Herbst-Irmer R.; Stalke D.; Roesky H. W. Synthesis and Coordination Behavior of a New Hybrid Bidentate Ligand with Phosphine and Silylene Donors. Chem.—Eur. J. 2021, 27, 1744–1752. 10.1002/slct.201600656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza J. A.; García-Álvarez P.; Laglera-Gándara C. J.; Pérez-Carreño E. Phosphane-functionalized heavier tetrylenes: synthesis of silylene- and germylene-decorated phosphanes and their reactions with Group 10 metal complexes. Dalton Trans. 2020, 49, 8331–8339. 10.1039/D0DT01727B. [DOI] [PubMed] [Google Scholar]
- Lentz N.; Cuevas-Chavez C.; Mallet-Ladeira S.; Sotiropoulos J.-M.; Baceiredo A.; Kato T.; Madec D. Germylene-β-sulfoxide Hemilabile Ligand in Coordination Chemistry. Inorg. Chem. 2021, 60, 423–430. 10.1021/acs.inorgchem.0c03101. [DOI] [PubMed] [Google Scholar]
- See, for example:; a Muck F. M.; Junold K.; Baus J. A.; Burschka C.; Tacke R. Donor-Stabilized Silylenes with Guanidinato Ligands. Eur. J. Inorg. Chem. 2013, 2013, 5821–5825. 10.1002/ejic.201301164. [DOI] [Google Scholar]; b Junold K.; Baus J. A.; Burschka C.; Tacke R. Bis[N,N’-diisopropylbenzamidinato(−)]silicon(II): A Silicon(II) Compound with Both a Bidentate and a Monodentate Amidinato Ligand. Angew. Chem., Int. Ed. 2012, 51, 7020–7023. 10.1002/anie.201203109. [DOI] [PubMed] [Google Scholar]; c Yeong H.-X.; Zhang S.-H.; Xi H.-W.; Guo J.-D.; Lim K. H.; Nagase S.; So C.-W. An Amidinate-Stabilized Germatrisilacyclobutadiene Ylide. Chem.—Eur. J. 2012, 18, 2685–2691. 10.1002/chem.201102201. [DOI] [PubMed] [Google Scholar]; d Matioszek D.; Katir N.; Saffon N.; Castel A. Halogermanium(II) Complexes Having Phenylamidinate As Supporting Ligands: Syntheses, Characterizations, and Reactivities. Organometallics 2010, 29, 3039–3046. 10.1021/om100347c. [DOI] [Google Scholar]; e Foley S. R.; Bensimon C.; Richeson D. S. Facile Formation of Rare Terminal Chalcogenido Germanium Complexes with Alkylamidinates as Supporting Ligands. J. Am. Chem. Soc. 1997, 119, 10359–10363. 10.1021/ja9719891. [DOI] [Google Scholar]
- Xiong Y.; Chen D.; Yao S.; Zhu J.; Ruzicka A.; Driess M. New Types of Ge2 and Ge4 Assemblies Stabilized by a Carbanionic Dicarborandiyl-Silylene Ligand. J. Am. Chem. Soc. 2021, 143, 6229–6237. 10.1021/jacs.1c01722. [DOI] [PubMed] [Google Scholar]
- Chen M.; Lei B.; Wang X.; Rong H.; Song H.; Mo Z. A Silylene-Stabilized Germanium Analogue of Alkynylaluminum. Angew. Chem., Int. Ed. 2022, 61, e202204495 10.1002/anie.202204495. [DOI] [PubMed] [Google Scholar]
- Akhtar R.; Kaulage S. H.; Sangole M. P.; Tothadi S.; Parvathy P.; Parameswaran P.; Singh K.; Khan S. First-Row Transition Metal Complexes of a Phosphine–Silylene-Based Hybrid Ligand. Inorg. Chem. 2022, 61, 13330–13341. 10.1021/acs.inorgchem.2c01233. [DOI] [PubMed] [Google Scholar]
- See references (5g,5j).
- Want W.; Inoue S.; Yao S.; Driess M. An Isolable Bis-Silylene Oxide (“Disilylenoxane”) and Its Metal Coordination. J. Am. Chem. Soc. 2010, 132, 15890–15892. 10.1021/ja106458p. [DOI] [PubMed] [Google Scholar]
- Zhang S.-H.; So C.-W. Synthesis and Characterization of an Amidinate-Stabilized Bisgermylene Oxide and Sulfide. Organometallics 2011, 30, 2059–2062. 10.1021/om101194f. [DOI] [Google Scholar]
- Wang W.; Inoue S.; Irran E.; Driess M. Synthesis and Unexpected Coordination of a Silicon(II)-Based SiCSi Pincerlike Arene to Palladium. Angew. Chem., Int. Ed. 2012, 51, 3691–3694. 10.1002/anie.201200632. [DOI] [PubMed] [Google Scholar]
- Krätschmer F.; Sun X.; Gillhuber S.; Kucher H.; Franzke Y. J.; Weigend F.; Roesky P. W. Fully Tin-Coated Coinage Metal Ions: A Pincer-Type Bis-stannylene Ligand for Exclusive Tetrahedral Complexation. Chem.—Eur. J. 2023, 29, e202203583 10.1002/chem.202203583. [DOI] [PubMed] [Google Scholar]
- Menezes P. W.; Yao S.; Beltrán-Suito R.; Hausmann N.; Menezes P. V.; Driess M. Facile Access to an Active γ-NiOOH Electrocatalyst for Durable Water Oxidation Derived From an Intermetallic Nickel Germanide Precursor. Angew. Chem., Int. Ed. 2021, 60, 4640–4647. 10.1002/anie.202014331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostenko A.; Driess M. Geometrically Compelled Disilene with λ4-Coordinate SiII Atoms. J. Am. Chem. Soc. 2018, 140, 16962–16969. 10.1021/jacs.8b11393. [DOI] [PubMed] [Google Scholar]
- Su B.; Kostenko A.; Yao A.; Driess M. Isolable Dibenzo[a,e]disilapentalene with a Dichotomic Reactivity toward CO2. J. Am. Chem. Soc. 2020, 142, 16935–16941. 10.1021/jacs.0c09040. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Kostenko A.; Hadlington T. J.; Luecke M.-P.; Yao S.; Driess M. Silicon-Mediated Selective Homo- and Heterocoupling of Carbon Monoxide. J. Am. Chem. Soc. 2019, 141, 626–634. 10.1021/jacs.8b11899. [DOI] [PubMed] [Google Scholar]
- Chen X.; Wang H.; Du S.; Driess M.; Mo Z. Deoxygenation of Nitrous Oxide and Nitro Compounds Using Bis(N-Heterocyclic Silylene)Amido Iron Complexes as Catalysts. Angew. Chem., Int. Ed. 2022, 61, e202114598 10.1002/anie.202114598. [DOI] [PubMed] [Google Scholar]
- Shan C.; Dong S.; Yao S.; Zhu J.; Driess M. Synthesis and Reactivity of an Anti-van’t Hoff/Le Bel Compound with a Planar Tetracoordinate Silicon(II) Atom. J. Am. Chem. Soc. 2023, 145, 7084–7089. 10.1021/jacs.3c00722. [DOI] [PubMed] [Google Scholar]
- See references (5c,5e,5f,5o,5p,5r,5t,5u,5v).
- Dehmel M.; Wünsche M. A.; Görls H.; Kretschmer R. Dinuclear Chlorotetrylenes of Silicon, Germanium, and Tin Based on a Backbone-Bridged Bis(amidine). Eur. J. Inorg. Chem. 2021, 2021, 4806–4811. 10.1002/ejic.202100692. [DOI] [Google Scholar]
- Garg P.; Dange D.; Jones C. s- and p-Block Dinuclear Metal(loid) Complexes Bearing 1,4-Phenylene and 1,4-Cyclohexylene Bridged Bis(amidinate) Ligands. Eur. J. Inorg. Chem. 2020, 2020, 4037–4044. 10.1002/ejic.202000737. [DOI] [Google Scholar]
- Garg P.; Dange D.; Jones C. Bulky arene-bridged bis(amide) and bis(amidinate) complexes of germanium(II) and tin(II). Dalton Trans. 2021, 50, 9118–9122. 10.1039/D1DT01642C. [DOI] [PubMed] [Google Scholar]
- Chlupatý T.; Brichová K.; Samsonov M. A.; Ru°žičková Z.; Ru°žička A. Reversible addition of tin(ii) amides to nitriles. Dalton Trans. 2022, 51, 1879–1887. 10.1039/D1DT04060J. [DOI] [PubMed] [Google Scholar]
- Feng Z.; Jiang Y.; Ruan H.; Zhao Y.; Tan G.; Zhang L.; Wang X. A diamidinatogermylene as a Z-type ligand in a nickel(0) complex. Dalton Trans. 2019, 48, 14975–14978. 10.1039/C9DT03803E. [DOI] [PubMed] [Google Scholar]
- Cabeza J. A.; García F.; García-Álvarez P.; García-Soriano R.; Pérez-Carreño E. Synthesis and Some Coordination Chemistry of Phosphane-Difunctionalized Bis(amidinato)-Heavier Tetrylenes: A Previously Unknown Class of PEP Tetrylenes (E = Ge and Sn). Inorg. Chem. 2023, 62, 15502–15509. 10.1021/acs.inorgchem.3c01953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See, for example:; a Sen S. S.; Roesky H. W.; Stern D.; Henn J.; Stalke D. High Yield Access to Silylene RSiCl (R = PhC(NtBu)2) and Its Reactivity toward Alkyne: Synthesis of Stable Disilacyclobutene. J. Am. Chem. Soc. 2010, 132, 1123–1126. 10.1021/ja9091374. [DOI] [PubMed] [Google Scholar]; b Nagendran S.; Sen S. S.; Roesky H. W.; Koley D.; Brubmüller H.; Pal A.; Herbst-Irmer R. RGe(I)Ge(I)R Compound (R = PhC(NtBu)2) with a Ge–Ge Single Bond and a Comparison with the Gauche Conformation of Hydrazine. Organometallics 2008, 27, 5459–5463. 10.1021/om800714f. [DOI] [Google Scholar]; c Sen S. S.; Kritzler-Kosch M. P.; Nagendran S.; Roesky H. W.; Beck T.; Pal A.; Herbst-Irmer R. Synthesis of Monomeric Divalent Tin(II) Compounds with Terminal Chloride, Amide, and Triflate Substituents. Eur. J. Inorg. Chem. 2010, 2010, 5304–5311. 10.1002/ejic.201000803. [DOI] [Google Scholar]
- See references (5d,5g,8−13,16).
- Sun X.; Röder C.; Roesky P. W. Zinc and Cadmium Complexes of Chelating N-Heterocyclic Silylene and Their Reactivity toward Elemental Chalcogens. Inorg. Chem. 2021, 60, 13861–13868. 10.1021/acs.inorgchem.0c03609. [DOI] [PubMed] [Google Scholar]
- Sun X.; Hinz A.; Gamer M. T.; Roesky P. W. Stable bidentate silylene adducts of alkaline-earth amides. Z. Anorg. Allg. Chem. 2022, 648, e202200104 10.1002/zaac.202200104. [DOI] [Google Scholar]
- Nazish M.; Bai H.; Legendre C. M.; Herbst-Irmer R.; Zhao L.; Stalke D.; Roesky H. W. A neutral vicinal silylene/phosphane supported six-membered C2PSiAu2 ring and a silver(i) complex. Chem. Commun. 2022, 58, 12704–12707. 10.1039/D2CC04163D. [DOI] [PubMed] [Google Scholar]
- See references (5d,5h,5l,5m,5o,5p,5r,5t,5u,5w,18,21−23).
- Sun X.; Simler T.; Reiter K.; Weigend F.; Roesky P. W. Synthesis and Reactivity of Bis(silylene)-Coordinated Calcium and Divalent Lanthanide Complexes. Chem.—Eur. J. 2020, 26, 14888–14895. 10.1002/chem.202003417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See references (5b,5c,5f,5n,5q,5r,26).
- See references (5s,5e,5v,20).
- Yamaguchi Y.; Yamanishi K.; Kondo M.; Tsukada N. Synthesis of Dinuclear (μ-η3-Allyl)palladium(I) and -platinum(I) Complexes Supported by Chelate-Bridging Ligands. Organometallics 2013, 32, 4837–4842. 10.1021/om400557p. [DOI] [Google Scholar]
- See, for example:; a Raut R. K.; Sahoo P.; Chimnapure D.; Majumdar M. Versatile coordinating abilities of acyclic N4 and N2P2 ligand frameworks in conjunction with Sn[N(SiMe3)2]2. Dalton Trans. 2019, 48, 10953–10961. 10.1039/C9DT00617F. [DOI] [PubMed] [Google Scholar]; b Flock J.; Suljanovic A.; Torvisco A.; Schoefberger W.; Gerke B.; Pottgen R.; Fischer R. C.; Flock M. The Role of 2,6-Diaminopyridine Ligands in the Isolation of an Unprecedented, Low Valent Tin Complex. Chem. -Eur. J. 2013, 19, 15504–15517. 10.1002/chem.201301340. [DOI] [PubMed] [Google Scholar]; c Olbert D.; Kalisch A.; Gorls H.; Ondik I. M.; Reiher M.; Westerhausen M. Syntheses, Crystal Structure and Reactivity of Tin(II) Bis[N-(diphenylphosphanyl)(2-pyridylmethyl)amide]. Z. Anorg. Allg. Chem. 2009, 635, 462–470. 10.1002/zaac.200801328. [DOI] [Google Scholar]
- Mantina M.; Chamberlin A. C.; Valero R.; Cramer C. J.; Truhlar D. G. Consistent van der Waals Radii for the Whole Main Group. J. Phys. Chem. A 2009, 113, 5806–5812. 10.1021/jp8111556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See for example:; Bursch M.; Mewes J.-M.; Hansen A.; Grimme S. Best-Practice DFT Protocols for Basic Molecular Computational Chemistry. Angew. Chem., Int. Ed. 2022, 61, e202205735 10.1002/anie.202205735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See, for example:; a Yadav D.; Siwatch R. K.; Sinhababu S.; Karwasara S.; Singh D.; Rajaraman G.; Nagendran S. Digermylene Oxide Stabilized Group 11 Metal Iodide Complexes. Inorg. Chem. 2015, 54, 11067–11076. 10.1021/acs.inorgchem.5b01436. [DOI] [PubMed] [Google Scholar]; b Alvarez-Rodriguez L.; Cabeza J. A.; García-Álvarez P.; Polo D. Amidinatogermylene Complexes of Copper, Silver, and Gold. Organometallics 2015, 34, 5479–5484. 10.1021/acs.organomet.5b00828. [DOI] [Google Scholar]; c Zhao N.; Zhang J.; Yang Y.; Chen G.; Zhu H.; Roesky H. W. Reactivity Studies of (Phenylethynyl)germylene LGeC≡CPh (L = HC[C(Me)N-2,6-iPr2C6H3]2) toward Pentafluorophenylcopper(I), -silver(I), and -gold(I) Complexes. Organometallics 2013, 32, 762–769. 10.1021/om300724t. [DOI] [Google Scholar]; d Leung W.-P.; So C.-W.; Chong K.-H.; Kan K.-W.; Chan H.-S.; Mak T. C. W. Reactivity of Pyridyl-1-azaallyl Germanium(II) Chloride: Synthesis of Novel Lithium Germinate [{(PhC⋮C)3Ge}3GeLi(Et2O)3] and Ge(II)–M(I) (M = Cu and Au) Adducts. Organometallics 2006, 25, 2851–2858. 10.1021/om060032g. [DOI] [Google Scholar]; e Matioszek D.; Kocsor T.-G.; Castel A.; Nemes G.; Escudie J.; Saffon N. Phosphaalkenyl germylenes and their gold, tungsten and molybdenum complexes. Chem. Commun. 2012, 48, 3629–3631. 10.1039/c2cc17551g. [DOI] [PubMed] [Google Scholar]; (f) Ref. (4d).
- See, for example:; a Nakane T.; Tanioka Y.; Tsukada N. Synthesis of Multinuclear Copper Complexes Bridged by Diquinolylamidinates and Their Application to Copper-Catalyzed Coupling of Terminal Alkynes and Aryl, Allyl, and Benzyl Halides. Organometallics 2015, 34, 1191–1196. 10.1021/om501139f. [DOI] [Google Scholar]; b Kahnes M.; Gorls H.; Westerhausen M. Synthesis and crystal structures of dinuclear zinc complexes with the 1,3-bis(2-pyridylmethyl)acetamidinato ligand. J. Organomet. Chem. 2011, 696, 1618–1625. 10.1016/j.jorganchem.2011.01.033. [DOI] [Google Scholar]; (c) Ref. (44).
- See, for example:; a Cabeza J. A.; Fernandez-Colinas J. M.; Garcia-Alvarez P.; Pérez-Carreño E.; Polo D. Reactivity Studies on a Binuclear Ruthenium(0) Complex Equipped with a Bridging κ2N,Ge-Amidinatogermylene Ligand. Inorg. Chem. 2015, 54, 4850–4861. 10.1021/acs.inorgchem.5b00412. [DOI] [PubMed] [Google Scholar]; b Álvarez-Rodríguez L.; Cabeza J. A.; García-Álvarez P.; Pérez-Carreño E.; Polo D. Amidinatogermylene Derivatives of Ruthenium Carbonyl: New Insights into the Reactivity of [Ru3(CO)12] with Two-Electron-Donor Reagents of High Basicity. Inorg. Chem. 2015, 54, 2983–2994. 10.1021/acs.inorgchem.5b00084. [DOI] [PubMed] [Google Scholar]; c Cabeza J. A.; Garcia-Alvarez P.; Polo D. Expanding the coordination chemistry of donor-stabilized group-14 metalenes. Dalton Trans. 2013, 42, 1329–1332. 10.1039/C2DT32654J. [DOI] [PubMed] [Google Scholar]
- Cabeza J. A.; García-Álvarez P.; Pérez-Carreño E.; Polo D. Ring Opening and Bidentate Coordination of Amidinate Germylenes and Silylenes on Carbonyl Dicobalt Complexes: The Importance of a Slight Difference in Ligand Volume. Chem.—Eur. J. 2014, 20, 8654–8663. 10.1002/chem.201402295. [DOI] [PubMed] [Google Scholar]
- CSD version 5.44 (Updated Sep 2023). See also,Allen F. H. The Cambridge Structural Database: A Quarter of a Million Crystal Structures and Rising. Acta Crystallogr. Sect. B 2002, 58, 380–388. 10.1107/S0108768102003890. [DOI] [PubMed] [Google Scholar]
- More than 35 hits were found for κ3E,N2-ligands (E = Si in no specific position within the ligand scaffold) in the Cambridge Crystallographic database (CSD version 5.44; updated Sep 2023).
- a Wen Z.-K.; Xie Y.-F.; Zhao S.-B.; Tan R.-Y.; Tang L.-F. Functionalized bis(pyrazol-1-yl)methanes by organotin halide on the methine carbon atom and their related reactions. J. Organomet. Chem. 2008, 693, 1359–1366. 10.1016/j.jorganchem.2008.01.041. [DOI] [Google Scholar]; b Tang L.-F.; Hong J.; Wen Z.-K. Unexpected Reactivity of CH(3,5-Me2Pz)2(CO)3WSnAr3 (Pz = pyrazol-1-yl; Ar = phenyl or p-tolyl) toward Hydrogen Halide. Organometallics 2005, 24, 4451–4453. 10.1021/om0503895. [DOI] [Google Scholar]; c Tang L.-F.; Zhao S.-B.; Jia W.-L.; Yang Z.; Song D.-T.; Wang J.-T. Reaction of Bis(pyrazol-1-yl)methanes Modified by Organotin Groups on the Methine Carbon with W(CO)5THF to Give Novel Heterodinuclear Organometallic Complexes. Organometallics 2003, 22, 3290–3298. 10.1021/om030221o. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




