Abstract
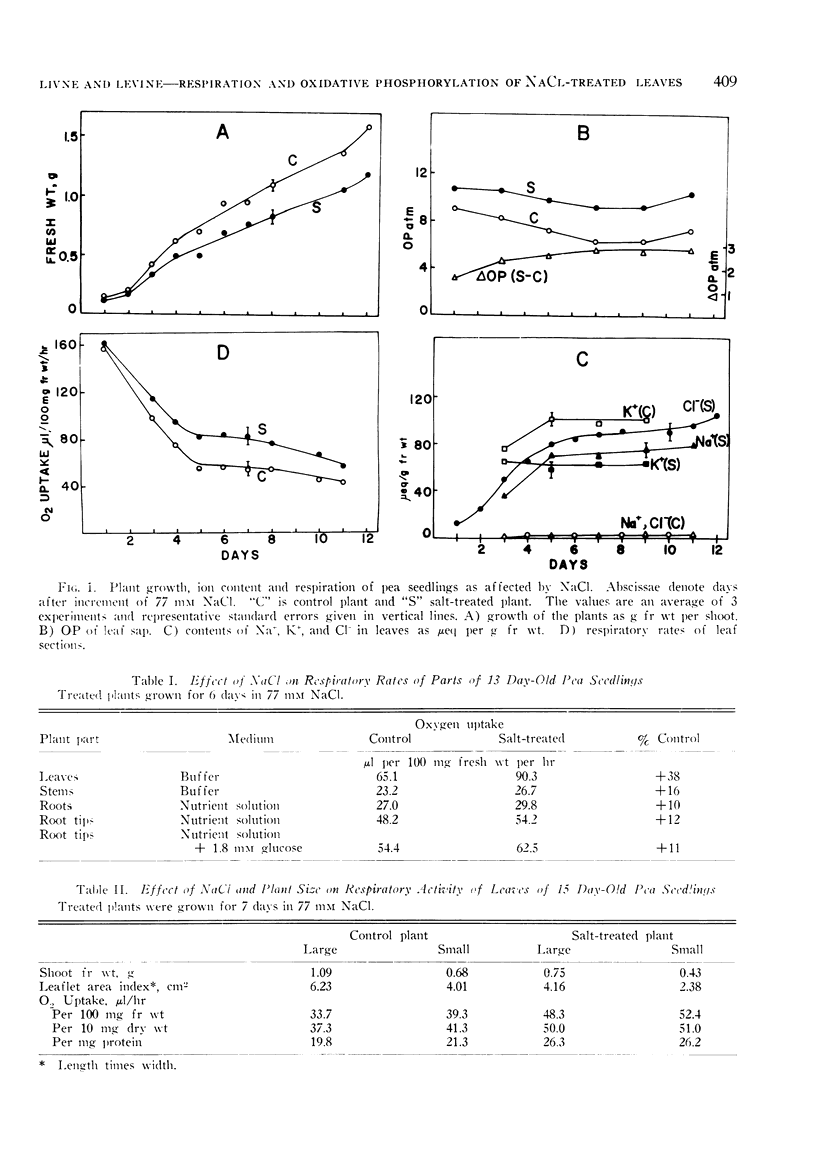
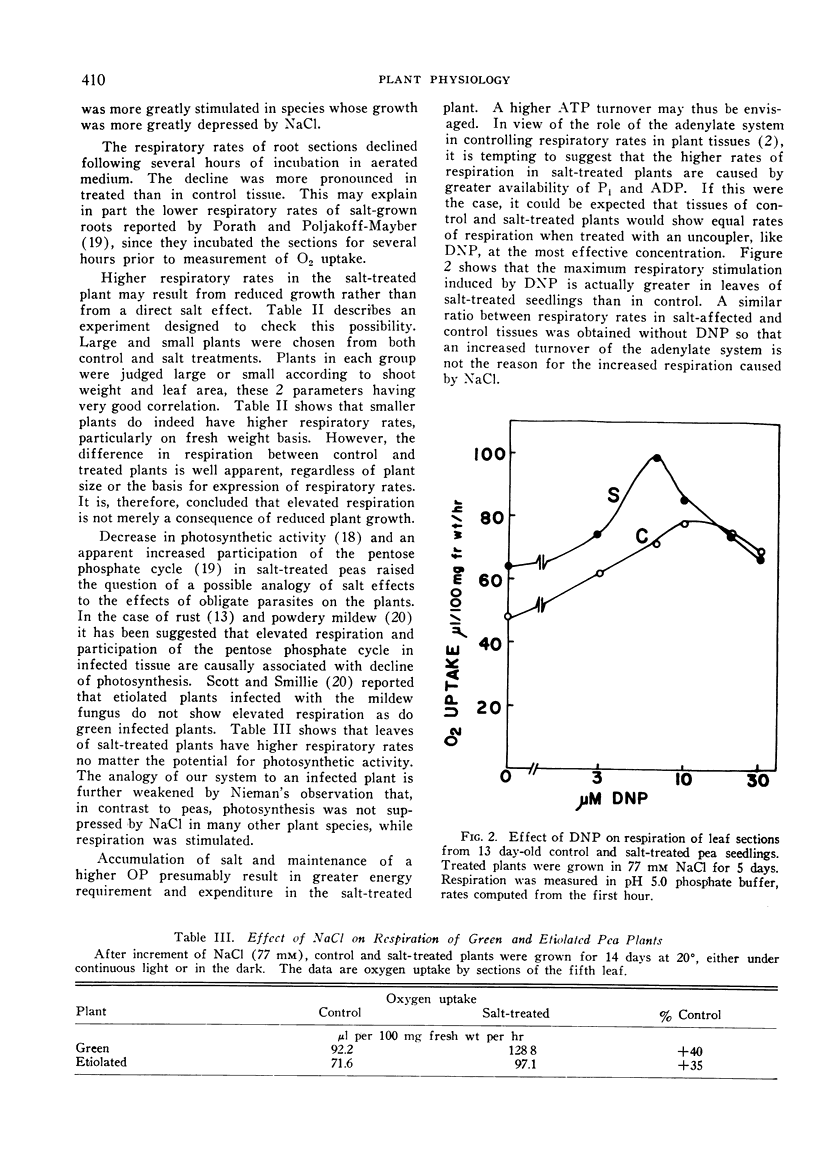
The effect of sodium chloride added to root medium of pea seedlings on respiratory activity of tissue segments and on isolated mitochondria was studied. Salinization enhances the respiration of leaves about one-third on a fresh weight, dry weight or protein basis. Roots and stems show only 10 to 15% respiratory stimulation. The onset of respiratory increase in leaves roughly parallels the increase in NaCl content and the decrease in growth rate. At a later stage the elevated respiration is apparent in treated plants even though the concentration of NaCl reaches a plateau and osmotic adjustment is being reached. Stimulation of respiration was found in both etiolated and green plants. Experiments with DNP show that simple uncoupling by salt is not involved; the respiratory increase in control and treated tissue is proportionally the same.
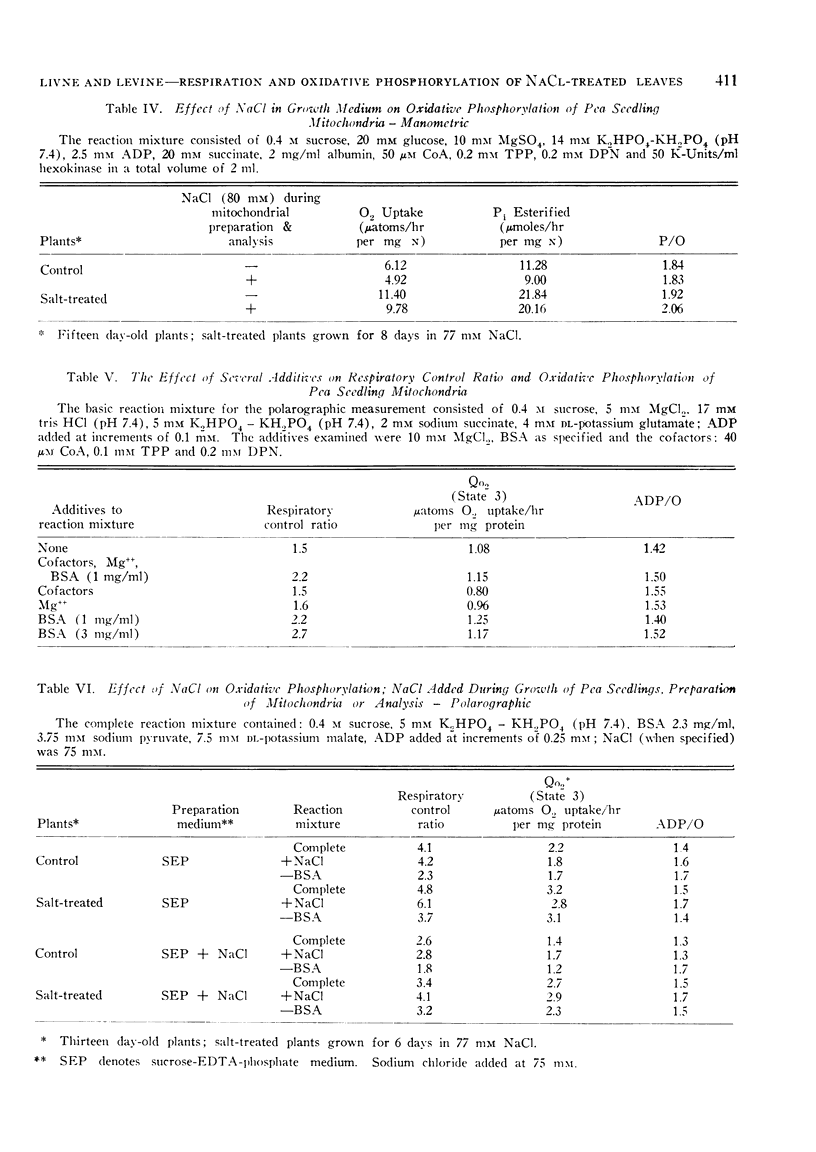
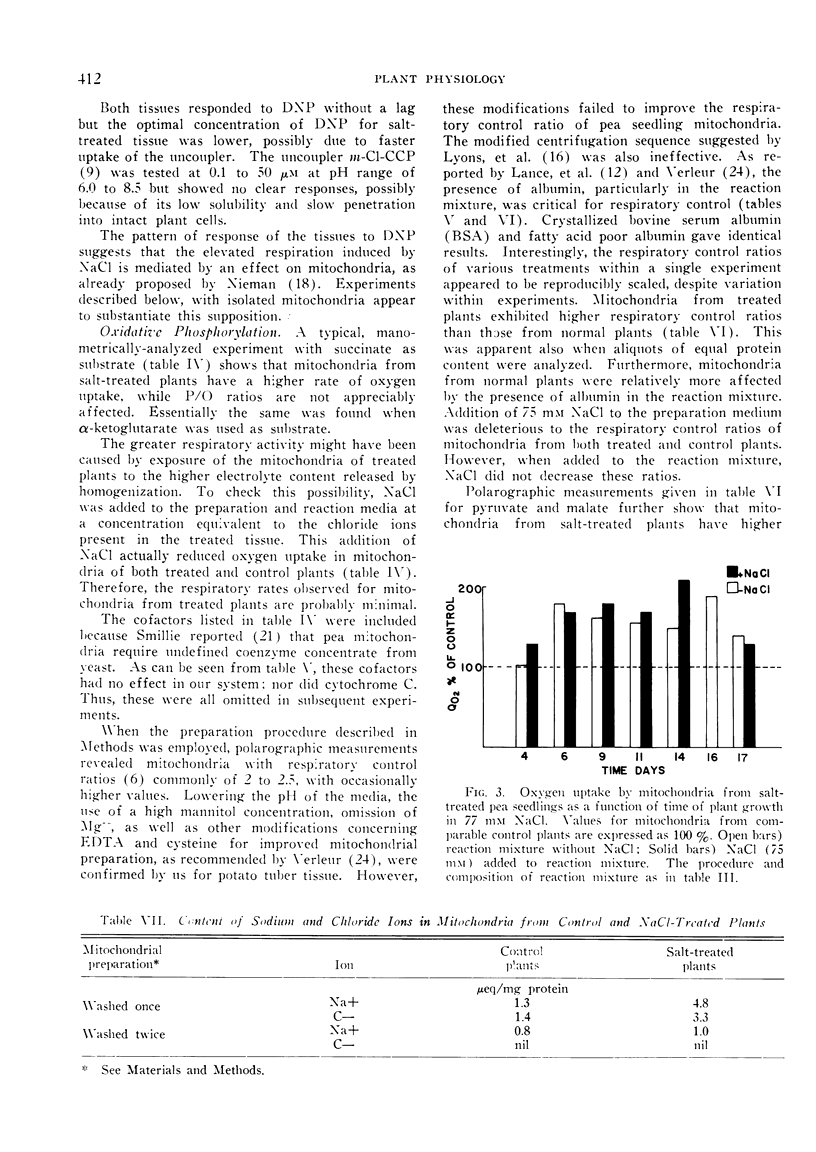
In accordance with increased respiration rates observed in vivo, mitochondria from salt-treated plants show higher rates of oxygen uptake on several substrates. The effect of NaCl added during growth is long term and is distinct from the effect of NaCl added to mitochondria isolated from control plants. Since P/O ratios are not affected by NaCl, the potential for oxidative phosphorylation in salt-affected tissue appears to increase. It is postulated that this increase may lead to changes in ADP and ATP content, and in turn, affect regulation of metabolic pathways.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. Biological feedback control at the molecular level. Science. 1965 Nov 12;150(3698):851–857. doi: 10.1126/science.150.3698.851. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. A method for the localization of sites for oxidative phosphorylation. Nature. 1955 Aug 6;176(4475):250–254. doi: 10.1038/176250a0. [DOI] [PubMed] [Google Scholar]
- Hiatt A. J., Evans H. J. Influence of Salts on Activity of Malic Dehydrogenase from Spinach Leaves. Plant Physiol. 1960 Sep;35(5):662–672. doi: 10.1104/pp.35.5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lance C., Hobson G. E., Young R. E., Biale J. B. Metabolic processes in cytoplasmic particles of the avocado fruit. VII. Oxidative and phosphorylative activities throughout the climacteric cycle. Plant Physiol. 1965 Nov;40(6):1116–1123. doi: 10.1104/pp.40.6.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. W., Evans H. J. The Influence of Salts on the Activity of Particulate Cytochrome Oxidase from Roots of Higher Plants. Plant Physiol. 1956 Sep;31(5):357–364. doi: 10.1104/pp.31.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K. J., Smillie R. M. Metabolic regulation in diseased leaves. I. The respiratory rise in barley leaves infected with powdery mildew. Plant Physiol. 1966 Feb;41(2):289–297. doi: 10.1104/pp.41.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleur J. D. Studies on the Isolation of Mitochondria from Potato Tuber Tissue. Plant Physiol. 1965 Nov;40(6):1003–1007. doi: 10.1104/pp.40.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]


