Abstract
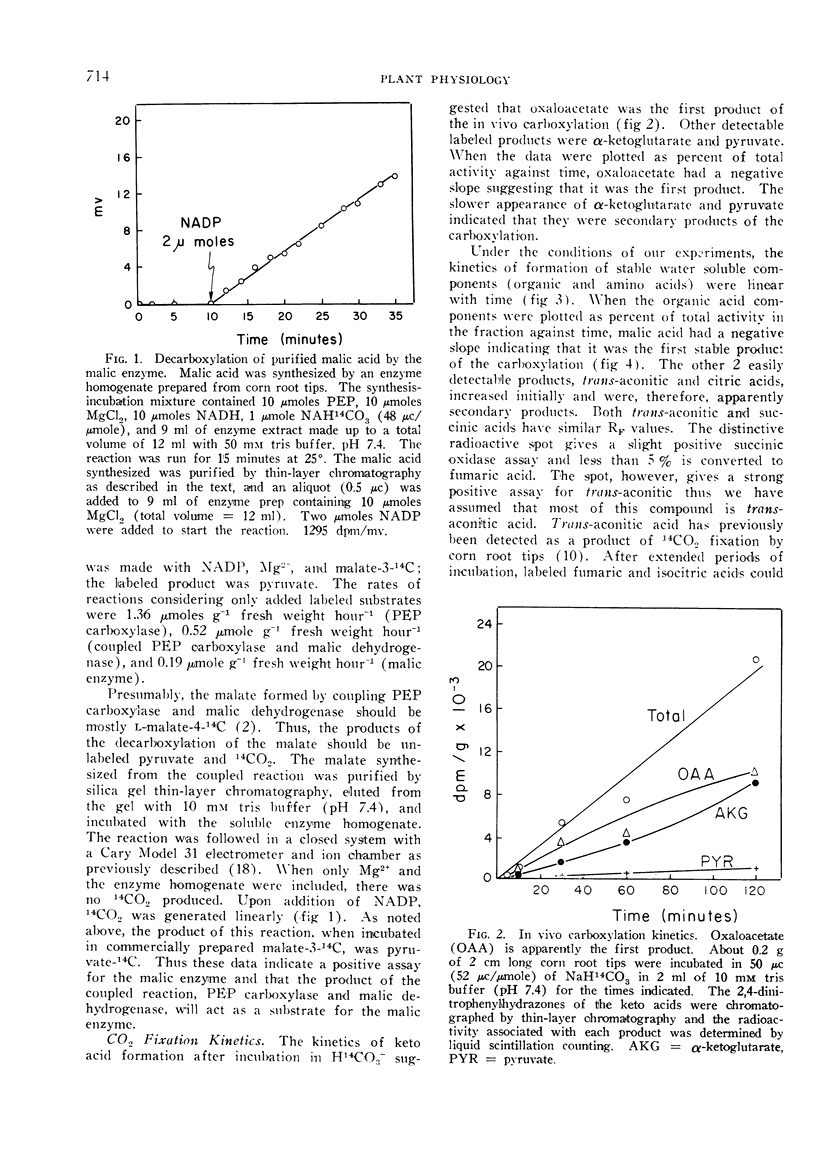
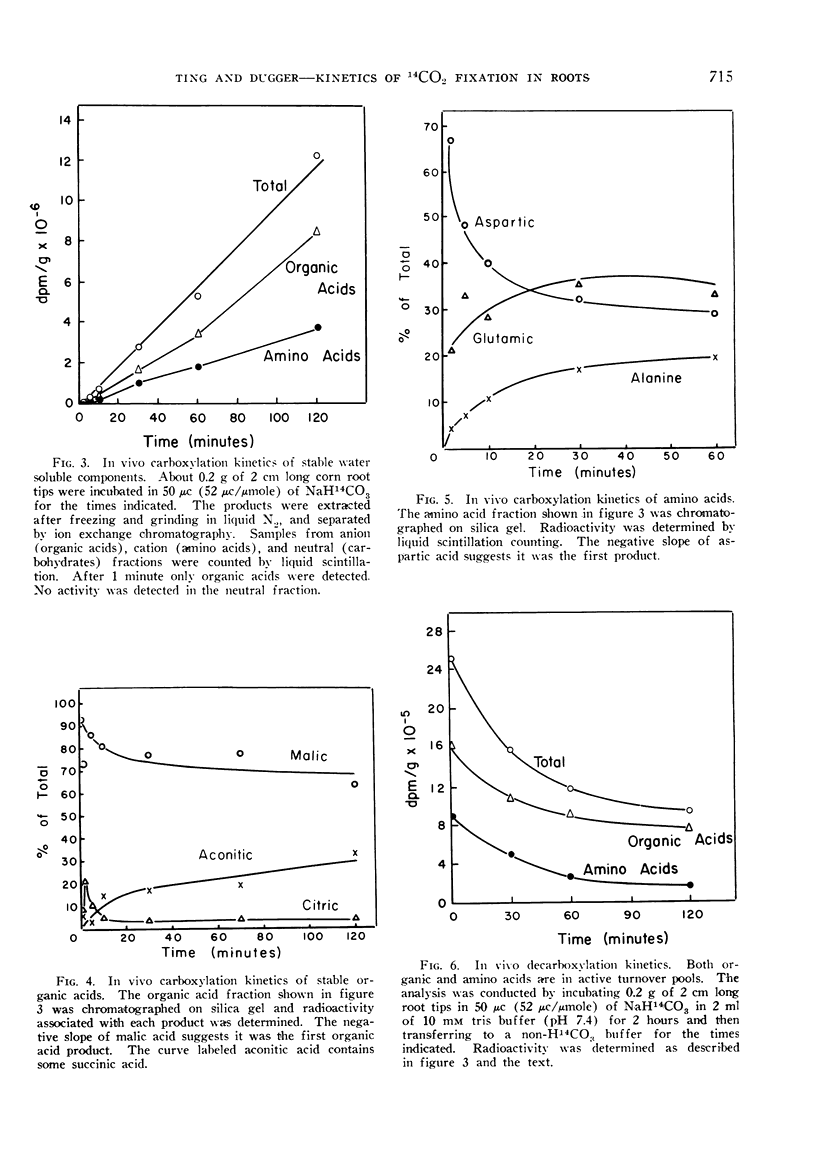
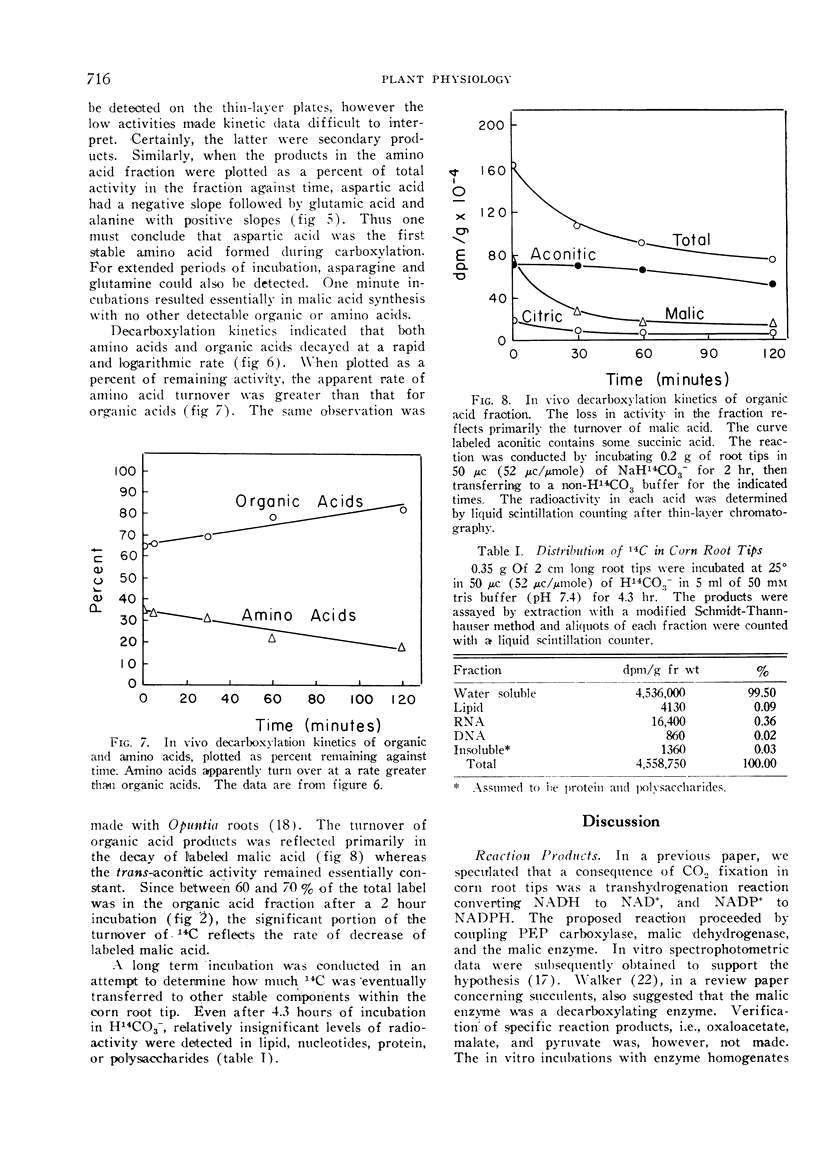
The kinetics of 14CO2 carboxylation and decarboxylation in corn root tips were determined to ascertain the sequence of product formation and subsequent utilization, and to obtain further evidence to predict the enzymes mediating the carboxylation and decarboxylations. The carboxylation data indicated that the first product was oxaloacetate followed by malate and aspartate. Malate was the first stable product which could be detected. Decarboxylation data indicated that a large fraction of the 14CO2 release and turnover of 14C was accountable for by a decrease in malate: however, essentially all labeled amino acids turned over rapidly and at a greater rate than organic acids. The data generally support the hypothesis that CO2 fixation in corn root tips is via P-enolpyruvate carboxylase and malic dehydrogenase and that subsequent malate metabolism is for the most part by direct decarboxylation, possibly by the malic enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S., GREINER C. M. The enzymatic synthesis of oxalacetate from phosphoryl-enolpyruvate and carbon dioxide. J Biol Chem. 1953 Oct;204(2):781–786. [PubMed] [Google Scholar]
- CANVIN D. T., BEEVERS H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem. 1961 Apr;236:988–995. [PubMed] [Google Scholar]
- Kunitake G., Stitt C., Saltman P. Dark Fixation of CO(2) by Tobacco Leaves. Plant Physiol. 1959 Mar;34(2):123–127. doi: 10.1104/pp.34.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips S. H., Beevers H. Compartmentation of Organic Acids in Corn Roots II. The Cytoplasmic Pool of Malic Acid. Plant Physiol. 1966 Apr;41(4):713–717. doi: 10.1104/pp.41.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips S. H., Beevers H. Compartmentation of organic acids in corn roots I. Differential labeling of 2 malate pools. Plant Physiol. 1966 Apr;41(4):709–712. doi: 10.1104/pp.41.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh H. V., Galmiche J. M., Gibbs M. Effect of Light on the Tricarboxylic Acid Cycle in Scenedesmus. Plant Physiol. 1965 Nov;40(6):1013–1022. doi: 10.1104/pp.40.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splittstoesser W. E. Dark CO(2) Fixation and its Role in the Growth of Plant Tissue. Plant Physiol. 1966 May;41(5):755–759. doi: 10.1104/pp.41.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Dugger W. M. CO(2) Fixation in Opuntia Roots. Plant Physiol. 1966 Mar;41(3):500–505. doi: 10.1104/pp.41.3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Dugger W. M., Jr Separation and detection of organic acids on silica gel. Anal Biochem. 1965 Sep;12(3):571–578. doi: 10.1016/0003-2697(65)90224-1. [DOI] [PubMed] [Google Scholar]
- Ting I. P., Dugger W. M., Jr Transhydrogenation in Root Tissue: Mediation by Carbon Dioxide. Science. 1965 Dec 24;150(3704):1727–1728. doi: 10.1126/science.150.3704.1727. [DOI] [PubMed] [Google Scholar]
- Tsay Y., Nishi A., Yanagita T. Carbon dioxide fixation in Aspergillus oryzae conidia at the initial phase of germination. J Biochem. 1965 Nov;58(5):487–493. doi: 10.1093/oxfordjournals.jbchem.a128229. [DOI] [PubMed] [Google Scholar]
- WALKER D. A. Pyruvate carboxylation and plant metabolism. Biol Rev Camb Philos Soc. 1962 May;37:215–256. doi: 10.1111/j.1469-185x.1962.tb01611.x. [DOI] [PubMed] [Google Scholar]


