Abstract
The possible involvement of nucleic acid and protein synthesis in light-regulated chlorophyll formation by rapidly greening leaves has been studied.
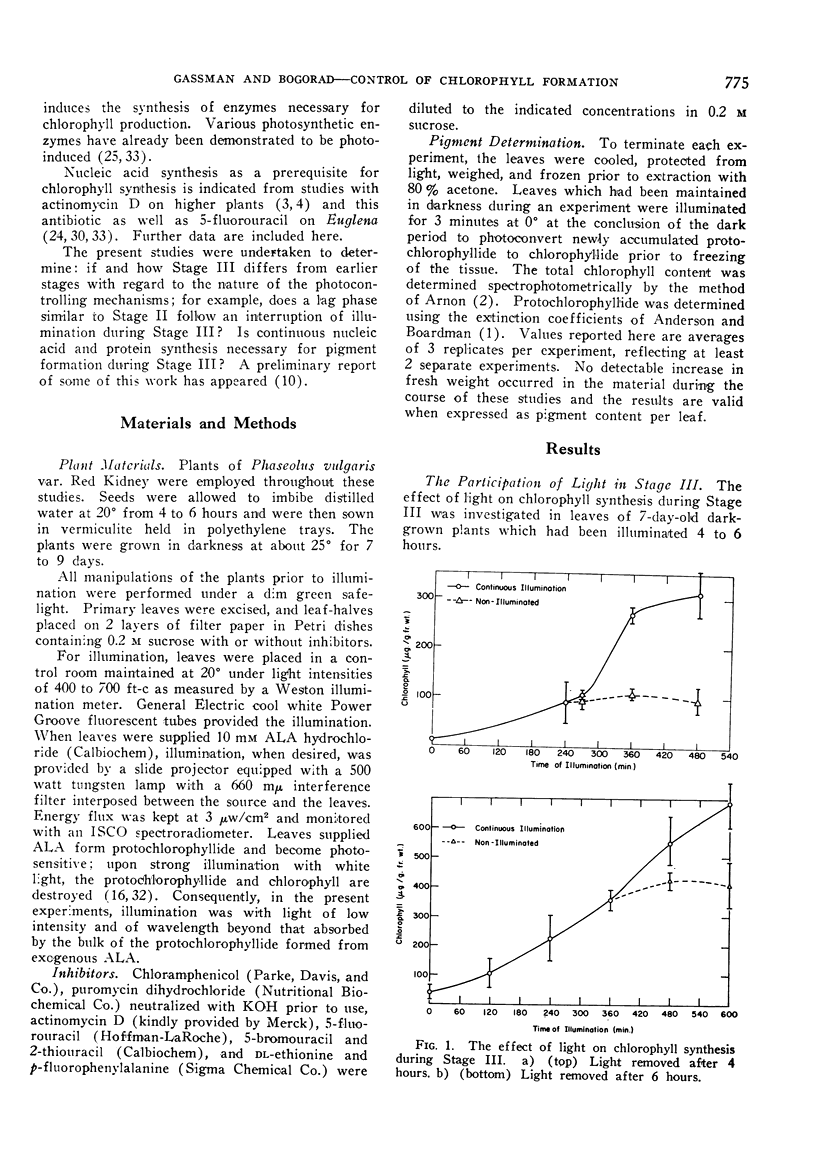
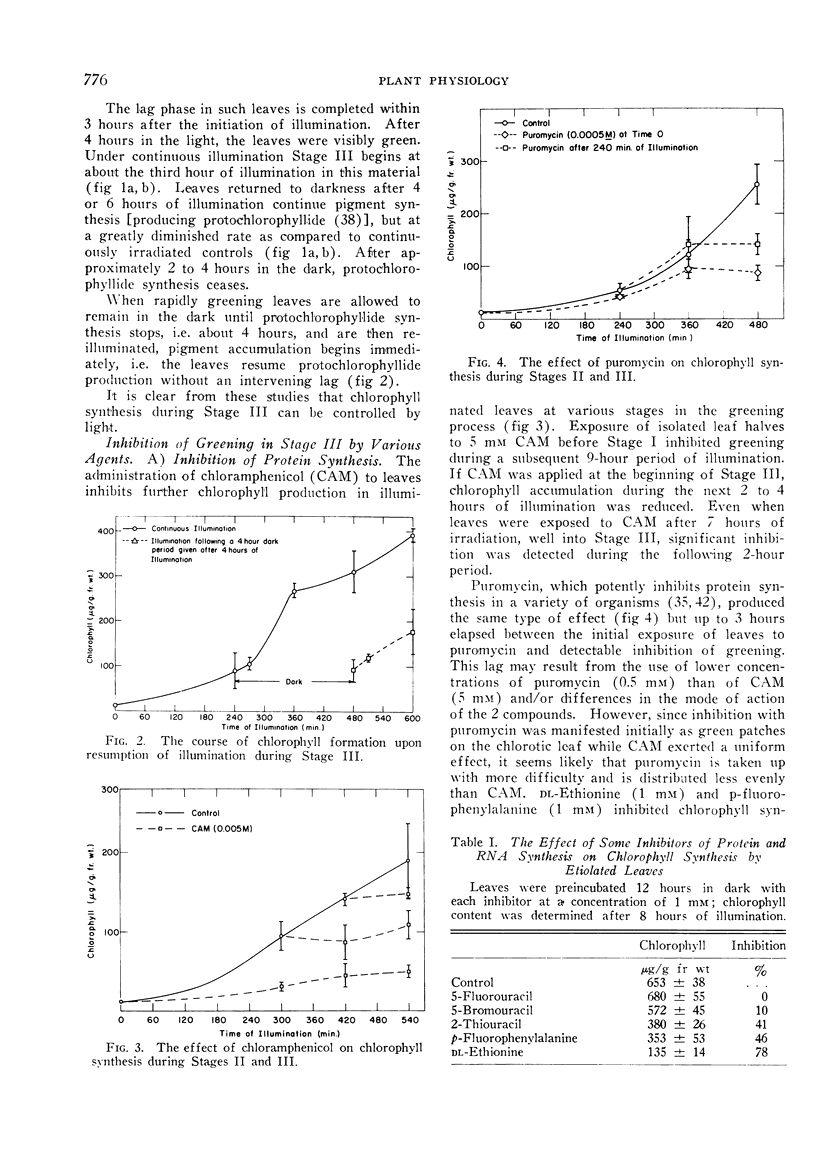
Removing leaves from illumination during the phase of rapid greening results in a reduction in the rate of pigment synthesis; cessation occurs within 2 to 4 hours. Etiolated leaves which exhibit a lag in pigment synthesis when first placed in the light do not show another lag after a 4 hour interruption of illumination during the phase of rapid greening.
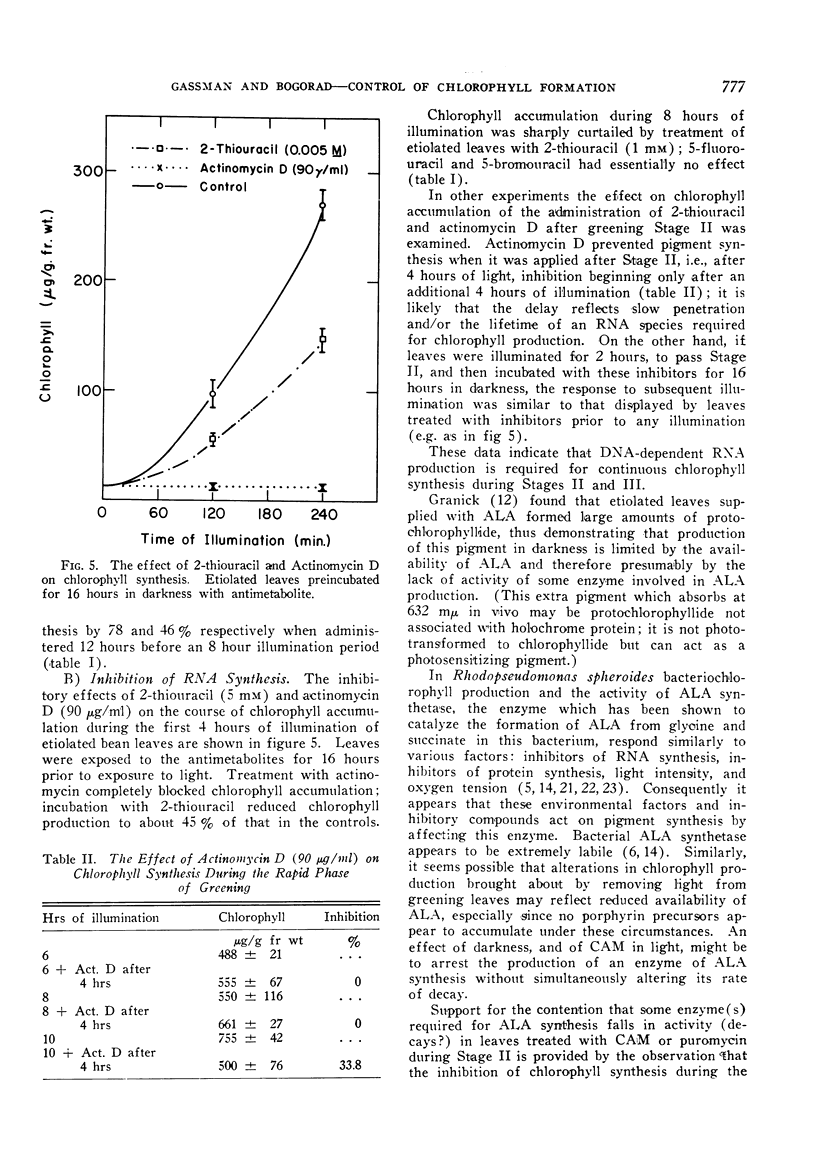
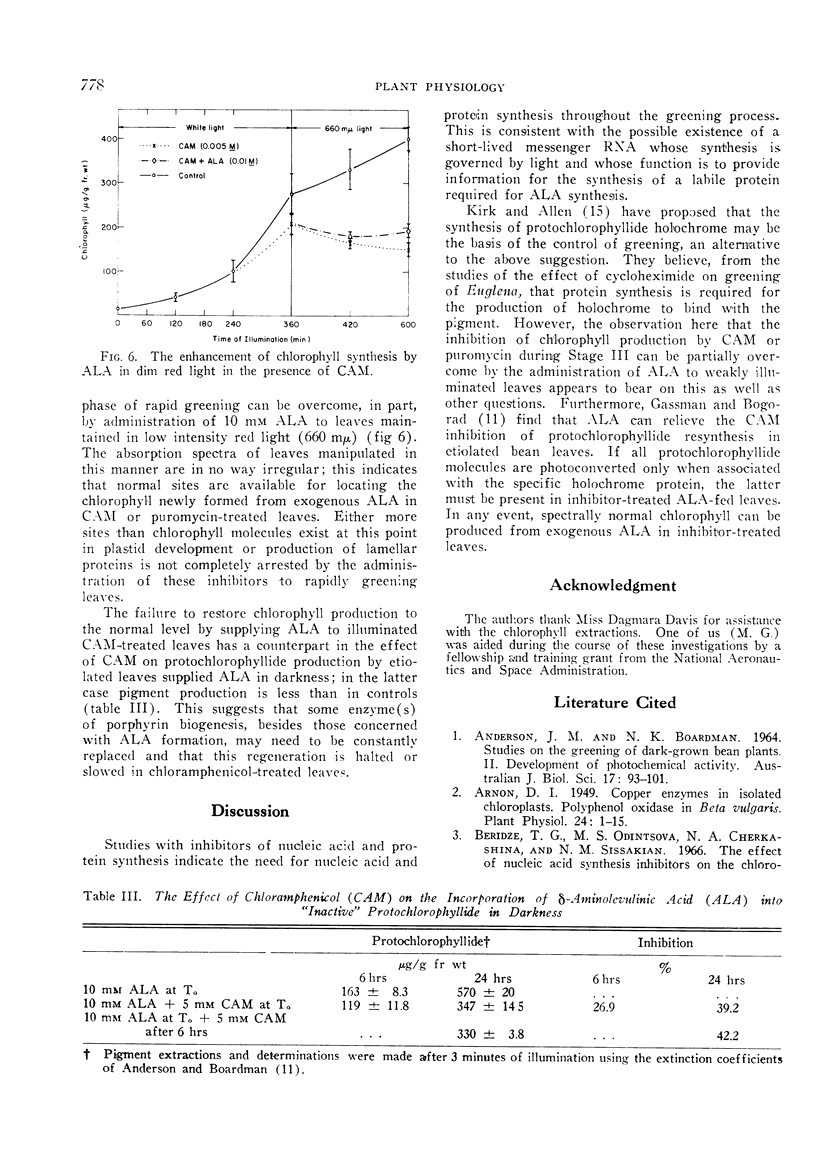
Actinomycin D, chloramphenicol, and puromycin inhibit chlorophyll synthesis when applied before or during the phase of rapid greening. Application of δ-amino-levulinic acid partially relieves the inhibition by chloramphenicol.
It is suggested that light regulates chlorophyll synthesis by controlling the availability of δ-aminolevulinic acid, possibly by mediating the formation of an enzyme of δ-aminolevulinate synthesis. This process may result from gene activation or derepression; the involvement of RNA synthesis of some sort is suggested by the inhibitory effect of actinomycin D on chlorophyll production by rapidly greening leaves.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULL M. J., LASCELLES J. The association of protein synthesis with formation of pigments in some photosynthetic bacteria. Biochem J. 1963 Apr;87:15–28. doi: 10.1042/bj0870015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogorad L., Jacobson A. B. Inhibition of greening of etiolated leaves by antinomycin D. Biochem Biophys Res Commun. 1964;14:113–117. doi: 10.1016/0006-291x(64)90239-6. [DOI] [PubMed] [Google Scholar]
- Brock T. D. CHLORAMPHENICOL. Bacteriol Rev. 1961 Mar;25(1):32–48. doi: 10.1128/br.25.1.32-48.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carell E. F., Price C. A. Porphyrins and the iron requirement for chlorophyll formation in Euglena. Plant Physiol. 1965 Jan;40(1):1–7. doi: 10.1104/pp.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DEKEN-GRENSON M. Grana formation and synthesis of chloroplastic proteins induced by light in portions of etiolated leaves. Biochim Biophys Acta. 1954 Jun;14(2):203–211. doi: 10.1016/0006-3002(54)90159-6. [DOI] [PubMed] [Google Scholar]
- Frank S. R. THE EFFECTIVENESS OF THE SPECTRUM IN CHLOROPHYLL FORMATION. J Gen Physiol. 1946 Jan 20;29(3):157–179. [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI M., GOTO K., FUJIMOTO M., NAMIKI O., KIKUCHI G. EFFECT OF INHIBITORS OF NUCLEIC ACID AND PROTEIN SYNTHESES ON THE INDUCED SYNTHESES OF BACTERIOCHLOROPHYLL AND DELTA-AMINOLEVULINIC ACID SYNTHETASE BY RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1965 Jan 11;95:94–110. doi: 10.1016/0005-2787(65)90215-7. [DOI] [PubMed] [Google Scholar]
- KOSKI V. M. Chlorophyll formation in seedlings of Zea mays L. Arch Biochem. 1950 Dec;29(2):339–343. [PubMed] [Google Scholar]
- KOSKI V. M., FRENCH C. S., SMITH J. H. C. The action spectrum for the transformation of protochlorophyll to chlorophyll a in normal and albino corn seedlings. Arch Biochem Biophys. 1951 Mar;31(1):1–17. doi: 10.1016/0003-9861(51)90178-6. [DOI] [PubMed] [Google Scholar]
- Kirk J. T., Allen R. L. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun. 1965 Dec 21;21(6):523–530. doi: 10.1016/0006-291x(65)90516-4. [DOI] [PubMed] [Google Scholar]
- Klein S., Bogorad L. FINE STRUCTURAL CHANGES IN PROPLASTIDS DURING PHOTODESTRUCTION OF PIGMENTS. J Cell Biol. 1964 Aug 1;22(2):443–451. doi: 10.1083/jcb.22.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S., Bryan G., Bogorad L. EARLY STAGES IN THE DEVELOPMENT OF PLASTID FINE STRUCTURE IN RED AND FAR-RED LIGHT. J Cell Biol. 1964 Aug 1;22(2):433–442. doi: 10.1083/jcb.22.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of enzymes concerned in bacteriochlorophyll formation in growing cultures of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Dec;23:487–498. doi: 10.1099/00221287-23-3-487. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of porphyrins and bacteriochlorophyll by cell suspensions of Rhodopseudomonas spheroides. Biochem J. 1956 Jan;62(1):78–93. doi: 10.1042/bj0620078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCALLA D. R., ALLAN R. K. EFFECT OF ACTINOMYCIN D ON EUGLENA CHLOROPLAST FORMATION. Nature. 1964 Feb 1;201:504–505. doi: 10.1038/201504a0. [DOI] [PubMed] [Google Scholar]
- MEGO J. L., JAGENDORF A. T. Effect of light on growth of Black Valentine bean plastids. Biochim Biophys Acta. 1961 Oct 28;53:237–254. doi: 10.1016/0006-3002(61)90437-1. [DOI] [PubMed] [Google Scholar]
- Marcus A. Photocontrol of Formation of Red Kidney Bean Leaf Triphosphopyridine Nucleotide Linked Triosephosphate Dehydrogenase. Plant Physiol. 1960 Jan;35(1):126–128. doi: 10.1104/pp.35.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M. Effect of Chloramphenicol on Light Dependent Development of Seedlings of Phaseolus vulgaris var. Black Valentine, With Particular Reference to Development of Photosynthetic Activity. Plant Physiol. 1962 Jul;37(4):473–480. doi: 10.1104/pp.37.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M. Effect of Chloramphenicol on Light-Dependent Synthesis of Proteins and Enzymes of Leaves and Chloroplasts of Phaseolus vulgaris. Plant Physiol. 1964 Jul;39(4):579–585. doi: 10.1104/pp.39.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M. Relationship Between Red Light Mediated Glyceraldehyde-3-Phosphate Dehydrogenase Formation and Light Dependent Development of Photosynthesis. Plant Physiol. 1965 Jan;40(1):57–61. doi: 10.1104/pp.40.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POGO B. T., POGO A. O. DNA DEPENDENCE OF PLASTID DIFFERENTIATION INHIBITION BY ACTINOMYCIN D. J Cell Biol. 1964 Jul;22:296–301. doi: 10.1083/jcb.22.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price L., Klein W. H. Red, far-red response & chlorophyll synthesis. Plant Physiol. 1961 Nov;36(6):733–735. doi: 10.1104/pp.36.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. H., Benitez A. The Effect of Temperature on the Conversion of Protochlorophyll to Chlorophyll a in Etiolated Barley Leaves. Plant Physiol. 1954 Mar;29(2):135–143. doi: 10.1104/pp.29.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D. Protein synthesis by isolated spinach chloroplasts. Arch Biochem Biophys. 1965 Aug;111(2):381–390. doi: 10.1016/0003-9861(65)90200-6. [DOI] [PubMed] [Google Scholar]
- WOLFF J. B., PRICE L. Terminal steps of chlorophyll A biosynthesis in higher plants. Arch Biochem Biophys. 1957 Dec;72(2):293–301. doi: 10.1016/0003-9861(57)90205-9. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky M. B., Haba G. L. INHIBITION BY PUROMYCIN OF AMINO ACID INCORPORATION INTO PROTEIN. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1721–1729. doi: 10.1073/pnas.45.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]


