Abstract
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) can occur at any age and are commonly caused by adverse drug events. Rapid diagnosis of SJS/TEN is imperative, followed by immediate cessation of offending agent and induction of appropriate treatment. Cyclosporine, a calcineurin inhibitor, has been reported to have a promising therapeutic effect in SJS/TEN patients with few side effects. Diagnosis of SJS/TEN in children is especially challenging as many of the symptoms mimic that of an upper respiratory infection, or other viral entities such as cocksackie A, roseola, or herpes simplex virus. We recommend initiating cyclosporine modified treatment, especially in children, upon any suspicion of SJS/TEN in a patient in order to halt the disease progression as early as possible.
Keywords: Cyclosporine modified, Stevens-Johnson syndrome, toxic epidermal necrolysis
Introduction
Stevens-Johnson syndrome (SJS) is a severe mucocutaneous reaction characterized by mucosal erosion, epidermal detachment, and necrosis that affects less than 10% of body surface area (BSA).[1] If more than 30% of BSA is detached, this is referred to as toxic epidermal necrolysis (TEN) syndrome. Stevens-Johnson can occur at any age and is most commonly caused by adverse drug events. Drug-associated SJS is a severe drug reaction with a mortality rate of up to 50% in adults and around 3.6% in children.[1,2] Although a number of drugs have been implicated in SJS/TEN, antibiotics are the most common culprit.[1] In addition to discontinuation of the suspected offending agent and a focus on symptomatic and supportive treatment, systemic glucocorticoids and intravenous immunoglobulin (IVIG) have been the mainstay for treatment in many studies.[3] However, in recent years, cyclosporine, a calcineurin inhibitor, has been reported to have a promising therapeutic effect in SJS/TEN patients with few side effects. To emphasize this, we present the case of a 12-year old child diagnosed with SJS successfully treated with early employment of oral cyclosporine modified.
Case Report
A 12-year-old girl with no significant medical history was seen in a dermatology private practice the day after a pediatric emergency department visit for a targetoid eruption. The eruption involved her trunk, arms, legs, palms, and soles following a 10-day course of trimethoprim/sulfamethoxazole and was diagnosed as hand, foot, and mouth disease. Upon arrival to the dermatology clinic, the patient was on oral hydroxyzine and prednisone. She continued to worsen and developed painful swallowing, difficulty urinating, periorbital edema, and conjunctival injection secondary to mucosal involvement the next day [Figures 1-3]. She also had associated malaise and subjective fevers. A diagnosis of SJS was made by the dermatologist. The risks, benefits, and alternatives of the diagnosis and treatments were discussed with the patient and the patient's mother and topical and oral therapies were chosen. The patient was started on cyclosporine modified 5 mg/kg (100 mg once daily orally) and the previously prescribed course of prednisone was continued (5 mg twice daily orally). A phone call with the parents on day 2 revealed a dramatic improvement of all symptoms. The patient was seen in follow-up on day 7. All symptoms present at onset had resolved. The targetoid eruption was fading, and the blistering and crust on her lips were almost gone [Figures 4 and 5]. A prednisone taper was initiated and cyclosporine modified was continued at the same dose. Ten days after the initial presentation, the patient was clear with complete re-epithelialization of all surfaces. Cyclosporine modified was stopped on day 14 with no taper. Four weeks after the initial presentation, the physical exam was notable only for mild hypopigmentation and hyperpigmentation with vascular change at the sites of inflammation. All therapies were discontinued, and the patient was advised to wear sunscreen to minimize the possibility of post-inflammatory hyperpigmentation.
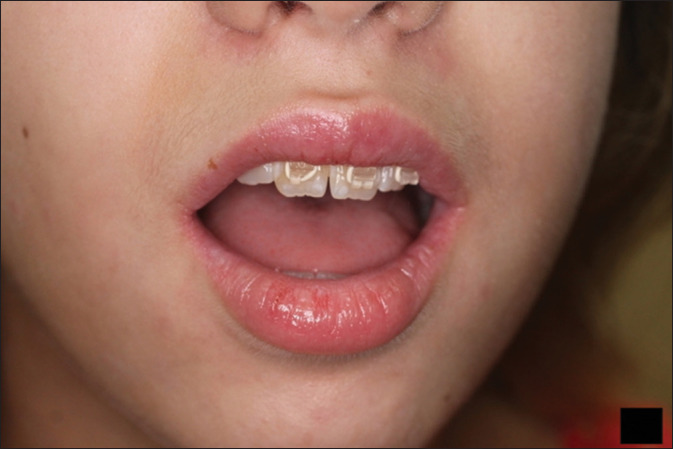
Figure 1.
Mucosal involvement and targetoid eruption at presenation
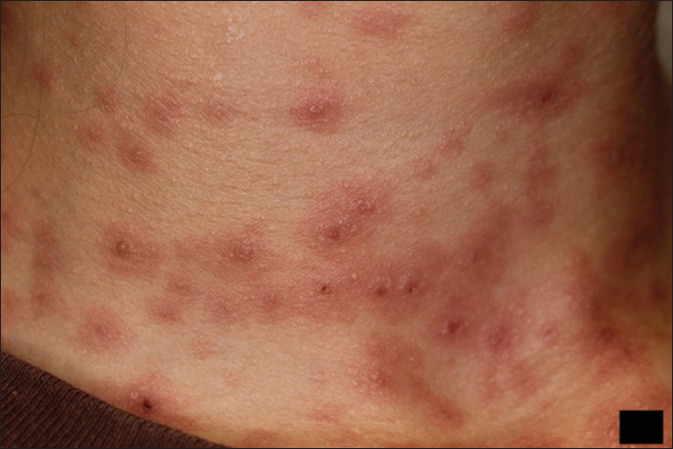
Figure 3.
Classic targetoid eruption on patient's neck
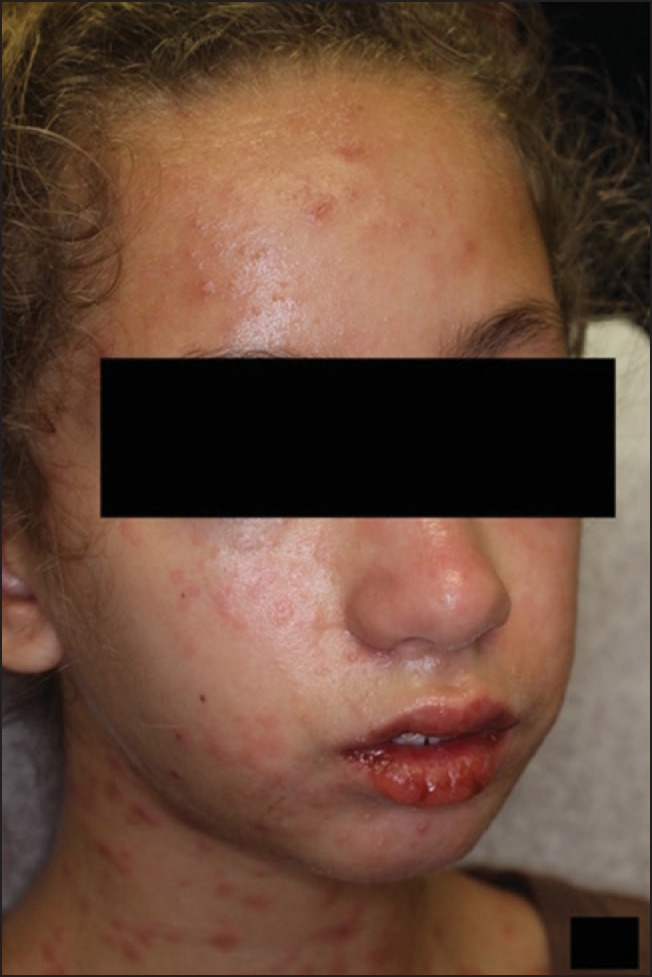
Figure 4.
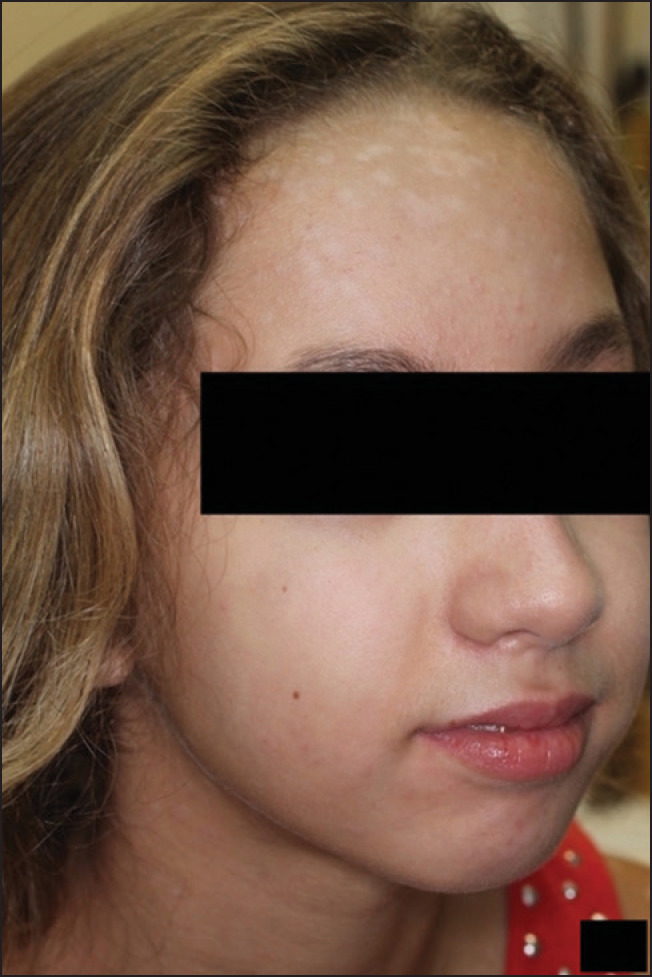
Complete resolution after 2 weeks of cyclosporine modified therapy. Mild hypopigmentation still present but resolving
Figure 5.
Complete resolution after 2 weeks of cyclosporine modified therapy. Mild hypopigmentation still present but resolving
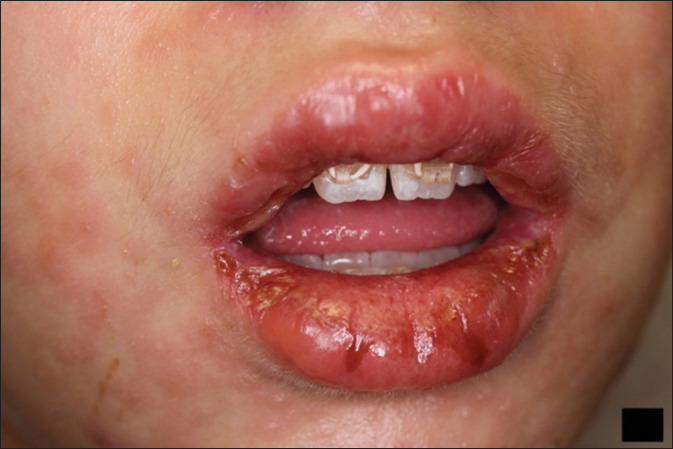
Figure 2.
Erythema, blistering, and erosions involving the mucosal surfaces along with perioral targetoid eruption
Discussion
SJS is a rare, severe cutaneous adverse reaction that has been shown to cause significant morbidity and mortality in children and adults.[4] The annual incidence is known to be 1-6 cases per million and, based on data from the national administrative database in Korea, is consistently rising with each new year.[4] Most cases of SJS/TEN are induced by ingestion of a drug.[5] Common culprits include allopurinol, aromatic anticonvulsants, antibiotics, and nonsteroidal anti-inflammatory drugs.[1] A systematic review and meta-analysis published in 2023 found that of the antibiotic-associated SJS/TEN, sulfonamides were the most commonly involved followed closely by penicillins, and then cephalosporins, fluoroquinolones, and macrolides.[1]
The clinical manifestations of SJS/TEN are the same for both children and adults. Findings may include skin and mucous membrane pain, erythema, blisters, epidermal exfoliation, and other skin lesions involving the conjunctiva, oral cavity, and/or urogenital mucosa.[6] Histopathologic examination, if chosen as a diagnostic modality, is characterized by extensive apoptosis and necrosis of keratinocytes.
Rapid diagnosis of SJS/TEN is imperative, followed by immediate cessation of the offending agent and induction of appropriate treatment. Although there is debate surrounding appropriate adjuvant therapy for SJS/TEN, several recent studies point toward cyclosporine modified as the most promising option.[7] Cyclosporine modified works to downregulate T-cell activation and thus inhibit T-cells from producing the cytokines that promote the pathogenesis and propagation of SJS/TEN, ultimately preventing keratinocyte apoptosis.[8] A case series performed by St John et al.[8] reported three pediatric patients successfully treated with cyclosporine who saw an average time of disease progression cessation of 2.2 days, and disease remission of reepithelialization of 13 days, following cyclosporine administration. A study performed in India found similar results with a mean time of disease stabilization following initiation of cyclosporine at 3.94 days and reepithelialization at 10.5 days, decreased hospital stays, reduced morbidity and mortality, and suggests the superiority of cyclosporine over other therapies such as IVIG, corticosteroid, cyclophosphamide, and supportive care only.[5] Although the optimal dosing of cyclosporine for SJS/TEN has not been standardized, literature suggests that 3-5 mg/kg body weight for 5-14 days proves successful.[5,7]
We would like to pay particular attention to early and proper diagnosis in pediatric patients. SJS/TEN manifest with prodromal symptoms 1-3 days prior to skin and mucosal lesions including fever, malaise, non-productive cough, stinging eyes, and a sore mouth.[9] Due to a plethora of bacteria and viruses that affect children, these symptoms can be misdiagnosed as an upper respiratory tract infection. Furthermore, the formation of erythema, red papules or patches, and even blistering can be mislabeled as a viral exanthem such as Coxsackie A, Roseola, or herpes simplex virus.[9] It is imperative to keep SJS/TEN in the differential when encountering these symptoms, particularly in children, so as to promptly remove the offending agent and begin systemic treatment. We recommend initiating cyclosporine-modified treatment, especially in children, upon any suspicion of SJS/TEN in a patient in order to halt the disease progression as early as possible.
Conclusion
Although SJS/TEN is generally considered a clinical emergency and warrants hospital admission, our case highlights the possibility of successful treatment in an outpatient setting. The clinical suspicion must remain high for SJS/TEN in any patient presenting with an acute outbreak of lesions or previously discussed symptoms, especially following recent medication initiation. Rapid cessation of the offending agent and employment of oral cyclosporine modified and oral prednisone, along with close monitoring, may eliminate the need for a hospital admission at bay. This case also highlights the possibility of treating SJS/TEN with oral therapies versus the traditional intravenous therapies. However, it is important to note that acute worsening of symptoms in our patient would have warranted prompt return to the emergency department and likely admission.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis. JAMA Dermatol. 2023;159:384–92. doi: 10.1001/jamadermatol.2022.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu DY, Brieva J, Silverberg NB, Paller AS, Silverberg JI. Pediatric Stevens-Johnson syndrome and toxic epidermal necrolysis in the United States. J Am Acad Dermatol. 2017:76811–7. doi: 10.1016/j.jaad.2016.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasegawa A, Abe R. Recent advances in managing and understanding Stevens-Johnson syndrome and toxic epidermal necrolysis. F1000 Res. 2020;9:F1000. doi: 10.12688/f1000research.24748.1. doi: 10.12688/f1000research.24748.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang MS, Lee JY, Kim J, Kim GW, Kim BK, Kim JY, et al. Incidence of Stevens-Johnson syndrome and toxic epidermal necrolysis: A nationwide population-based study using national health insurance database in Korea. PLoS One. 2016;11:e0165933. doi: 10.1371/journal.pone.0165933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balai M, Meena M, Mittal A, Gupta LK, Khare AK, Mehta S. Cyclosporine in Stevens-Johnson syndrome and toxic epidermal necrolysis: Experience from a tertiary care centre of South Rajasthan. Indian Dermatol Online J. 2021;12:116–22. doi: 10.4103/idoj.IDOJ_326_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye Z, Li C, Zhang H, Zhang C, Lu X. Effectiveness and safety of early short-course, moderate- to high-dose glucocorticoids for the treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: A retrospective study. Clic Cosmet Investig Dermatol. 2022;15:1979–90. doi: 10.2147/CCID.S378106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heymann WR. Cyclosporine reach the top of the SJS/TEN leaderboard. AAD Archive: Dermatology World Insights and Inquiries. Available from: https://www.aad.org/dw/dw-insights-and-inquiries/medical-dermatology/cyclosporine-reaches-the-top-of-the-sjs-ten-leaderboard . [Google Scholar]
- 8.St John J, Ratushny V, Liu KJ, Bach DQ, Badri O, Gracey LE, et al. Successful use of cyclosporine A for Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Pediatr Dermatol. 2017;34:540–6. doi: 10.1111/pde.13236. [DOI] [PubMed] [Google Scholar]
- 9.Ramien M, Goldman JL. Pediatric SJS-TEN: Where are we now? F1000 Res. 2020;9:F1000. doi: 10.12688/f1000research.20419.1. doi: 10.12688/f1000research.20419.1. [DOI] [PMC free article] [PubMed] [Google Scholar]