Abstract
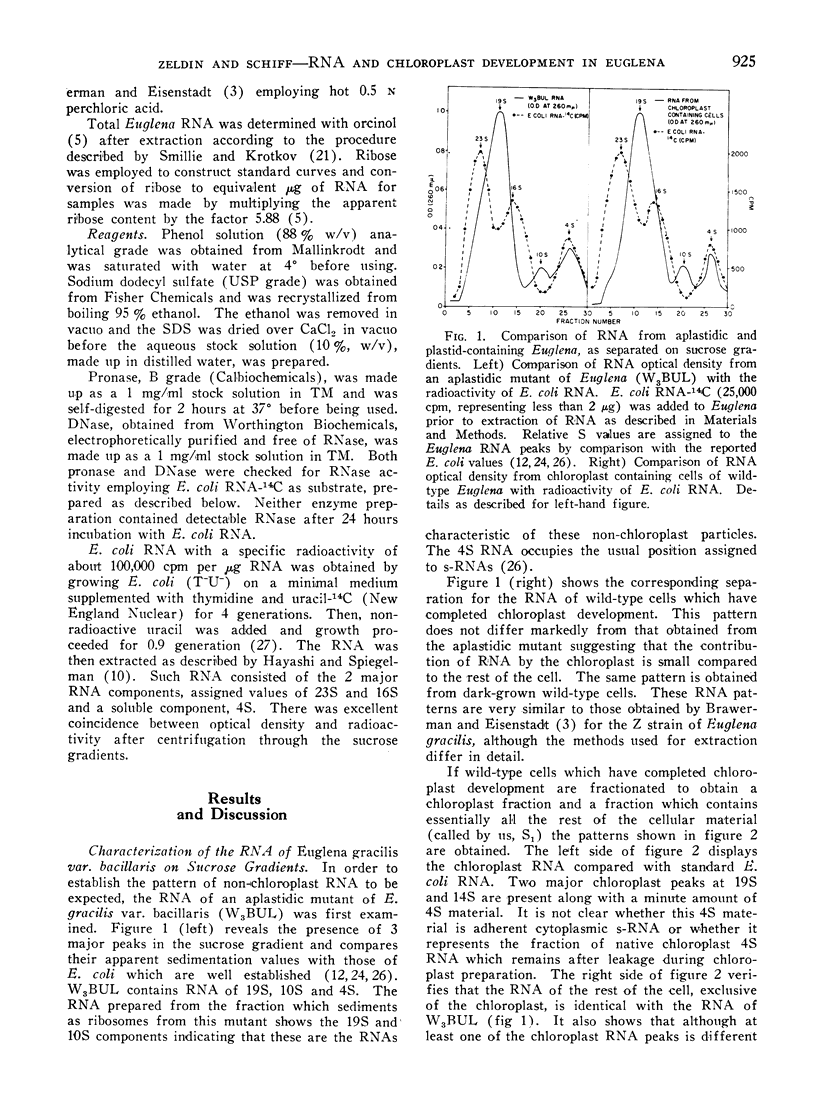
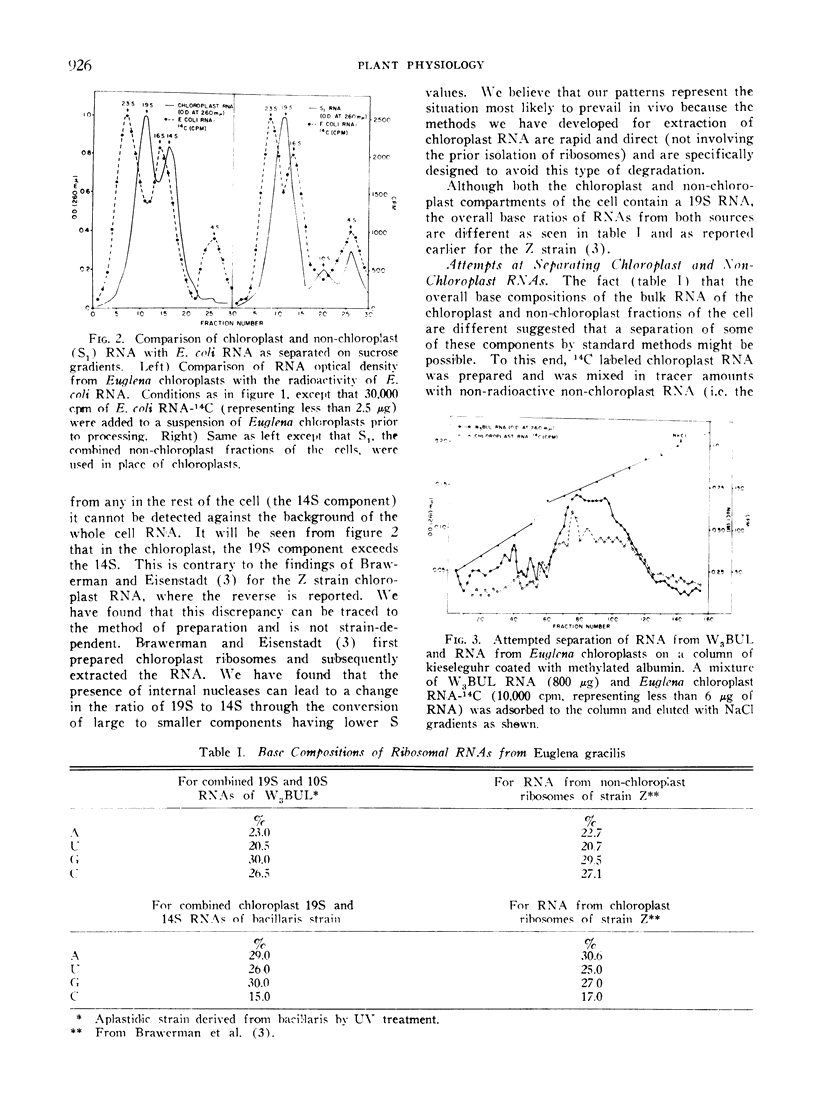
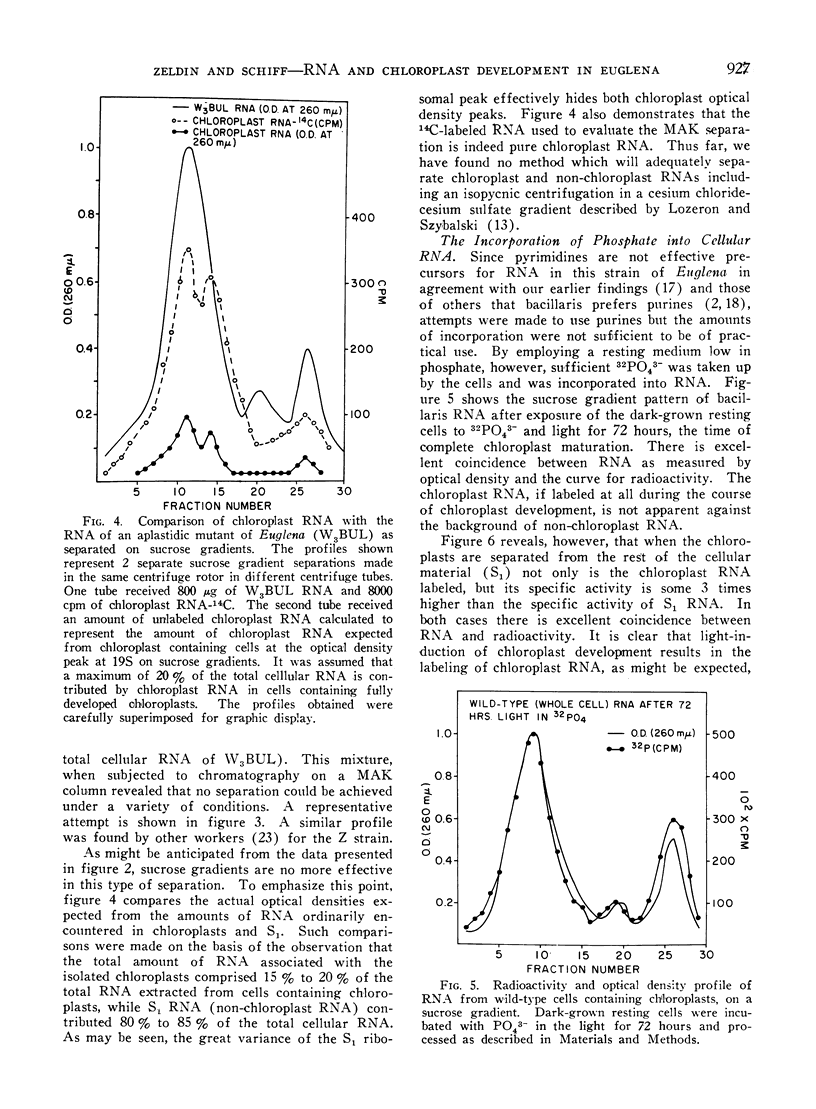
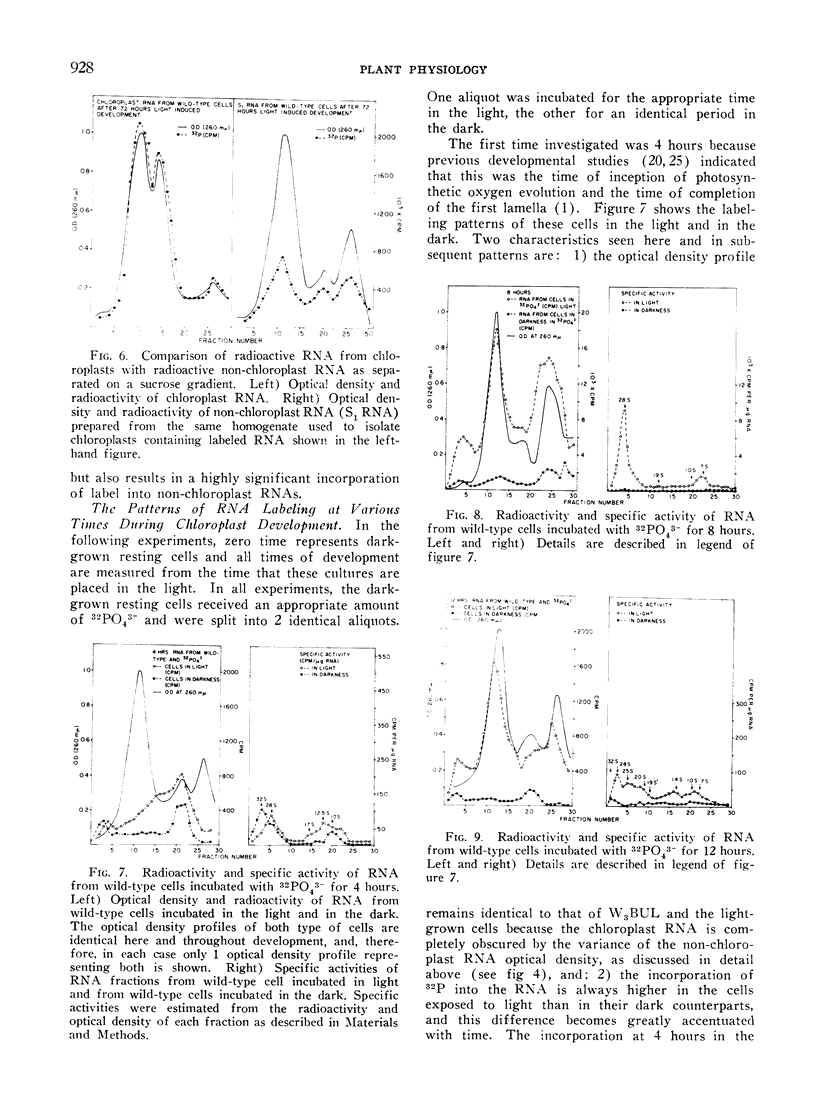
Methods are described which provide good recoveries of non-degraded chloroplast and non-chloroplast RNAs from Euglena gracilis var. bacillaris. These have been characterized by comparing the RNA from W3BUL (an aplastidic mutant of Euglena), with that of wild-type cells which have been resolved into chloroplast and non-chloroplast fractions. Using E. coli RNA as a standard, the RNAs from W3BUL and from the non-chloroplast fraction of green cells exhibit optical density peaks, upon sucrose gradient centrifugation, at 4S, 10S, and 19S. The chloroplast fraction exhibits optical density peaks at 19S and 14S with the 19S component predominating. Application of various techniques for the separation of RNAs to the problem of separating the chloroplast and non-chloroplast RNAs, without prior separation of the organelle, have not proven successful.
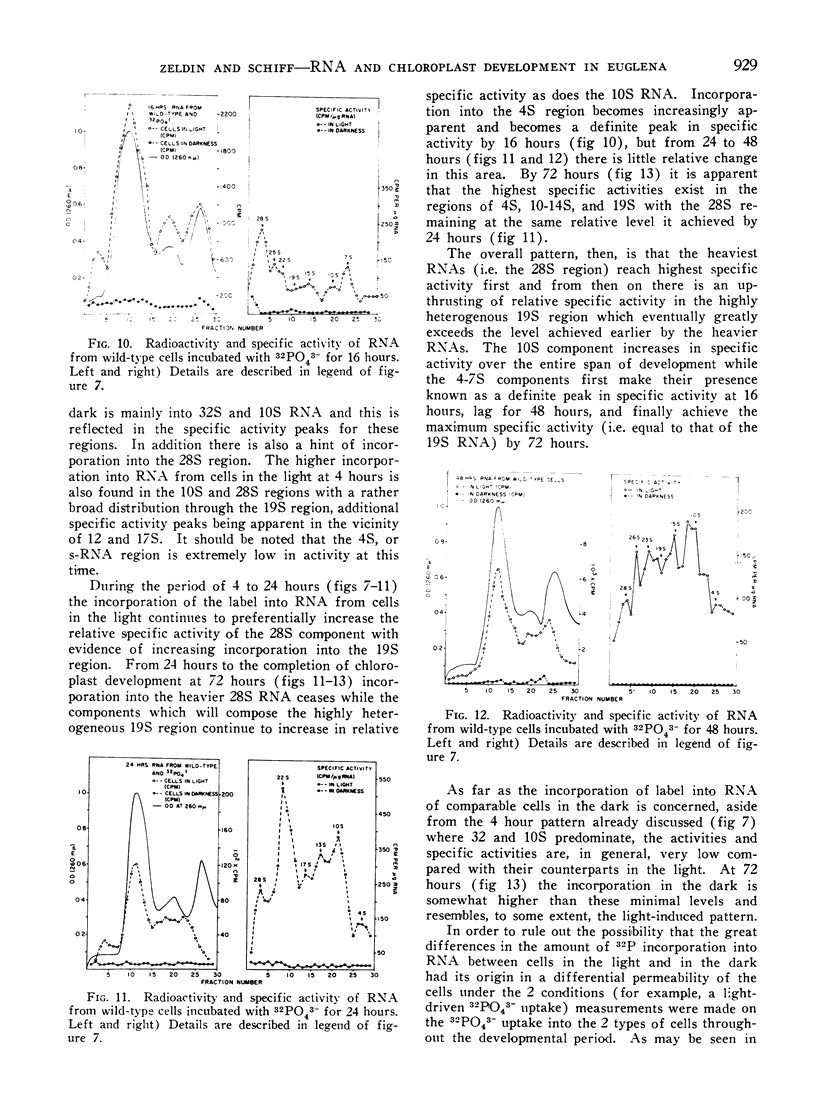
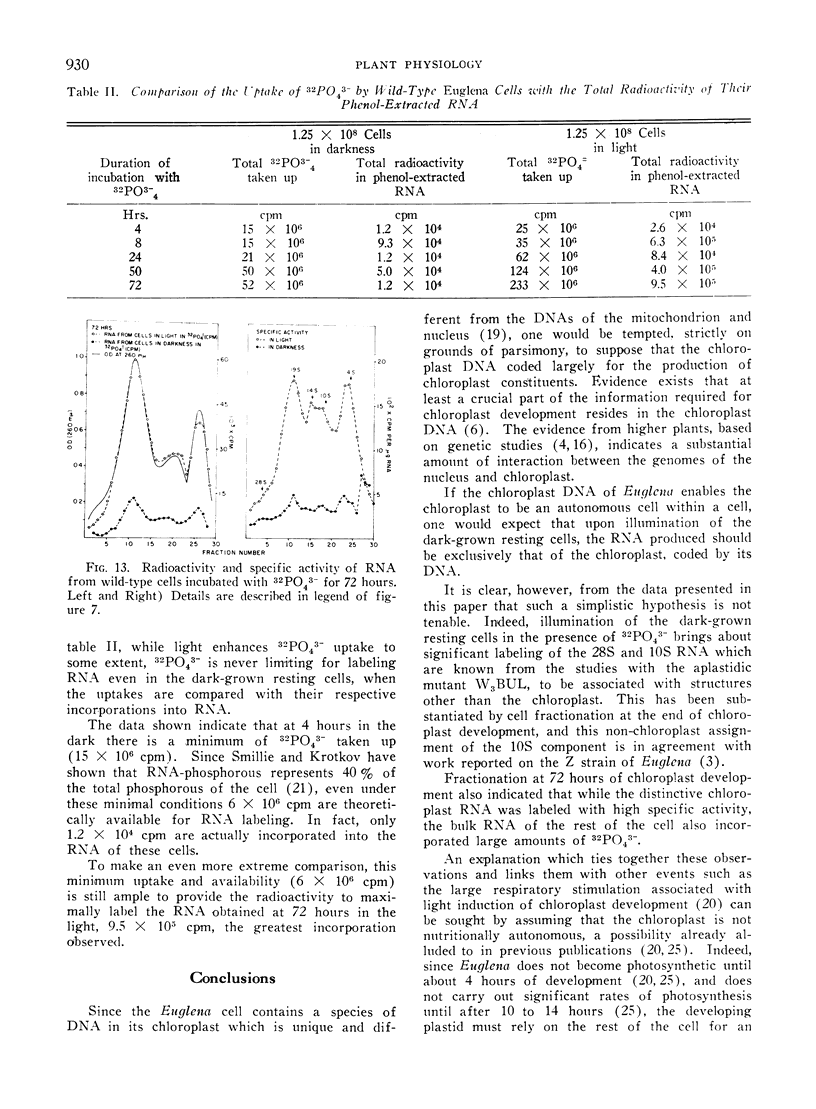
32Pi is readily incorporated into RNA by cells undergoing light-induced chloroplast development, and fractionation at the end of development reveals that although chloroplast RNAs have a higher specific activity, the other RNAs of the cells are significantly labeled as well. The succession of labeling patterns of total cellular RNA as light-induced chloroplast development proceeds are displayed and reveal that all RNA species mentioned above eventually become labeled. In contrast, cells kept in darkness during this period incorporate little 32Pi into any RNA fraction. In addition, a heavy RNA component, designated as 28S, while representing a negligible fraction of the total RNA, becomes significantly labeled during the first 24 hours of illumination. While there is light stimulated uptake of 32Pi into the cells, this uptake is never limiting in the light or dark, for RNA labeling.
On the basis of these findings, we suggest that extensive activation of non-chloroplast RNA labeling during chloroplast development is the result of the activation of the cellular synthetic machinery external to the chloroplast necessary to provide metabolic precursors for plastid development. Thus the plastid is viewed as an auxotrophic resident within the cell during development. Other possibilities for interaction at this and other levels are also discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAWERMAN G., EISENSTADT J. M. TEMPLATE AND RIBOSOMAL RIBONUCLEIC ACIDS ASSOCIATED WITH THE CHLOROPLASTS AND THE CYTOPLASM OF EUGLENA GRACILIS. J Mol Biol. 1964 Dec;10:403–411. doi: 10.1016/s0022-2836(64)80061-9. [DOI] [PubMed] [Google Scholar]
- Ben-Shaul Y., Schiff J. A., Epstein H. T. Studies of Chloroplast Development in Euglena. VII. Fine Structure of the Developing Plastid. Plant Physiol. 1964 Mar;39(2):231–240. doi: 10.1104/pp.39.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN M., SCHIFF J. A., EPSTEIN H. T. STUDIES OF CHLOROPLAST DEVELOPMENT IN EUGLENA. XII. TWO TYPES OF SATELLITE DNA. J Mol Biol. 1965 Apr;11:769–774. doi: 10.1016/s0022-2836(65)80034-1. [DOI] [PubMed] [Google Scholar]
- EISENSTADT J. M., BRAWERMAN G. THE PROTEIN-SYNTHESIZING SYSTEMS FROM THE CYTOPLASM AND THE CHLOROPLASTS OF EUGLENA GRACILIS. J Mol Biol. 1964 Dec;10:392–402. doi: 10.1016/s0022-2836(64)80060-7. [DOI] [PubMed] [Google Scholar]
- Gebicki J. M., Freed S. Microdetermination of nucleotides in hydrolyzates of RNA. Anal Biochem. 1966 Feb;14(2):253–257. doi: 10.1016/0003-2697(66)90133-3. [DOI] [PubMed] [Google Scholar]
- HAYASHI M., SPIEGELMAN S. The selective synthesis of informational RNA in bacteria. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1564–1580. doi: 10.1073/pnas.47.10.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYMAN H., EPSTEIN H. T., SCHIFF J. A. Studies of chloroplast development in Euglena. I. Inactivation of green colony formation by u.v. light. Biochim Biophys Acta. 1961 Jun 24;50:301–309. doi: 10.1016/0006-3002(61)90328-6. [DOI] [PubMed] [Google Scholar]
- Lozeron H. A., Szybalski W. Suppression of RNA precipitation during Cs2SO4 density gradient centrifugation. Biochem Biophys Res Commun. 1966 Jun 13;23(5):612–618. doi: 10.1016/0006-291x(66)90443-8. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- SAGAN L. AN UNUSUAL PATTERN OF TRITIATED THYMIDINE INCORPORATION IN EUGLENA. J Protozool. 1965 Feb;12:105–109. doi: 10.1111/j.1550-7408.1965.tb01822.x. [DOI] [PubMed] [Google Scholar]
- SMILLIE R. M., KROTKOV G. Phosphorus-containing compounds in Euglena gracilis grown under different conditions. Arch Biochem Biophys. 1960 Jul;89:83–90. doi: 10.1016/0003-9861(60)90016-3. [DOI] [PubMed] [Google Scholar]
- Sagan L., Ben-Shaul Y., Epstein H. T., Schiff J. A. Studies of chloroplast development in Euglena. XI. Radioautographic localization of chloroplast DNA. Plant Physiol. 1965 Nov;40(6):1257–1260. doi: 10.1104/pp.40.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess E., Richter G. Die Nucleinsäuren grüner und gebleichter Zellen von Euglena gracilis. Arch Mikrobiol. 1966 Mar 31;53(3):195–207. [PubMed] [Google Scholar]
- Stanley W. M., Jr, Bock R. M. Isolation and physical properties of the ribosomal ribonucleic acid of Escherichia coli. Biochemistry. 1965 Jul;4(7):1302–1311. doi: 10.1021/bi00883a014. [DOI] [PubMed] [Google Scholar]
- Stern A. I., Schiff J. A., Epstein H. T. Studies of Chloroplast Development in Euglena. V. Pigment Biosynthesis, Photosynthetic Oxygen Evolution and Carbon Dioxide Fixation during Chloroplast Development. Plant Physiol. 1964 Mar;39(2):220–226. doi: 10.1104/pp.39.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANKOFSKY S. A., SPIEGELMAN S. The identification of the ribosomal RNA cistron by sequence complementarity. I. Specificity of complex formation. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1069–1078. doi: 10.1073/pnas.48.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]


