Abstract
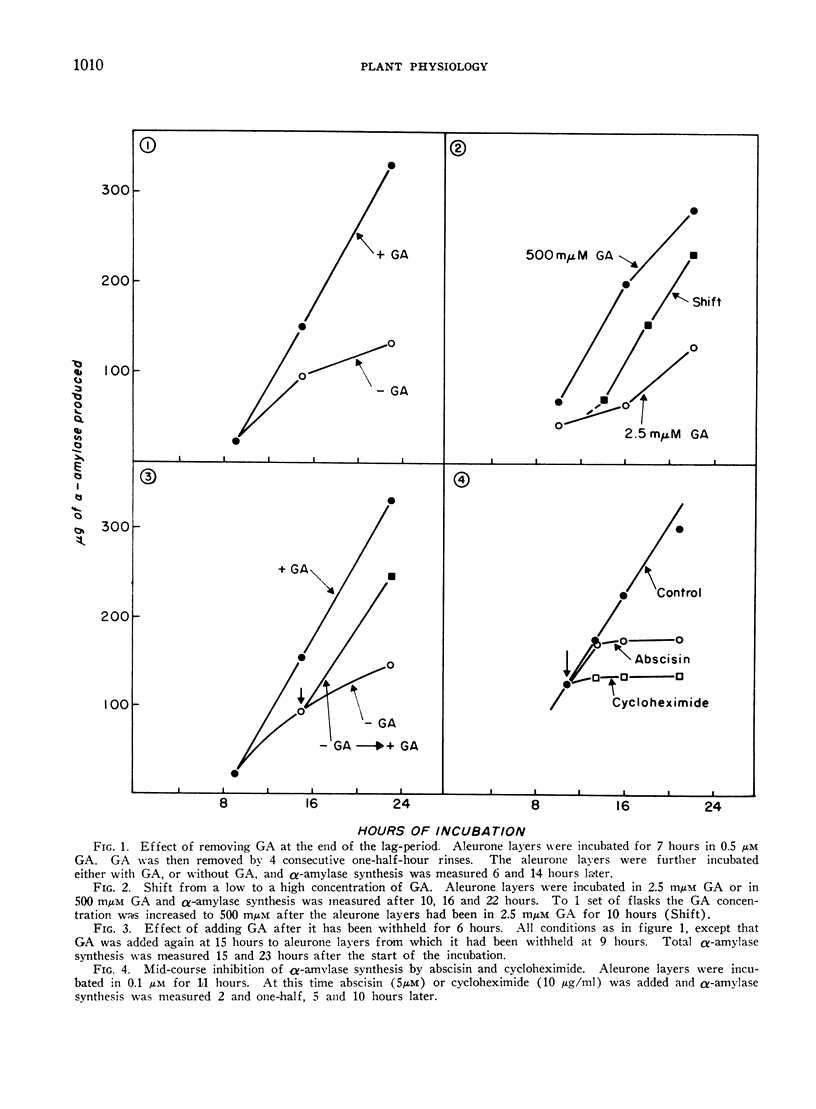
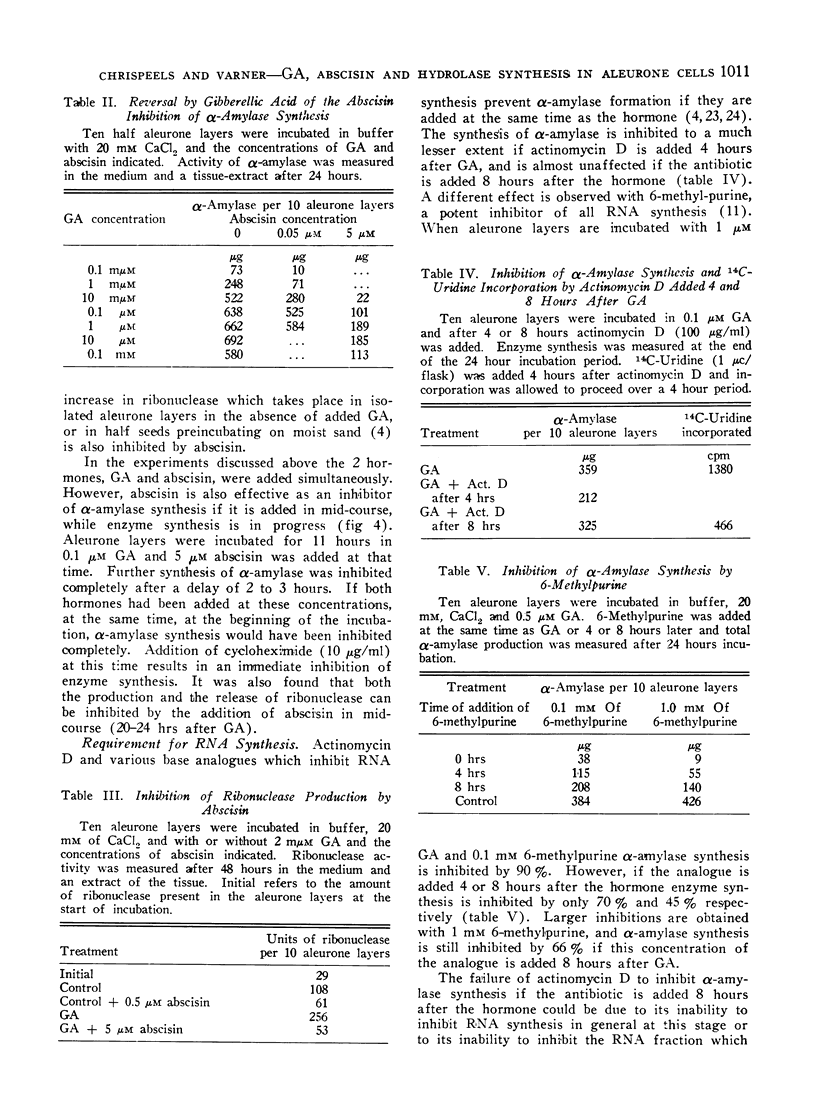
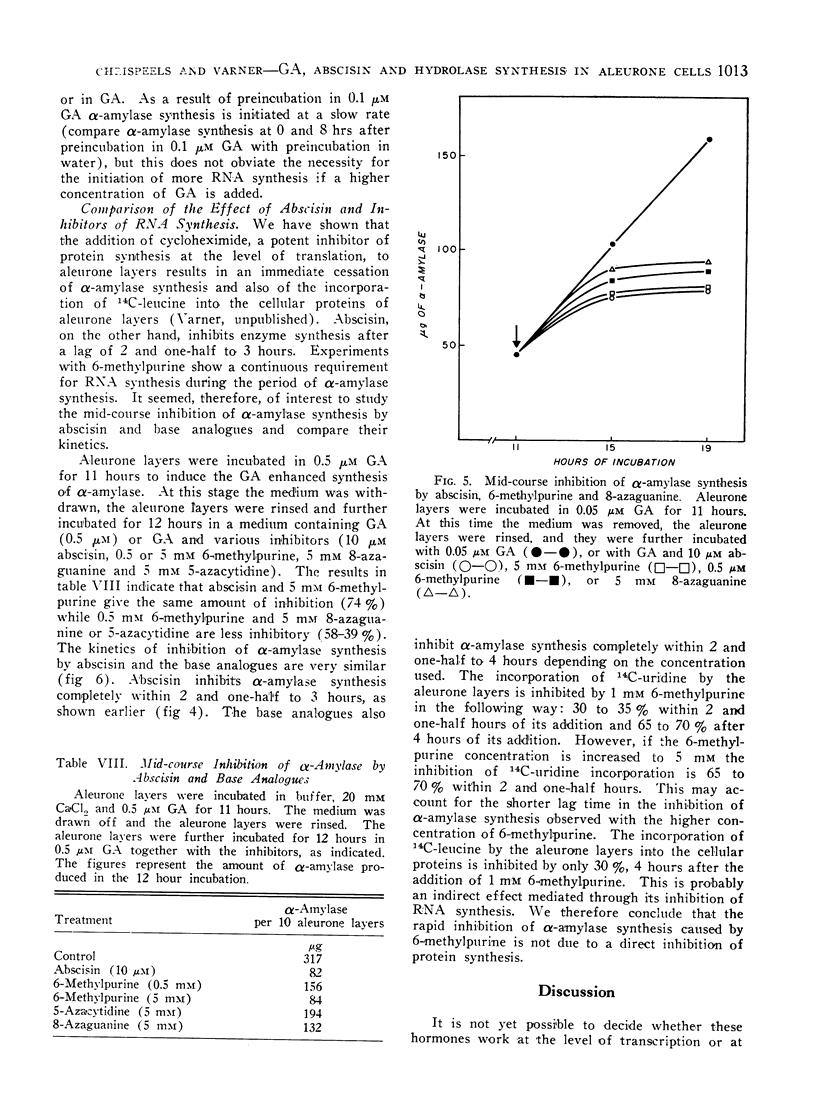
Gibberellic acid (GA) enhances the synthesis of α-amylase and ribonuclease in isolated aleurone layers and this process is inhibited by abscisin. Removal of gibberellic acid in mid-course of α-amylase production results in a slowing down of α-amylase synthesis, suggesting a continued requirement of GA for enzyme synthesis. This is paralleled by a continuous requirement for RNA synthesis. Addition of 6-methylpurine or 8-azaguanine in mid-course results in an inhibition of α-amylase synthesis within 3 to 4 hours. However, actinomycin D added in mid-course is almost without effect. This is not due to its failure to enter the cells, because it does inhibit 14C-uridine incorporation at this stage. Addition of abscisin to aleurone layers which are synthesizing α-amylase results in an inhibition of this synthesis within 2 to 3 hours. Cycloheximide on the other hand inhibits enzyme synthesis immediately upon its addition. These data are consistent with the hypothesis that the expression of the GA effect requires the synthesis of enzyme-specific RNA molecules. The similarity in the kinetics of inhibition between abscisin on the one hand and 8-azaguanine or 6-methylpurine on the other suggests that abscisin may exert its action by inhibiting the synthesis of these enzyme-specific RNA molecules or by preventing their incorporation into an active enzyme-synthesising unit.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B., Holm R. E. Enhancement of RNA synthesis, protein synthesis, and abscission by ethylene. Plant Physiol. 1966 Oct;41(8):1337–1342. doi: 10.1104/pp.41.8.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra G. R., Varner J. E. Gibberellic acid-controlled metabolism of RNA in aleurone cells of barley. Biochim Biophys Acta. 1965 Dec 9;108(4):583–592. doi: 10.1016/0005-2787(65)90055-9. [DOI] [PubMed] [Google Scholar]
- Hamilton T. H., Moore R. J., Rumsey A. F., Means A. R., Schrank A. R. Stimulation of synthesis of ribonucleic acid in sub-apical sections of Avena coleoptile by indolyl-3-acetic acid. Nature. 1965 Dec 18;208(5016):1180–1183. doi: 10.1038/2081180a0. [DOI] [PubMed] [Google Scholar]
- KIRBY K. S. ISOLATION AND CHARACTERIZATION OF RIBOSOMAL RIBONUCLEIC ACID. Biochem J. 1965 Jul;96:266–269. doi: 10.1042/bj0960266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L. Effect of purine and pyrimidine analogues on growth and RNA metabolism in the soybean hypocotyl-the selective action of 5-fluorouracil. Plant Physiol. 1966 Oct;41(8):1257–1264. doi: 10.1104/pp.41.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L. Ribonucleic Acid and Protein Synthesis as Essential Processes for Cell Elongation. Plant Physiol. 1964 May;39(3):365–370. doi: 10.1104/pp.39.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Shannon J. C. Enhancement by Auxin of Ribonucleic Acid Synthesis in Excised Soybean Hypocotyl Tissue. Plant Physiol. 1964 May;39(3):360–364. doi: 10.1104/pp.39.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsan J., Lang A. DNA synthesis in the elongating nondividing cells of the lentil epicotyl and its promotion by gibberellin. Plant Physiol. 1966 Jun;41(6):965–970. doi: 10.1104/pp.41.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noodén L. D., Thimann K. V. EVIDENCE FOR A REQUIREMENT FOR PROTEIN SYNTHESIS FOR AUXIN-INDUCED CELL ENLARGEMENT. Proc Natl Acad Sci U S A. 1963 Aug;50(2):194–200. doi: 10.1073/pnas.50.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleg L. G. Physiological Effects of Gibberellic Acid: I. On Carbohydrate Metabolism and Amylase Activity of Barley Endosperm. Plant Physiol. 1960 May;35(3):293–299. doi: 10.1104/pp.35.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENIS M. A. INDUCTION OF ENZYMATIC ACTIVITY BY INDOLYL-3-ACETIC ACID AND ITS DEPENDENCE ON SYNTHESIS OF RIBONUCLEIC ACID. Nature. 1964 May 30;202:900–901. doi: 10.1038/202900b0. [DOI] [PubMed] [Google Scholar]
- Varner J. E., Chandra G. R. HORMONAL CONTROL OF ENZYME SYNTHESIS IN BARLEY ENDOSPERM. Proc Natl Acad Sci U S A. 1964 Jul;52(1):100–106. doi: 10.1073/pnas.52.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner J. E. Gibberellic Acid Controlled Synthesis of alpha-Amylase in Barley Endosperm. Plant Physiol. 1964 May;39(3):413–415. doi: 10.1104/pp.39.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]


