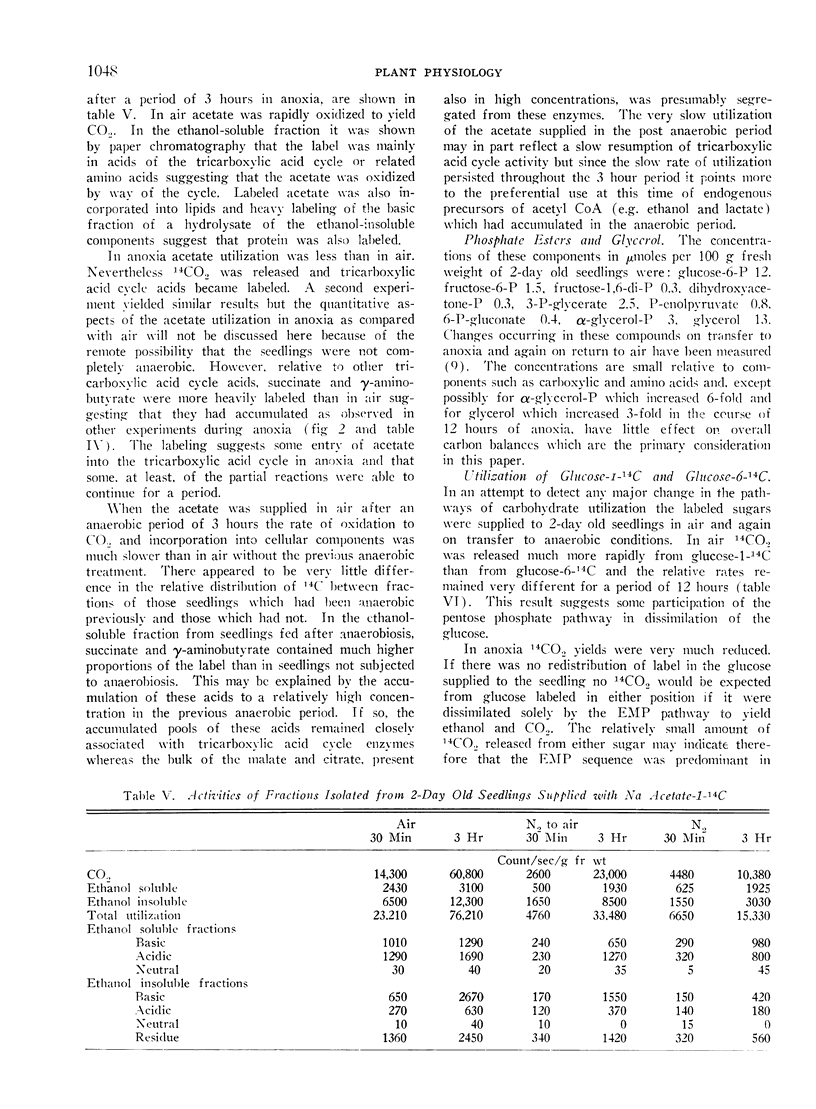
Abstract
Young seedlings of buckwheat (Fagopyrum esculentum) respire in air with an RQ of unity. Analysis of respiratory substrates coupled with a study of the utilization of acetate-14C and glucose-14C suggest that both the Embden-Meyerhof-Parnas, tricarboxylic acid and pentose phosphate sequences participate in the total respiratory catabolism.
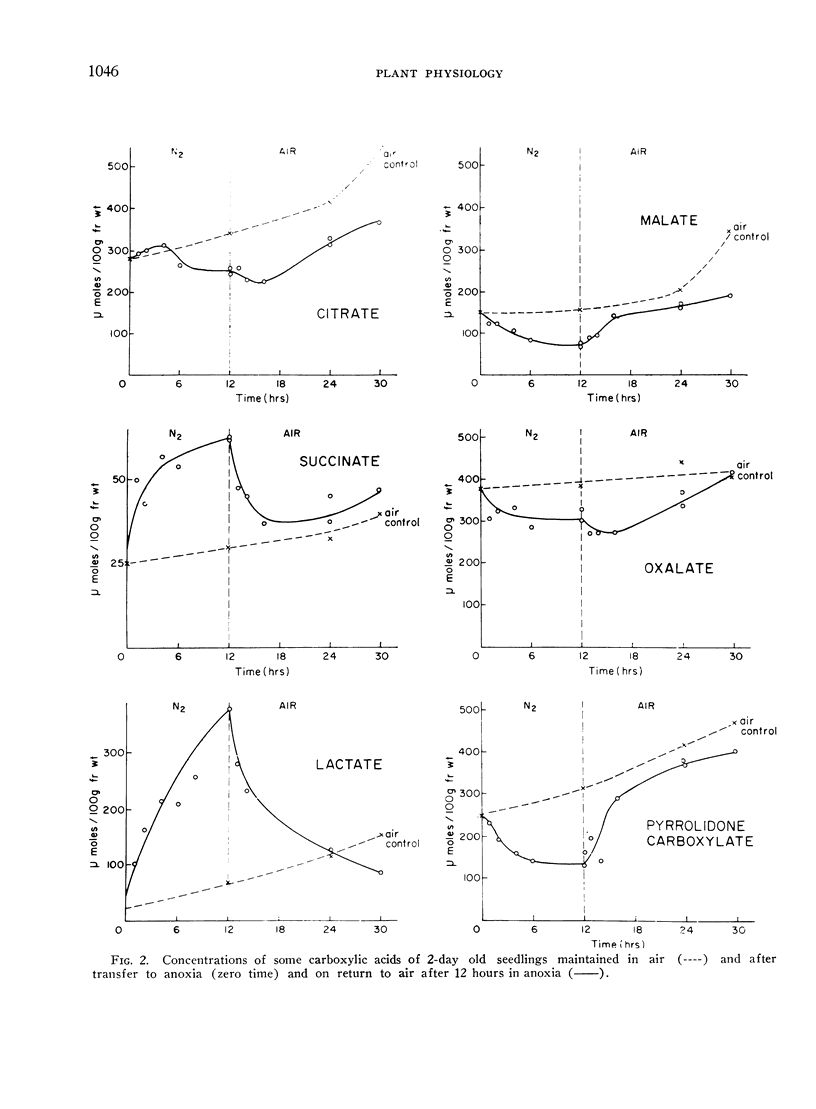
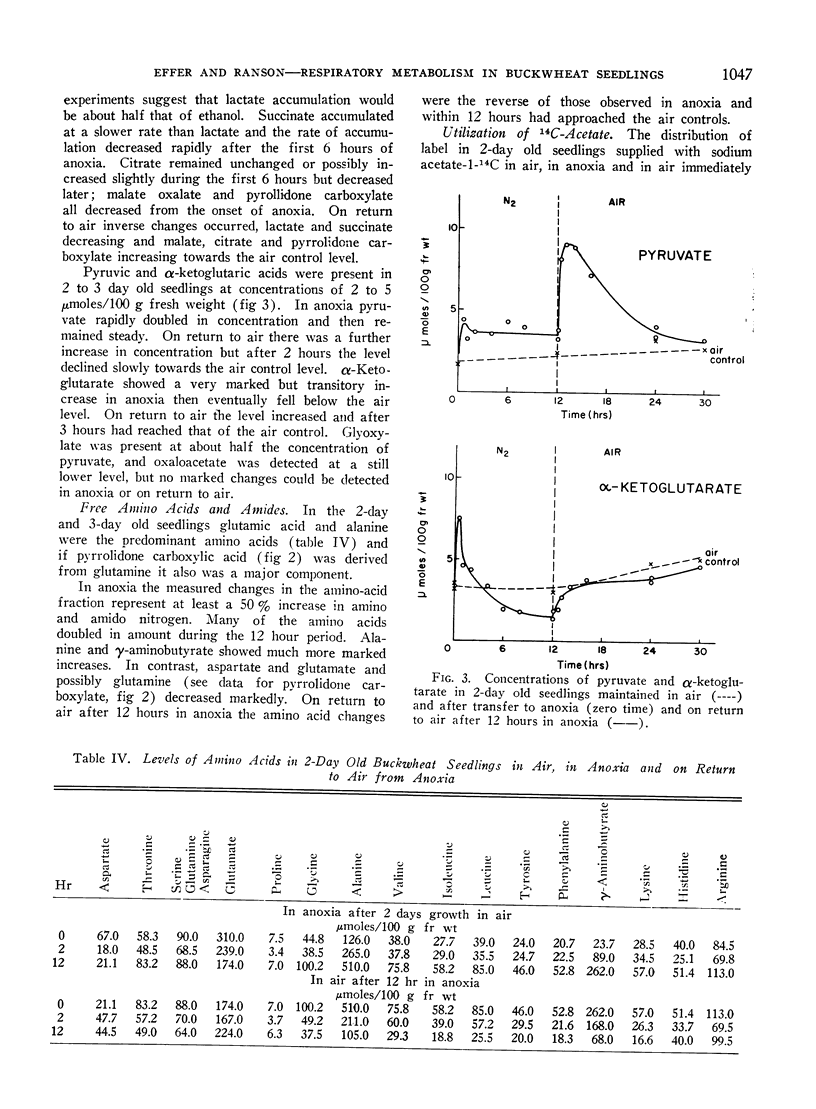
In anoxia CO2 dropped to one third of the aerobic rate and ethanol accumulated to only about one half the rate of CO2 output on a molar basis. Smaller amounts of lactate, succinate and free amino acids (particularly alanine and γ-aminobutyric acid) accumulated, carboxylic acids decreased and there were initial increased in pyruvate and α-ketoglutarate. The observed changes are consistent with residual tricarboxylic acid and pentose phosphate cycle activity in anoxia and may account for the excess CO2 production over ethanol accumulation. CO2, ethanol and lactate production did not account for all of the carbohydrate consumed in anoxia.
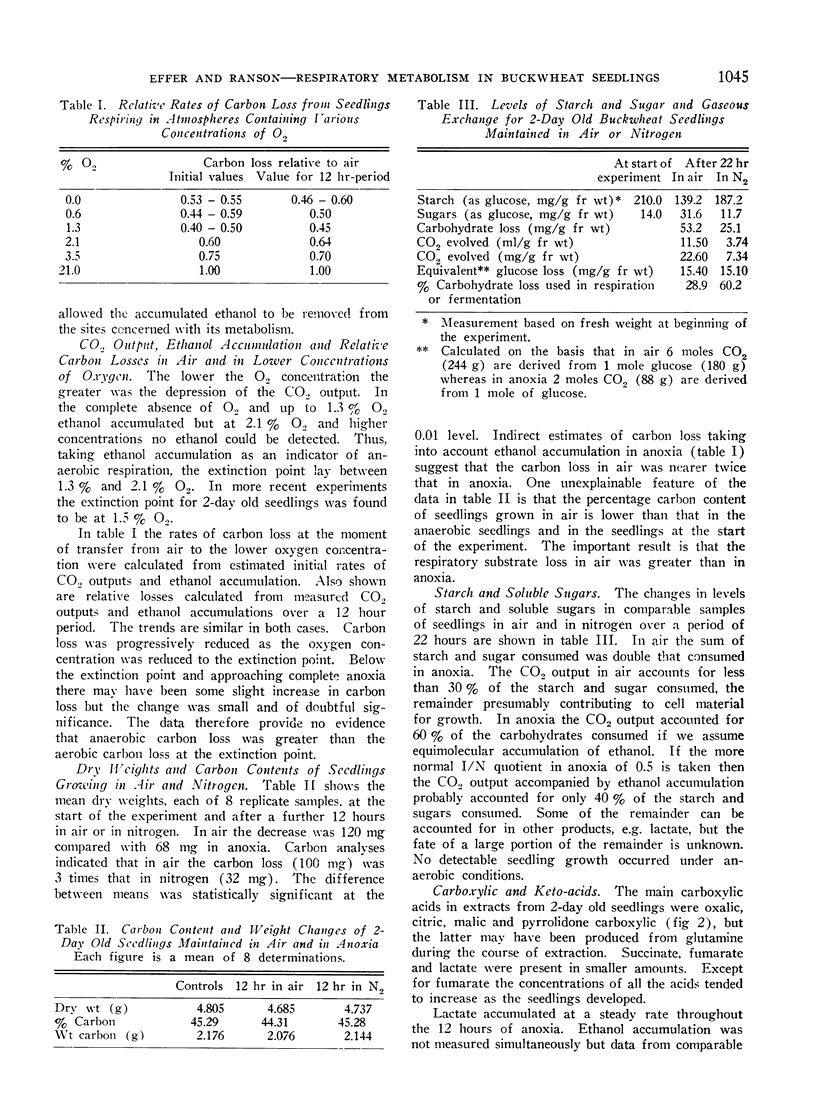
Relative rates of carbon loss were measured in air and in atmospheres containing 3.5%, 2.1%, 1.3% and 0.6% oxygen. The extinction point of anaerobic metabolism was 1.5%.
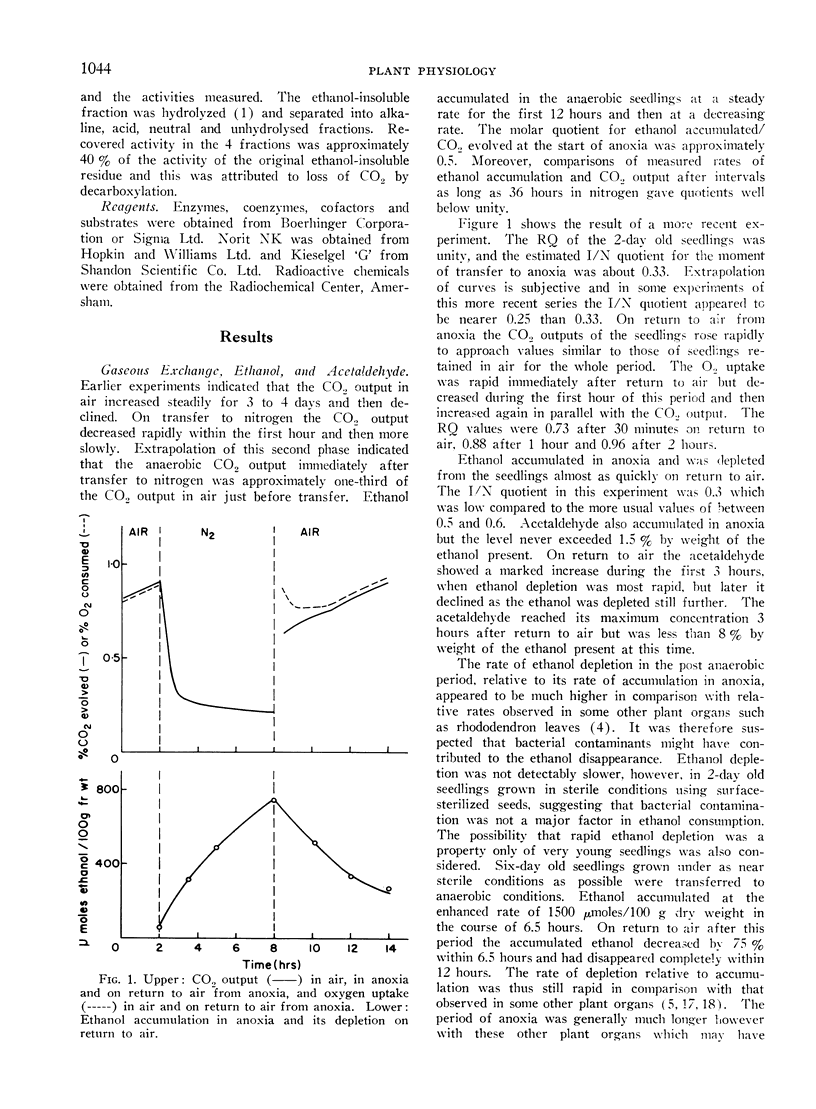
On return to air from anoxia the CO2 output increased and the RQ rose from 0.8 to 1.0 over the first 2-hour period. Ethanol, lactate and succinate were consumed and other constituents returned to their previous aerobic level. Some of these changes suggest a rather slow resumption of tricarboxylic acid cycle activity on return to air.
Carbon loss as CO2 in air was greater than the carbon loss as CO2 at the extinction point. Carbon loss in anoxia as CO2, ethanol and lactate was similar to carbon loss at the extinction point. Assessed in this orthodox manner buckwheat seedlings show no Pasteur effect but the complex nature of the changes in levels of metabolic substrates and intermediates do not allow firm conclusions to be drawn on the effects of oxygen on the rates of glycolysis and other respiratory processes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AISENBERG A. C. Sugar phosphate levels in the mitochondrial Pasteur effect. J Biol Chem. 1959 Mar;234(3):441–444. [PubMed] [Google Scholar]
- Bourne D. T., Ranson S. L. Respiratory Metabolism in Detached Rhododendron Leaves. Plant Physiol. 1965 Nov;40(6):1178–1190. doi: 10.1104/pp.40.6.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins E. A., Beevers H. Ethanol Metabolism in Plant Tissues. Plant Physiol. 1963 Jul;38(4):375–380. doi: 10.1104/pp.38.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effer W. R., Ranson S. L. Some effects of oxygen concentration on levels of respiratory intermediates in buckwheat seedlings. Plant Physiol. 1967 Aug;42(8):1053–1058. doi: 10.1104/pp.42.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAWAZ E. N., FAWAZ G. Inhibition of glycolysis in rat skeletal muscle by malonate. Biochem J. 1962 Jun;83:438–445. doi: 10.1042/bj0830438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees T. A., Beevers H. Pathways of Glucose Dissimilation in Carrot Slices. Plant Physiol. 1960 Nov;35(6):830–838. doi: 10.1104/pp.35.6.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINEX F. M., PLAZIN J., CLAREUS D., BERNSTEIN W., VAN SLYKE D. D., CHASE R. Determination of total carbon and its radioactivity. II. Reduction of required voltage and other modifications. J Biol Chem. 1955 Apr;213(2):673–680. [PubMed] [Google Scholar]
- VAN SLYKE D. D., PLAZIN J., WEISIGER J. R. Reagents for the Van Slyke-Folch wet carbon combustion. J Biol Chem. 1951 Jul;191(1):299–304. [PubMed] [Google Scholar]


