Summary
Atrial fibrillation (AF) is the most common supraventricular arrhythmia affecting up to 1% of the general population. Its prevalence dramatically increases with age and could reach up to ∼10% in the elderly. The management of AF is a complex issue that is object of extensive ongoing basic and clinical research, it depends on its genetic and epigenetic causes, and it varies considerably geographically and also according to the ethnicity. Mechanistically, over the last decade, Genome Wide Association Studies have uncovered over 100 genetic loci associated with AF, and have shown that European ancestry is associated with elevated risk of AF. These AF-associated loci revolve around different types of disturbances, including inflammation, electrical abnormalities, and structural remodeling. Moreover, the discovery of epigenetic regulatory mechanisms, involving non-coding RNAs, DNA methylation and histone modification, has allowed unravelling what modifications reshape the processes leading to arrhythmias. Our review provides a current state of the field regarding the identification and functional characterization of AF-related genetic and epigenetic regulatory networks, including ethnic differences. We discuss clear and emerging connections between genetic regulation and pathophysiological mechanisms of AF.
Keywords: Atrial fibrillation, Epigenetics, Genetics, Mechanisms
Introduction
Atrial fibrillation (AF) is a problem of global scope and increasing dimension.1 AF is diagnosed by electrocardiographic recording. The typical finding is rapid (>300/min), irregular and often low-amplitude atrial activity, with an irregular ventricular response.
Key messages.
-
•
Atrial fibrillation (AF) is the most common supraventricular arrhythmia affecting up to 1% of the general population: its prevalence reaches up to ∼10% in the elderly.
-
•
AF insurgence depends on intertwined genetic and epigenetic causes.
-
•
The risk of AF is significantly lower in non-White races and ethnicities (including Blacks, Hispanics and Asians) than Whites.
-
•
Genome Wide Association Studies (GWAS) and Epigenome Wide Association Study (EWAS) in multiethnic studies of AF in underrepresented populations are powerful approaches to investigate the genetic basis of racial and ethnic variation in AF.
-
•
GWAS and EWAS help in generating clinically potentially usable polygenic risk scores and epigenetic clocks for AF, with most studies conducted in individual of European ancestry.
-
•
Integration of “omics” (genomics, transcriptomics, proteomics, and metabolomics) and artificial intelligence-driven network approaches are increasingly robust tools for detecting AF and for stratifying patients.
-
•
A prototype case of a molecular link between epigenetic, genetic and pathophysiological mechanisms influencing AF and its racial and ethnic variations is PITX2, a homeobox transcription factor regulating cardiac conduction, modulation of ion channels and cardiac development.
Search strategy and selection criteria.
We identified data for this Series paper by searching MEDLINE, Current Contents, PubMed, and references from relevant articles using the search terms “atrial fibrillation, genetics”, “atrial fibrillation, epigenetics”, “atrial fibrillation, artificial intelligence”, “atrial fibrillation black”, “atrial fibrillation asian”, “atrial fibrillation racial differences”, and “expression of PITX2 and atrial fibrillation”. We considered data published in English between Jan 1, 1990, and June 16th, 2023.
The risk of AF is significantly lower in non-White races and ethnicities (including Blacks, Hispanics and Asians) than Whites.2, 3, 4, 5 These differences occur despite a comparable or higher burden of risk factors (especially in Blacks) and point to a possible genetic basis.6 Indeed, there is an decreased risk of AF in first-degree relatives of non-White (Black or Hispanic) early-onset AF-patients compared to first-degree relations of Whites, pointing to genetic factors.7 In addition to genetic factors, epigenetics (factors controlling gene expression rather than gene sequence variations per se) have the capacity to influence racial/ethnic and regional occurrence and consequences of AF. Finally, emerging work points to an important role of inflammation, including inflammatory signaling in non-inflammatory cells, as playing an important role in AF occurrence.8 This paper aims to review the rapidly evolving areas of genetic and epigenetic control of AF, with a view to considering their possible implication in the regional distribution and occurrence of AF as well as future developments in research and therapeutic innovation.
Genetic basis of AF and ethnic/racial variability
AF is increasingly recognized as having a strong genetic component.9,10 Three genetic approaches have historically been applied to AF: (i) linkage analysis using families with Mendelian forms of AF, (ii) Genome Wide Association Studies (GWAS), and (iii) coding variation from genome sequence data. The oldest approach, linkage analysis, was performed in families with a clear hereditary pattern, based on the tendency for a genetic marker near a disease-causing gene to be inherited together with the causal gene. AF-associated genes that were discovered through family based or gene-based studies include the ion channel subunit KCNQ1,11 atrial natriuretic peptide ANP,12 the transcription factor TBX5,13 cytoskeletal/contractile proteins MYL414 and TTN15 in different ethnicities (Table 1). Genetic linkage studies have been very informative but the percentage of even early-onset AF (<60 years-old) cases that reveal mutations is only ∼10%, with mutations located predominantly in the TTN gene, among others.33,34 GWAS were applied to AF for the first time in 2007 in people of European and Chinese descent and identified variants in chromosome 4q25, for which the closest gene is Pitx2, associated to AF.19 Since then, GWAS studies have identified >100 AF candidate genes and risk loci, with protein-coding genes implicated in fibrosis and extracellular matrix remodeling, cardiomyogenesis, cell–cell coupling, ion-channel function or nuclear structure. In fact, most AF-associated GWAS variants are located in the noncoding genome, with a range of genes, such as Pitx2, ZFHX3, PRRX1, TBX5, NKX2–5, HAND2, MYL4, TTN and SYNPO2L that have been implicated in mediating effects.35, 36, 37, 38, 39 A study based on Finnish biobanks from 12,859 AF cases and 73,341 controls, recently identified the loss-of-function splice site variant with a substantial effect size in the SYNPO2L gene, encoding a cytoskeletal/contractile protein involved in sarcomere organization, which was associated with a significantly increased risk of AF.40 A larger GWAS, comparing a total of 60,620 cases and 970,216 controls of European ancestry from six contributing studies (including the UK Biobank and the AFGen Consortium), identified risk variants that fall near genes where more deleterious mutations have been reported to cause serious heart defects in humans (i.e., PITX2, TBX5), or near genes important for cardiac muscle function and integrity20 (Table 1). Coding variants can be inherited, but also acquired, or somatic, which historically has been a focus of cancer genomics. A potential role of somatic mutations in connexin (40 and 43) genes has been shown in the pathogenesis of some AF cases.41,42 However, other recent small scale studies deep sequencing DNA from AF patients found no evidence of somatic variants within the coding regions of LA posterior wall tissue, suggesting that atrial-specific mutations might be rare and unlikely to exert a prominent role in AF pathogenesis; or they might be technical artifacts linked to sample storage conditions.43,44
Table 1.
Genetic components of AF pathogenesis, identified by technological advance and by geography of ancestry.
| Technological advance | European descent (targets) | African descent (targets) | Asian descent (targets) | Other descents (targets) |
|---|---|---|---|---|
| Genetics | ||||
| Familial AF and linkage analysis | ANP12; TBX513; MYL414 | |||
| Genome Wide Association Studies (GWAS) | MYOZ116; PLEC17; MYL418; PITX219, 20, 21, 22, 23; TTN15,24; GATA420; MYH620,25; NKX2–520; CFL220; MYH720; PKP220; RBM2020; SGCG20; SSPN20; ZFHX326; KCCN321,27; CEP6821; KCNN221; SOX521; SH3PXD2A21; METTL11B21; CEP6821; KLHL3–WNT8A–FAM13B21; ASAH121; KCNJ521; SCN22; CDK628; EPHA328; GOSR228; UBE4B28; CASZ128; CASQ228; GJA528; NUCKS128; KIF3C28; XP0128; REEP128; KDM3A28; WIPF128; CHRNA28; SPATS2L28; LRIG128; PHLDB228; GNB428; WDR128; SLC9B128; CAMK2D28; ARHGAP1028; ARHGAP2628; NR3C128; NKX2–528; ATXN128; KDM1B28; CDKN1A28; UST28; DGKB28; CREB528; GTF2I28; COG528; KCNH228; XP0728; FBX03228; PTK228; SLC24A228; MLLT328; ZNF46228; PSMB728; REEP328; NAV228; SSPN28; PKP228; NACA28; BEST328; KRR128; PHLDA128; TBX5-AS128; TBX3 intergenic28; DNAH1028; MYH728; AKAP628; SNX628; CFL228; LRRC 7428; IRF2BPL28; USP328; TLE328; UACA28; IGF1R28; POLR2A,28 TNFSF1 228; MYOCD28; MAPT28; KCNJ2,28CASC1728; SMAD728 CASC2028; BMP2,28 PRRX123; CAV123; SYNE323; FBP1/223; HCN423; SYNPO2L23; WNT8A23; SCN10A29; TUBA828; ZFHX323 |
MYOZ116; PITX121; UBE4B21; CASZ121; CASQ221; GJA521; NUCKS121; KIF3C21; XP0121; REEP121; KDM3A21; WIPF121; CHRNA21; SPATS2L21; LRIG121; PHLDB221; GNB421; WDR121; SLC9B121; CAMK2D21; ARHGAP1021; ARHGAP2621; NR3C121; NKX2–521; ATXN121; KDM1B21; CDKN1A21; UST21; DGKB21; CREB521; GTF2I21; COG521; KCNH221; XP0721; FBX03221; PTK221; SLC24A221; MLLT321; ZNF46221; PSMB721; REEP321; NAV221; SSPN21; PKP221; NACA21; BEST321; KRR121; PHLDA121; TBX5-AS1,21 TBX3 intergenic21; DNAH1021; MYH721; AKAP621; SNX621; CFL221; LRRC 7421; IRF2BPL21; USP321; TLE321; UACA21; IGF1R21; POLR2A21; TNFSF1 221; MYOCD21; MAPT21; KCNJ221; CASC1721; SMAD721; CASC2021; BMP221; TUBA821 |
KCNQ111; CAV123,30; PITX219, 20, 21, 22, 23; PRRX123; SH3PXD2A21; UBE4B21; ZFHX323; CASZ121; CASQ221; GJA521; NUCKS121; KIF3C21; XP0121; REEP121; DM3A21; WIPF121; CHRNA21; SPATS2L21; LRIG121; PHLDB221; GNB421; WDR121; SLC9B121; CAMK2D21; ARHGAP1021; ARHGAP2621; NR3C121; NKX2–521; ATXN121; KDM1B21; CDKN1A21; UST21; DGKB21; CREB521; GTF2I21; COG521; KCNH221; XP0721; FBX03221; PTK221; SLC24A221; MLLT321; ZNF462; PSMB721 REEP321; NAV221; SSPN21; PKP221; NACA21; BEST321; KRR121; PHLDA121; TBX5-AS121; TBX3 intergenic21; DNAH1021; MYH721; AKAP621; SNX621; CFL221; LRRC 7421; IRF2BPL21; USP321 TLE321; UACA21; IGF1R21; POLR2A21; TNFSF1 221; MYOCD21; MAPT21 KCNJ221; CASC1721; SMAD721; CASC2021; BMP221; TUBA821 |
PITX231; DTNA32; UBE4B21; CASZ121; CASQ221; GJA521; NUCKS121; KIF3C21; XP0121; REEP121; KDM3A21; WIPF121; CHRNA21; SPATS2L21; LRIG121; PHLDB221; GNB421; WDR121; SLC9B121; CAMK2D21; ARHGAP1021; ARHGAP2621; NR3C121; NKX2–521; ATXN121; KDM1B21; CDKN1A21; UST21; DGKB21; CREB5; GTF2I21; COG521; KCNH221; XP0721; FBX032;21 PTK221; SLC24A2,21 MLLT321; ZNF46221; PSMB721; REEP321; NAV221; SSPN21 PKP221; NACA21; BEST321; KRR121; PHLDA121; TBX5-AS121; TBX3 intergenic21; DNAH1021; MYH721; AKAP621; SNX621; CFL221; LRRC 7421; IRF2BPL21; USP321; TLE321; UACA21; IGF1R21; POLR2A21; TNFSF1 221; MYOCD21; MAPT21; KCNJ221; CASC1721; SMAD721; CASC2021,21; TUBA821 |
GWAS in multiethnic studies of AF genetics in underrepresented populations represent an invaluable approach to investigate the genetic basis of racial and ethnic variation in AF. Genome-wide admixture analysis of CHS (n = 4173), the ARIC (n = 12,341) study, and the Health ABC (n = 1015) showed that the protective allele of the AF SNP rs10824026 was more common among Black than White patients.16 This SNP is located 5 kb upstream of the SYNPO2L gene.16 Investigation of both common and rare variants in a large collection of individuals in the AFGen Consortium, by meta-analyses of 33 GWAS studies, including 22,346 individuals with AF and 132,086 referents, identified 12 new AF loci.21 Among these, only variants in PITX2 genomic region significantly associated with AF in European, African and Japanese ancestries21 (Table 1). The largest multi-ethnic meta-analysis of GWAS for AF to date has been performed by Roselli et al., and consisted of >500,000 individuals, including 65,446 with AF from the UK Biobank, AFGen Consortium, Broad AF Study and Biobank Japan.28 The study identified 97 loci significantly associated with AF, including 67 that were novel, in a combined-ancestry analysis, and 3 that were novel in a European-specific analysis.28 The latter 3 loci were located close to or within the genes CDK6 involved in cell cycle control, EPHA3 (a receptor involved in tyrosine kinase signaling), and GOSR2 (involved in intracellular vesicular transport) (Table 1). Importantly, single nucleotide variations identified by GWAS helped in generating clinically usable polygenic risk scores (PRS) for AF.45, 46, 47 In more detail, PRS is a single value estimate of an individual's genetic liability to a disease. It is calculated by the sum of an individual's risk alleles, weighted by risk allele effect sizes derived from GWAS data. Several private companies nowadays use PRS to offer a comprehensive and integrated approach to enhance care management for CVD, including AF, as companion diagnostics. Genetic mutations revealed by genetic linkage studies, GWAS, and coding variation studies may also help to elucidate the sex bias in AF towards men48 and the increased prevalence/risk of AF due to habitual alcohol consumption in East Asians compared to Europeans.49,50 In addition, they have shown a substantial overlap between genetic variants implicated in AF and atrial cardiomyopathy.37 In this respect, pathway and functional transcriptomic enrichment analyses suggested that putative AF genes act via cardiac structural remodeling, as observed in atrial cardiomyopathy.20,51 It emerges from the studies discussed in this section that the majority of genetic analyses for AF have been performed in individuals of European descent. European ancestry per se is an independent risk factor for incident AF,52 and the heritability of AF based on common genetic variants in individuals of European ancestry has been estimated to be ∼22%.53 However, the tendency to focus on European ancestry has led to a non-representative distribution of ancestries in genetic studies compared with the real-world diversity, which may negatively impact the applications of scores to other ethnicities.
Epigenetic basis of AF
Epigenetics includes three main regulatory systems that determine chromatin remodelling and gene transcription regulation: DNA methylation, regulation of transcription and translation by non-coding RNAs, and chromatin remodelling (histone modifications). The functional crosstalk among these epigenetic processes contributes strongly to determining cell phenotype in AF (Fig. 1). All epigenetic processes have been implicated in AF pathogenesis (Table 2). During DNA methylation, a methyl group is catalyzed by DNA methyltransferases (DNMTs) to shift from the S-adenosyl- l-methionine to the 5′ carbon of cytosine, which are mostly located in cytosine-phosphate-guanine (CpG) islands. Gene-promoter hypermethylation causes transcriptional silencing, whereas hypomethylation leads to increased expression of the gene. AF might be associated with global DNA hypermethylation.60 The concept of Epigenome Wide Associated Studies (EWAS) was first introduced in 2011,71 and led to spurring of many correlative studies. In 2016, a report on whole genome methylation in the Offspring Cohort of the Framingham Heart Study, including 183 participants of European descent with prevalent AF and 220 with incident AF with up to 9 year follow up revealed that a CpG site locus in CUX2, a transcription factor, was the most significant SNP associated with AF.54 The promoter of the PITX2 gene, which consistently emerges from most AF-related GWAS, was found hypermethylated in the regions encompassing LA or pulmonary veins-LA junctions of affected individuals of European descent in a small sample size study.55 The promoter of KIF15, a cytoskeletal protein is hypermethylated in a small size Chinese AF cohort, and predictive tools computed a concomitant regulation of PSMC3, TINAG, and NUDT6 genes.61 New-onset AF is a known postoperative complication after cardiac surgery: Fischer et al. identified two loci associated with AF in this setting, namely EYS and LINC01683,56 and developed a DNA methylation biomarker-based prediction model for the future occurrence of postoperative AF associated with cardiac surgery in a European cohort.72 The latter prospective study was conducted on a medium size cohort (n = ∼200), with ROC curves displaying AUC values in the range of 0.7–0.8, thus demonstrating the potential of precision medicine to develop models combining epigenomic and clinical data to predict AF. Epigenetic clocks feed fine-tuned algorithms to calculate biological age based on a read-out of the extent to which dozens of CpG sites across an individual's DNA are methylated.73 A multi-ethnic study involving 5600 participants with 905 incident AF cases during a mean follow-up of 12.9 years revealed that some epigenetic clocks were associated with a statistically significant higher incidence of AF after adjusting for chronological age, race, sex, and smoking variables, demonstrating that biological (epigenetically measured) aging might play a role in AF development independent of chronological age.73,74 However, a potential causal relationship between epigenetic clocks (or DNA methylome), aging and AF remains unexplored. A recurrent feature of DNA methylation studies is that profiles were measured from blood, which could vary from the ones found in atria or specific tissues/cell types. However, invasive specimen collection is often not feasible in human studies, and blood is frequently used and accepted as a proxy tissue to assess DNA methylation.
Fig. 1.
Major epigenetic mechanisms regulating gene expression and cell phenotypes in AF.
Table 2.
Epigenetic components of AF pathogenesis, identified by technological advance and by geography of ancestry.
| Technological advance | European descent (targets) | African descent (targets) | Asian descent (targets) | Other descents (targets) |
|---|---|---|---|---|
| Epigenetics | ||||
| Epigenome Wide Association Study (EWAS) | CUX254; PITX255; EYS,56 LINC01683.56 | |||
| Chromatin Immunoprecipitation sequencing (ChIP-Seq) | αSMA57; PITX258 | MYOZ116; PITX121; UBE4B21; CASZ121; CASQ221; GJA521; NUCKS121; KIF3C21; XP0121; REEP121; KDM3A21; WIPF121; CHRNA21; SPATS2L21; LRIG121; PHLDB221; GNB421; WDR121; SLC9B121; CAMK2D21; ARHGAP1021; ARHGAP2621; NR3C121; NKX2–521; ATXN121; KDM1B21; CDKN1A21; UST21; DGKB21; CREB521; GTF2I21; COG521; KCNH221; XP0721; FBX03221; PTK221; SLC24A221; MLLT321; ZNF46221; PSMB721; REEP321; NAV221; SSPN21; PKP221; NACA21; BEST321; KRR121; PHLDA121; TBX5-AS1,21 TBX3 intergenic21; DNAH1021; MYH721; AKAP621; SNX621; CFL221; LRRC 7421; IRF2BPL21; USP321; TLE321; UACA21; IGF1R21; POLR2A21; TNFSF1 221; MYOCD21; MAPT21; KCNJ221; CASC1721; SMAD721; CASC2021; BMP221; TUBA821 |
KCNQ111; CAV123,30; PITX219, 20, 21, 22, 23; PRRX123; SH3PXD2A21; UBE4B21; ZFHX323; CASZ121; CASQ221; GJA521; NUCKS1 (21; KIF3C21; XP0121; REEP121; DM3A21; WIPF121; CHRNA21; SPATS2L21; LRIG121; PHLDB221; GNB421; WDR121; SLC9B121; CAMK2D21; ARHGAP1021; ARHGAP2621; NR3C121; NKX2–521; ATXN121; KDM1B21; CDKN1A21; UST21; DGKB21; CREB521; GTF2I21; COG521; KCNH221; XP0721; FBX03221; PTK221; SLC24A221; MLLT321; ZNF462; PSMB721 REEP321; NAV221; SSPN21; PKP221; NACA21; BEST3 (21; KRR121; PHLDA121; TBX5-AS121; TBX3 intergenic21; DNAH1021; MYH721; AKAP621; SNX621; CFL221; LRRC 7421; IRF2BPL21; USP321 TLE3 2221; UACA21; IGF1R21; POLR2A21; TNFSF1 221; MYOCD21; MAPT21 KCNJ221; CASC1721; SMAD721; CASC2021; BMP221; TUBA821 |
PITX231; DTNA32; UBE4B21; CASZ121; CASQ221; GJA521; NUCKS121; KIF3C21; XP0121; REEP121; KDM3A21; WIPF121; CHRNA21; SPATS2L21; LRIG121; PHLDB221; GNB421; WDR121; SLC9B121; CAMK2D21; ARHGAP1021; ARHGAP2621; NR3C121; NKX2–521; ATXN121; KDM1B21; CDKN1A21; UST21; DGKB21; CREB5; GTF2I21; COG521; KCNH221; XP0721; FBX032;21 PTK221; SLC24A2,21 MLLT321; ZNF46221; PSMB721; REEP321; NAV221; SSPN21 PKP221; NACA21; BEST321; KRR121; PHLDA121; TBX5-AS121; TBX3 intergenic21; DNAH1021; MYH721; AKAP621; SNX621; CFL221; LRRC 7421; IRF2BPL21; USP321; TLE321; UACA21; IGF1R21; POLR2A21; TNFSF1 221; MYOCD21; MAPT21; KCNJ221; CASC1721; SMAD721; CASC2021,21; TUBA821 |
| Integrated omics approaches | ||||
| ChIP-Seq, transcriptomics | NKX2-559; TBX359; ZFHX359; SYNPO2L59; MYH759 | NPRA60; KIF1561 | ||
| ChIP-Seq, mass spectrometry | ATP5L62; CES1D62; MYL762; NDUFA1262; NDUFA862; NDUFS762; PDHA162; TNNI362; UQCR1062 | |||
| GWAS, CHiP-Seq, ATAC-Seq, transcriptomics | NKX2.563 | |||
| GWAS, ChIP-Seq, transcriptomics, mass spectrometry | NKX2.5, TNNT2, CYB5R3, NDUFB3, HIBADH NDUFA9, DLAT, PPIF, MYL4, CKM, NKX2-5, MYL764; PGAM264; TNNC164; CYC164; ETFB64; PRDX564; AK164; ALDOA64; TCAP64; TOM1L264 | UBADC1,65 Quinic acid,65Diosmetin,65 IGK,65 alpha-Ergocryptine,65 Fludrocortisone acetate,65 2,6-Di-tert-butylbenzoquinone,65 NBL1,65 NPC2,65 SNCA65 | ||
| GWAS, EWAS, transcriptomics | ADORA166; ATP1A366; ATP1B266; CACNA1D66; KCNQ466; NR3C266; THRA66 | |||
| ATAC-Seq, transcriptomics | BMP1035; SMYD235; PITX235; MYOT, TBX535; GJA135; CAV135; HCN435; SPATS2L35; PLN35; KCNH267 | |||
| ATAC-Seq, Cut&Run, transcriptomics | TBX5-p.G125R variant68 | |||
| Network/artificial intelligence | ||||
| CNN GWAS | PITX269; SCN5A70; TTN70 | PITX269 | PITX269 |
Within the structure of the nuclear chromatin, the N-terminal histone tails of all histones and the C-terminal tails of H2A histones stem from the nucleosome core structure. These tails are preferentially accessible and target sites for many post-translational modifications (PTMs).75 PTMs such as phosphorylation, acetylation, and methylation can strongly modify chromatin conformation and affect gene expression. Each PTM type is regulated by ad hoc enzymes. For instance, histone deacetylase (HDACs)-related epigenetic mechanisms have been implicated in AF regulation, by ventricular electrical remodeling (reviewed in76,77). Few examples related to enzymes regulating specific PTM in the context of AF are illustrated below.
Both rare and common variants in the gene encoding KCNN3, a calcium-activated K+ channel, have been associated with AF.21,27 KCNN3 expression is reducedin AFvia HDAC2-dependent histone PTM in atrial cardiomyocytes.78, 79, 80 The expression of EZH2, encoding the histone methyltransferase responsible for methylation of histone H3 lysine 27, a hallmark of epigenetic gene silencing is upregulated in permanent AF patients with atrial fibrosis, and its repression/activation correlated with anti-fibrotic/pro-fibrotic mechanisms, respectively.57 In this respect, chromatin immunoprecipitation (ChIP) is a powerful tool to analyze protein-DNA interactions in vivo. ChIP-seq, which combines ChIP with next generation sequencing (NGS) technology, can efficiently detect genome-wide DNA segments that interact with histones or other epigenetic players. A ChIP assay showed increased specific binding of EZH2 to the α-SMA promoter, providing evidence that EZH2 causes atrial fibroblast activation through this binding.57 However, it remains unexplained how deposition of H3K27me3 would reconcile with increased with pro-fibrogenic α-SMA gene expression in atrial fibroblasts. Decreased expression of targets of the CREB/CREM/ATF transcription factor family was previously linked to AF susceptibility in humans, and the CREM repressor isoform CREM-IbΔC-X is upregulated in human AF.62 Heart-specific transgenic CREM-IbΔC-X mice represent a model of spontaneous onset AF. Prolonged treatment with valproic acid (VPA, an anticonvulsant drug and a potent HDAC inhibitor) treatment increased H4-acetylation (a readout of HDAC activity) in atria of CREM-IbΔC-X mice.62 A ChIP-PCR assay directed against the CREM and its targets in CREM-IbΔC-X versus wild type mice, revealed a counter-regulatory effect of VPA in this AF mouse model, supporting the VPA-dependent functional delay of AF onset.62
This approach identified nine VPA-upregulated genes/proteins (Atp5l, Ces1d, Myl7, Ndufa12, Ndufa8, Ndufs7, Pdha1, Tnni3, Uqcr10), which were downregulated in the atria of a transgenic mouse model of AF (CREM-IbΔC-X)62 (Table 2). ChIP-Seq analysis revealed that Pitx2 directly binds to conserved chromatin upstream of two specific microRNAs (miRs): miR-17-92 and miR-106b-2558 (see also section “Genetic regulators operating via pathophysiological mechanisms that remain to be clarified- the prototype case of PITX2”). In turn miRs are a class of non-coding RNAs [ncRNAs, which also include long ncRNAs (lncRNAs) and circular RNAs (circRNAs)], epigenetic modulators of gene expression recognized as major regulatory gatekeepers of protein-coding genes in the human genome. The role of post-transcriptional ncRNA regulators in specific events related with AF has started to be unveiled in the last years and has been extensively reviewed.76,77,81,82 There is evidence that miRs are involved in both electrical and structural remodeling in AF. MiR-26 is an example of the former: its increased expression in AF resulted in augmented expression of KCNJ2 encoding a potassium channel, causing an enhanced vulnerability to AF in mice.83 Conversely, miR-21 is an example for the involvement of miRs in structural remodeling. Upregulation of miR-21 in AF indirectly promotes fibrosis via downregulation of SPRY1, a negative regulator of profibrotic cellular signaling pathways.84,85 MiRs therapeutics for AF appear promising but they are still in their infancy. One of the primary concerns is miRs' ability to target multiple pathways in multiple tissues. Further research must be carried out to confirm their safety and therapeutic potential for AF. Overall, a better understanding of the mechanisms of epigenetic gene regulation may provide improved tools to define the links between genetic variation and atrial function in AF across different ethnicities.
Integrated and artificial intelligence-driven analysis in AF
Technologies such as NGS and mass spectrometry will help enormously in understanding molecular pathophysiology, and relating the results obtained in animal models to those obtained in man, to ultimately translate basic information into for clinical application. “Integrated omics” combine multiple omics datasets from sources like genomics, transcriptomics, proteomics, and metabolomics. By integrating three types of omics data (i.e., GWAS, EWAS and transcriptome-wide association study), many more relevant AF-related genes were identified than using GWAS alone from the AFGen consortium.66,86 Most integrated omics studies have focused on individuals of European ancestry. Benaglio et al. analyzed transcriptomic and epigenomic data from induced pluripotent stem cell-derived cardiomyocytes from seven related individuals and identified ∼2000 single-nucleotide variants associated with NKX2-5 binding—as assessed by ChIP-Seq, showing that differential binding at numerous regulatory variants across the genome might contribute to ECG phenotypes in AF.63 The same study experimentally and functionally validated two NKX2-5 binding sites in these variants (rs3807989, within the locus of CAV1; and rs590041, within the locus of SSBP3), showing that they underlie ECG GWAS signals.63
Van Ouwerkerk et al. unraveled the genetic predisposition for AF by probing the non-coding regions of the genome. Using available and newly generated data, they prioritized candidate genes that may be affected by 104 SNP previously associated to AF,28 by cross-referencing transcriptomic, epigenomic, and chromatin conformation datasets, and studied the impact of SNP-containing regulatory elements (RE) on putative target gene expression.35 RE activity is limited to topologically associating domain, which in turn is a tissue independent feature. The authors assessed which gene promoters are contacted by the putative RE by integrating available chromatin conformation data (measured with Hi-C, a method to study the three-dimensional architecture of genomes) and the levels of genes expressed in the human left atrial posterior wall that play a role in cardiac conduction, force of contraction, and heart development.35 Finally, they also identified new potential cardiac regulatory elements by Assay for Transposase Accessible Chromatin with high-throughput sequencing (ATAC-seq), a method for genome-wide mapping of chromatin accessibility at the regions associated with AF, generating a dataset for transcription factor motif enrichment, and deployed an enhancer prediction tool (EMERGE). This integrative study ultimately identified BMP10, SMYD2, PITX2, MYOT, TBX5, GJA1, CAV1, HCN4, SPATS2L, and PLN genes, among others, to be likely affected by AF associated variants and proposed a role for new regulatory region variants in modulating the expression of the potential AF genes.35 This sophisticated approach is able to prioritize potentially AF-related functional variants and, interestingly, it was validated for some AF-associated regions (i.e., GJA1) in the mouse using genome editing. Nevertheless, the involvement of such variant REs in AF-relevant gene regulation and pathophysiology was based on a number of assumptions and prompts further studies. Weber Hall et al. recently created a model of left atrium (LA) chromatin states (profiling 7 histone post-translational modifications by ChIP-Seq: H3K4me3, H3K4me2, H3K4me1, H3K27ac, H3K36me3, H3K27me3, H3K9me3), integrated with transcriptomics (RNA-Seq), DNA methylation, long range chromatin interaction data from primary human left atrium and ventricle derived from Hi-C, GWAS data and electrocardiographic traits in AF patients.59 In more detail, the authors first profiled histone modifications in 5 healthy human LA tissues and produced a model of potential chromatin states. This model was then integrated with gene expression profiles, and DNA methylation to validate the functionality of chromatin states. They then identified active enhancers (RE) defined by H3K27ac and H3K4me1 colocalization and by bioinformatics tools. They identified gene-enhancer connections using HiC performed in LA, analyzed these genes for enriched pathways, and prioritized CVD effector genes found at GWAS. Adopting this workflow, they have defined a robust gene regulatory network underlying AF susceptibility, composed of known players such as NKX2-5, TBX3, ZFHX3, SYNPO2L and MYH7.59 Although not leading to the identification of new AF-targets, the latter integrated genetic/epigenetic/transcriptomic pipeline could be applied to large AF cohorts and to study inter-ethnic/racial differences. Technological advances also led to combine single cell ATAC-Seq with RNA-Seq, allowing in parallel chromatin accessibility and transcriptome, in adult human hearts. This approach led to the identification and characterization of AF variants associated to the locus of KCNH2, a potassium channel67; and of the pathogenic missense variant p. G125R in TBX5 causing Holt–Oram syndrome and early onset of atrial fibrillation.68 A landmark study by Assum et al. performed a multi-omics analysis integrating genomics, transcriptomics and proteomics in human atrial tissue in a AF case control cohort (n = 118).64 In genetics, cis and trans regulatory elements differ from their ability to regulate nearby and distant genes, respectively. Using published data or data newly obtained with Affymetrix GeneChip Arrays, Assum et al. first integrated cis-expression quantitative trait loci (cis-QTL) and cis-protein quantitative trait loci (pQTL) analysis to allow the distinction between functional regulatory mechanisms with consequences for mRNA and protein levels. Second, they extended the cis-QTL analysis by combining it with a PRS for AF, a concept previously applied for blood-derived gene expression and termed eQTS.87 Integration of partially divergent eQTS and pQTS, trans-associations with AF GWAS SNPs and gene ontology analysis led to identify core gene pathways for AF. Third, they focused on the key AF gene NKX2-5, largely based on the above-mentioned functional study of Benaglio et al.,63 and they observed a strong correlation between the AF disease variant SNP rs9481842 and the NKX2-5 transcript. Finally, the authors showed that in addition to cis-regulatory elements, trans-acting mechanisms are important for the NKX2-5-mediated link between the genetic variant rs9481842 and AF. Through integration of NKX2-5 ChIP-Seq, HiC and the genetic variant rs9481842 data, NKX2-5 specific targets were identified and proposed as AF priority putative core genes, for further experimental research: TNNT2, CYB5R3, NDUFB3, HIBADH NDUFA9, DLAT, PPIF, MYL4, CKM, MYL7, PGAM2, TNNC1, CYC1, ETFB, PRDX5, AK1, ALDOA, TCAP, TOM1L2.64
Artificial intelligence (AI), and in particular machine learning (ML, the application of AI into a system or machine, which helps it to self-learn and improve continually), research has become increasingly common and popular for AF diagnosis and patient stratification.64,88 Neural networks are a branch of ML models that are built using principles of neuronal organization constituting animal brains. In this context, neural network (CNN) analysis of large-scale genetic data such as GWAS and EWAS is expected to be faster, more efficient and accurate than other types of neural networks, such as artificial neural network (ANN) and recurrent neural network (RNN). Application of a CNN analysis to a multi-ethnic AF network GWAS for early-onset AF, including 6358 subjects from four independent cohorts (Korean, Japanese, European, multi-ethnic), led to moderate-to-high predictive power and confirmed the known target PITX2 by assigning it a high saliency score among the AF associated SNPs.69 Libiseller-Egger et al. used a previously published CNN89 to predict the cardiovascular age of 36,349 participants of the UK biobank from their ECGs, and performed a GWAS on the difference between predicted and chronological age (delta age). The analysis identified eight loci associated with delta age including genes linked to AF, such as SCN5A and TTN, indicating a potentially increased disease susceptibility.70 AI-driven network approaches are increasingly robust and useful tools for detecting polygenic diseases such as AF, by capturing the cumulative effects and interactions between omics.
Connecting genetic regulation to pathophysiological mechanisms
Genetic regulators operating via clear pathophysiological mechanisms
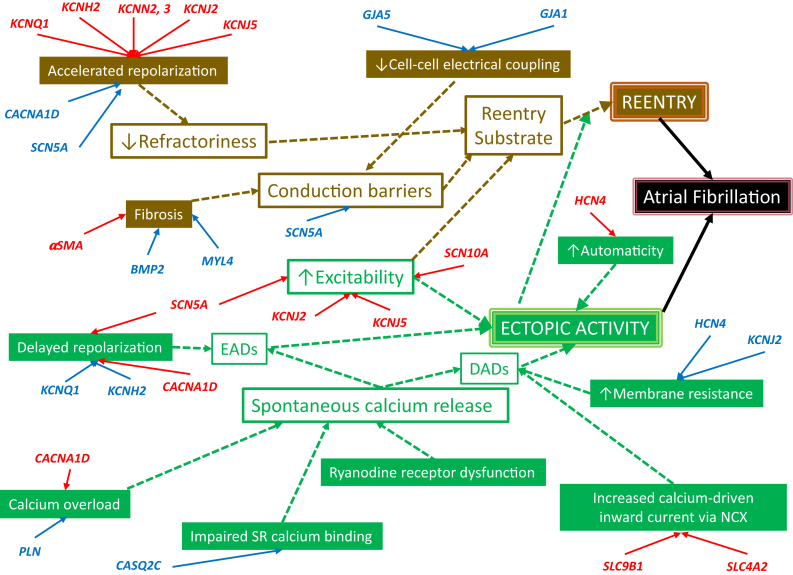
Many of the identified genetic and epigenetic factors controlling AF risk operate through well-identified pathophysiological mechanisms (Fig. 2). The details of the mechanisms shown in the figure go beyond the scope of the present manuscript-the interested reader is referred to some detailed reviews.90,91 In brief, 2 main arrhythmogenic mechanisms lead to AF: atrial reentry and ectopic atrial activity. These principal mechanisms and their underlying promoters are color-coded brown and green, respectively, in Fig. 2.
Fig. 2.
Pathophysiological mechanisms linking functional changes caused by specific AF-associated gene-variants to AF. Gain-of-function is indicated by red, Loss-of-function by blue. Green boxes are associated with AF-promotion by enhanced spontaneous ectopic activity, brown boxes are associated with AF-promotion by forming a vulnerable substrate for reentry. Open boxes represent final common proximal changes in key electrical properties, whereas filled boxes are underlying changes that lead to final common proximal changes. The upward and downward arrows indicate the direction of change in a specific property; the connecting arrows indicate a causal relationship.
Reentry occurs when ectopic activity encounters a substrate that can support reentry and continues if the reentrant impulse continues to propagate without being stopped by an inexcitable barrier. Several proximal drivers create a favorable substrate for reentry: 1) Abbreviated refractoriness, generally due to accelerated repolarization, makes it easier for the reentering impulse to encounter excitable tissue throughout the circuit; 2) The presence of conduction barriers favors the initiation of reentry by promoting unidirectional block and makes it easier for reentry to be maintained by stabilizing reentry around a barrier; 3) Increased excitability stabilizes reentry by increasing the energy of the propagating reentrant wave. Conduction barriers are established by structural obstacles like tissue fibrosis, by impaired cell-to-cell coupling and by reduced strength of the Na+ current (INa) responsible for impulse propagation. Increased excitability occurs when INa is increased (leading to the paradoxical reality that both gain or loss of INa function can promote reentry, via different mechanisms) or when the resting membrane potential is made more negative by enhanced background potassium current (inward rectifier IK1 or acetylcholine-dependent current IKACh).
Ectopic activity leads to AF primarily by acting as a trigger to initiate reentry, but sustained repeated rapid activity can also maintain AF even in the absence of reentry. Ectopic activity typically results from spontaneous after depolarisations, either early after depolarisations (EADs), occurring before, or delayed after depolarisations (DADs), occurring after full repolarization. DADs are likely the most common precipitant of AF-related ectopic activity. DADs typically result from spontaneous diastolic Ca2+-release events from the sarcoplasmic reticulum (SR), with the released Ca2+ being exchanged for extracellular Na+ via the Na+/Ca2+-exchanger (NCX), causing during diastole a depolarizing inward current carried by Na+. SR Ca2+ release is controlled by the cardiac ryanodine receptor (RyR2) gating mechanism, which is Ca2+ sensitive. Increased cell Ca2+ can trigger abnormal RyR2 Ca2+ releases, while impaired SR Ca2+ binding or abnormalities of RyR2 (e.g., phosphorylation) can make it hypersensitive to Ca2+. Other changes that can enhance the risk of ectopic activity are increases in NCX function, which enhance the inward current generated by any given quantity of released Ca2+. Furthermore, increased membrane resistance increases the degree of depolarization for any given depolarizing current carried by NCX.
The genes identified to be associated with AF risk (Tables 1 and 3) that have known or strongly suspected pathophysiological mechanisms are indicated in red (for consequences of gain of function) or blue (for loss of function) in Fig. 2. Gain-of-function mutations in several K+-channel genes accelerates repolarization, abbreviating refractoriness and leading to a reentrant substrate, as does loss of Ca+- or Na+ channel function variants. The opposite effects lead to delayed repolarization and risk of EAD-related ectopic activity. Gain-of-function in background K+-channels enhance excitability by hyperpolarizing the membrane and removing Na+-channel inactivation, as does gain-of-function in Na+-channels. Conduction barriers are produced by impaired intercellular coupling due to loss-of-function variants in connexin genes GJA5 and GJA1, as well as through tissue fibrosis caused by mutations in fibroblast genes (like αSMA gain of function or BMP2 loss of function) or atrial-selective contractile genes (loss of MYL4 function110). Spontaneous SR Ca2+-release is favoured by Ca2+-loading due to gain of Ca2+-channel function or loss-of-function of phospholamban (PLN), which inhibits SR Ca2+-uptake via the SR Ca2+-ATPase. Impaired SR Ca2+-binding by dysfunctional or downregulated calsequestrin (CASQ) increases free SR Ca2+-activity, functionally mimicking Ca2+-loading. Gain-of-function mutations in NCX (encoded by SLC genes) increase the current for any given amount of Ca2+ released and loss-of-function channel mutations that reduce diastolic current flow and increase membrane resistance, increase the degree of voltage change caused by a given depolarizing NCX current, causing larger DADs for any given amount of Ca2+-release induced current.
Table 3.
Key studies determining atrial PITX2 expression in patients and in experimental models and systems (upper part); key studies linking 4q25 rs13143308T allele to atrial cardiomyocyte function of AF patients (lower part).
| Study first author surname and year | Pubmed ID | Details and quality of evidence |
Summary of key findings |
||||
|---|---|---|---|---|---|---|---|
| Species | Model system | Key inclusion & exclusion criteria | Relevant outcome(s) to atrial remodeling | Key findings and important biases | Conclusion(s) | ||
| Key studies determining atrial PITX2 expression in patients and in experimental models and systems | |||||||
| Kahr et al., 2011 | 92 | Human | Right and left atrial appendages (RAA, LAA) | Patients in sinus rhythm undergoing heart surgery for CABG or valve replacement | Protein levels of PITX2 two-fold higher in LA vs RA | Systematic differences between LA and RA gene expression exist and support a potential role of PITX2 in shaping LA | |
| Donate Puertas et al., 2017 | 55 | Human | LAA and pulmonary veins-LA junction | Patients with or without AF undergoing heart surgery for valve replacement | Increased LA surface in AF is inversely correlated with PITX2 mRNA levels |
|
AF is associated with epigenetic LA changes including PITX2 promoter hypermethylation |
| Kao et al., 2013 | 93 | Rat Mouse |
Rat LA Mouse atrial HL-1 cells |
Rats with isoprenaline-induced heart failure (HF) vs control animals | HF and Ang-II decrease atrial PITX2c protein levels |
|
Heart failure and Ang-II promote atrial PITX2c promoter hypermethylation |
| Scridon et al., 2015 | 94 | Rat | Rat LA | Young (14 weeks-old), adult (24 weeks-old) and aged (48 weeks-old) spontaneously hypertensive rats (SHRs) | Hypertension decreases atrial PITX2c mRNA levels |
|
In SHRs PITX2 down-regulation is an age-dependent process that starts before the occurrence of atrial arrhythmias |
| Torrado et al., 2015 | 95 | Pig Mouse |
Pig LA Mouse atrial HL-1 cells |
Pigs with AF induced by rapid atrial pacing | AF itself decreases LA PITX2 (and TBX5) protein levels |
|
Rapid atrial pacing mimicking AF decreases atrial PITX2c and TBX5 protein levels by upregulating miR-21 |
| Key studies assessing the consequences of genetic manipulation of PITX2 expression levels for atrial function and AF susceptibility | |||||||
| Wang et al., 2010 | 96 | Mouse | Whole hearts and LA | Mice with global PITX2 deficiency (PITX2null ± mice) | PITX2 deficiency creates an atrial arrhythmogenic substrate |
|
PITX2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification |
| Chinchilla et al., 2011 | 97 | Human Mouse |
Human RAA and LAA Mouse RA and LA Mouse atrial HL-1 cells |
Patients with or without AF undergoing heart surgery for valve replacement Conditional atrial-specific PITX2 deficient mice (NppaCre + PITX2−/−) |
Atrial-specific PITX2 deficiency causes atrial electrical and structural changes |
|
PITX2 could be an upstream transcriptional regulator of atrial function left-sided pacemaker specification |
| Kirchhof et al., 2011 | 98 | Human Mouse |
Human RAA and LAA Mouse RA and LA |
Patients in sinus rhythm with or without AF undergoing heart surgery for CABG or valve replacement PITX2c deficient mice (PITX2c+/−) |
PITX2c deficiency causes atrial electrical disturbances (action potential shortening) but no structural changes |
|
Reduction of atrial PITX2c expression promotes AF inducibility by causing action potential shortening and is associated with complex changes in gene expression in the atria |
| Tao et al., 2014 | 99 | Mouse | Whole atria | Conditional PITX2 deficient mice (PITX2 CKO) | Conditional PITX2 deficiency causes abnormal cardiac conduction, sinoatrial node dysfunction, and alterations in cardiomyocyte ultrastructure |
|
PITX2 directly regulates ion transport, calcium handling and intercalated dics genes |
| Wang et al., 2014 | 58 | Mouse | Whole atria | Different genetically modified mouse lines | PITX2 deficiency upregulates miR-17-92 and miR-106b-25 and upregulation of these miRs promote pacing-induced AF in mice |
|
PITX2 suppresses expression of miR-17-92 and miR-106b-25 and inhibits predisposition to AF |
| Nadadur et al., 2016 | 100 | Mouse | Whole LA Isolated atrial myocytes |
TBX5 and PITX2 deficient and double mutant mouse lines | PITX2 deficiency causes atrial APD abbreviation, but does not induce cellular triggered activity |
|
The TBX5 deficiency associated susceptibility to inducible AF was rescued by PITX2 haploinsufficiency in mice |
| Syeda et al., 2016 | 101 | Human Mouse |
Human LAA Mouse whole hearts Mouse LA Mouse LA myocytes |
Patients undergoing bilateral thoracoscopic AF ablation PITX2c deficient mice (PITX2c+/−) |
Pitx2 deficiency causes APD shortening and a more depolarized RMP |
|
PITX2 deficiency results in more depolarized RMP, which causes greater sodium channel inactivation, thereby potentiating the antiarrhythmic effects of flecainide |
| Kao et al., 2019 | 102 | Human | Cultured human atrial fibroblasts | PITX2c knockdown in human atrial fibroblasts | PITX2c deficiency increases activity of atrial fibroblasts, promoting their transition to collagen-secreting myofibroblasts |
|
PITX2c deficiency increases human atrial fibroblast activity potentially promoting atrial fibrosis |
| Collins et al., 2019 | 103 | Zebra fish | Whole heart | PITX2c deficient zebra fish (PITX2c ± and PITX2c−/−) | PITX2c deficiency causes atrial conduction defects and alterations in cardiomyocyte metabolism and ultrastructure |
|
PITX2c deficiency in zebra fish induces sarcomere and metabolic defects that precede the development of atrial conduction disturbances and arrhythmias |
| Holmes et al., 2021 | 104 | Human Mouse |
Human IPSCs HEK293 cells Mouse LA |
HEK293 cells expressing Scn5a + Scn1b Human IPSC-derived myocytes PITX2c deficient mice (PITX2c+/−) |
PITX2c deficiency causes a more positive RMP |
|
The more depolarized RMP due to PITX2c deficiency increases the efficacy of dronedarone to prolong atrial action potential |
| Tarifa et al., 2023 | 105 | Mouse | Pooled LA and RA myocytes | Atrial-specific PITX2 deficient mice (NppaCre + PITX2+/−; NppaCre + PITX2−/−) | Atrial-specific PITX2 deficiency causes atrial electrical and Ca2+ handling alterations that may promote atrial ectopy |
|
Atrial-specific PITX2 deficiency reduces ICa,L that may cause re-entry promoting action potential shortening and induces cellular Ca2+ handling abnormalities that may cause atrial ectopy and triggered activity |
| Kim et al., 2023 | 106 | Mouse | Atrial myocytes | PITX2 deficient mice (PITX2+/−) | PITX2 deficiency causes Ca2+-dependent cellular triggered activity |
|
The higher susceptibility to AF of PITX2 ± mice is suppressed by selective RyR2 inhibition suggesting that ectopic (triggered) activity is one potential arrhythmogenic mechanism of PITX2 deficiency |
| Schulz et al., 2023 | 107 | Human | Humans IPSCs | CRISPER/Cas9-mediated PITX2 deletion in healthy human atrial-like IPSCs Atrial engineered heart tissue |
Atrial-specific PITX2 deficiency causes atrial electrical changes |
|
PITX2 knockout induces key findings of electrical (shorter triangular AP with decreased Ito and ICa,L) and contractile (reduced force of contraction) remodeling typical of persistent AF |
| Perez-Hernandez et al., 2016 | 108 | Human Mouse |
Human RAA Mouse atrial HL-1 cells |
Patients in sinus rhythm with or without AF undergoing heart surgery for CABG or valve replacement Mouse atrial HL-1 cells |
Increased PITX2c is associated with reduced ICa,L and increased IKs, both potentially abbreviating action potential duration |
|
Human chronic AF is associated with increased PITX2c expression and a reduction of ICa,L and an increase in IKs in RA cardiomyocytes that may contribute to re-entry promoting AP shortening |
| Key study linking 4q25 rs13143308T allele to atrial cardiomyocyte function of AF patients | |||||||
| Herraiz-Martinez et al., 2019 | 109 | Human | Human RAA | Patients in sinus rhythm with or without AF undergoing heart surgery for CABG or valve replacement | The risk variant rs13143308T associates with DAD-dependent triggered activity, but not with ICa,L function |
|
The 4q25 variant rs13143308T increase the risk for AF by causing ectopic (triggered) activity |
Genetic regulators operating via pathophysiological mechanisms that remain to be clarified-the prototype case of PITX2
For some of the genetic control mechanisms, their role is much less clear, despite a great deal of accumulated data. Because of space limitations, we will discuss in detail here only one genetic mechanism to illustrate the challenges in linking identified genetic mechanisms with clear pathophysiological mediators that mediate their influence on AF. The specific element to be discussed is the genetically controlled variation in the expression of PITX2, selected because it is the gene closest to the strongest AF-linked region, on chromosome 4q25 and because a large number of studies have addressed potential underlying mechanisms.
Although many SNPs on chromosome 4q25 associate with a higher risk for AF, causality is difficult to demonstrate in the case of PITX2. Because PITX2 is the closest gene to the discovered AF-associated SNPs, it is assumed that these SNPs could causally promote AF by regulating the atrial expression levels of PITX2. This idea is further supported by data showing that PITX2 is a suppressor of sinoatrial node-specific gene expression exclusively located in the left atrium (LA), with PITX2 deficiency increasing the mRNA levels of HCN4, SHOX2 and TBX3.96 However, published work did not find a significant correlation between the presence of risk SNPs on chromosome 4q25 and the atrial expression levels of PITX2.98,111 Although at the mRNA level the expression of PITX2 is 100-fold higher in human LA than in right atrium (RA),98 the PITX2 protein is only 2-fold higher in LA than in RA,92 suggesting a much smaller LA-RA PITX2 protein gradient in the human atrium. In addition, despite some evidence at the mRNA level that patients with AF may have lower levels of PITX2 in LA compared to sinus rhythm controls,55,97 PITX2 mRNA is increased in RA cardiomyocytes from AF patients.108 In addition, PITX2 mRNA levels vary substantially in LA appendages of AF patients requiring rhythm control therapy with ablation,101 there is no confirmation of reduced PITX2 in AF patients at the protein level, and no studies employed immunostaining to show and quantify the protein abundance of PITX2 in atrial cardiomyocytes or in other cell types of the human atrium. Rats with heart failure (HF)93 or hypertension94 reveal reduced PITX2 mRNA in LA, pointing to a potential influence of the AF-accompanying co-morbidities on PITX2 expression. Rapid atrial pacing also causes a decrease of PITX2 mRNA in pig LA,95 suggesting that AF itself could modify atrial PITX2 expression. These findings are consistent with the hypermethylation of the PITX2 promoter in LA of AF vs sinus rhytm patients55 and in LA of HF rats.93 However, whether the protein levels of Pitx2 are reduced in the atria or in atrial cardiomyocytes of patients with AF remain to be demonstrated.
The consequences of PITX2 deficiency for atrial function and AF susceptibility were studied in many mouse lines with global or cardiac-restricted genetic knockdown of PITX2 (summarized in Table 3). There is a universal finding that PITX2 deficiency in mice is associated with either ECG changes resembling AF or with a higher susceptibility to pacing-induced AF (Table 3). Genetic knockdown of PITX2 or its major cardiac isoform PITX2c in mice and zebrafish or in isolated atrial cardiomyocytes and fibroblasts provided inconsistent and sometimes contradictory results about the underlying proarrhythmogenic substrate. For instance, action potential (AP) duration (APD) was abbreviated in some studies,98,100,101 but unaltered in one study employing PITX2 deficient mice.97 PITX2 deficiency in human IPSCs resulted in APD abbreviation.107 AP upstroke velocity was unchanged in PITX2 deficient mice,97 but was faster in human IPSCs with genetic PITX2 knockdown.97 Similarly, resting membrane potential (RMP) was more depolarized97,101 in PITX2 deficient mice, but hyperpolarized in human IPSCs with genetic PITX2 knockdown.101 Surprisingly, the basal inward rectifier K+ current IK1 was unchanged in both PITX2 deficient mice101 and human IPSCs with genetic PITX2 knockdown.101 Likely because of the more depolarized RMP, the antiarrhythmic drugs dronedarone and flecainide caused a stronger APD prolongation in PITX2c deficient mice.101,104 Ca2+ influx appears disturbed, because PITX2 deficiency caused a reduction of L-type Ca2+ current (ICa,L) in mouse atrial cardiomyocytes105 and in human IPSCs.107 In contrast, the presence of the Rs13143308T risk allele did not associate with changes in ICa,L.109 Atrial conduction defects were detected in PITX2 deficient zebrafish112 and in one study using PITX2 deficient mice,99 but conduction was unaltered in other PITX2 deficient mice studies.98,101
Atrial structure appears differentially affected in the individual studies using PITX2 deficient mouse lines. There is evidence for atrial enlargement97 and alterations in cellular ultrastructure with disrupted intercalated discs and swollen and vacuolated mitochondria in LA cardiomyocytes113 in PITX2 deficient mice, which are consistent with data from PITX2 knockout zebrafish, whereby atrial enlargement and fibrosis, sarcomere disassembly, altered cardiac metabolism, and increased ROS production occurred before the onset of cardiac arrhythmias.103 Incidence and severity of PITX2c deficiency-related arrhythmias in zebrafish were reduced by antioxidant treatment with the ROS scavenger N-acetyl-cysteine, pointing to oxidative stress as a driver of the arrhythmia in this model.103 PITX2 knockout in mice could also cause cardiomyocyte hypertrophy.105 However, another study could not find differences in cardiac dimensions and structure or contractile function.98 In addition, it is important to note that some mouse lines with PITX2 deficiency developed sinoatrial node99 or ventricular dysfunction,97 which might have confounded the results at the atrial level, potentially contributing to the inconsistent results between studies.
One study in cultured human atrial fibroblasts showed that PITX2 knockdown resulted in increased fibroblast migration, with no change in proliferation, which was associated with enhanced Ca2+ influx and increased protein levels of autophosphorylated (activated) CaMKII, α-smooth muscle actin, and MMP2, but not MMP9 or collagen-1.102 Inhibition of CaMKII normalized fibroblast migration and rescued the phenotype, suggesting that PITX2 deficiency promotes the transition of fibroblasts to collagen-secreting myofibroblasts by activation of CaMKII, potentially causing structural remodeling.102
There is evidence for cellular triggered activity in PITX2 deficient mice.102,106 PITX2 deficiency increased SR Ca2+ load, the frequency of RyR2-mediated Ca2+ sparks and waves and the resulting NCX-mediated transient inward current ITI in LA and RA cardiomyocytes, thereby causing DAD-mediated triggered activity.102 As already mentioned, PITX2 suppresses expression of miR-17-92 and miR-106b-25 thereby inhibiting the predisposition to AF,58 whereas loss of miR-106b-25 cluster promotes triggered activity and AF by enhancing RyR2 expression and SR Ca2+ release,114 consistent with data from patients with paroxysmal AF.115 Other miRs involved in the pathophysiology of PITX2-dependent AF include miR-29a, miR-200, miR-203, miR-21, miR-208ab, miR-1 and miR-26b.95,116 In particular,miR-21, miR-106a, miR-203 and miR-208ab were down-regulated, whereas miR-1, miR-26b, miR-29a, miR-106b and miR-200 were up-regulated by the absence of PITX2. A miR-dependent gene regulatory network has been described leading to PITX2-dependent AF.117 However, another study could not detect DAD-mediated cellular triggered activity in PITX2 deficient mice.100 The selective RyR2 inhibitor ent-verticulide suppressed spontaneous Ca2+ waves and the related cellular triggered activity in PITX2 deficient mice, pointing to a critical role of RyR2 dysfunction.106 Another study performed in human RA cardiomyocytes showed that the presence of the Rs13143308T risk allele was associated with increased SR Ca2+ load, frequency of Ca2+ sparks and transient inward current ITI, and DADs, but no changes in ICa,L.109 Although the putative association of the risk rs13143308T allele with human atrial PITX2 expression levels was not assessed, these data support the possibility that Ca2+-dependent cellular triggered activity, as observed in AF patients,115 could represent a potential arrhythmogenic mechanism associated with PITX2 deficiency.
In summary, the studies assessing the putative role of PITX2 in AF pathophysiology clearly illustrate the big challenges of defining and establishing causal mechanisms of PITX2 in atrial arrhythmogenesis. Although PITX2 deficiency could cause reentry-promoting APD abbreviation, the findings related to atrial electrical and structural remodeling are rather inconsistent among the individual studies (Table 3). This may also be due to species-specific differences and differences in the genetic background (e.g., in determinants of APD, calcium handling etc.), which may further hinder the comparison between experimental models (mice, pigs, zebrafish, iPSC). Whether PITX2 deficiency related DAD-mediated triggered activity could be translated to clinical AF requires direct demonstration. There are still many important gaps in our knowledge about the direction of change of PITX2 and the underlying arrhythmogenic mechanisms in humans with AF that will require extensive additional work to foster translation to the clinical management of AF.
Conclusions
A review of AF mechanisms in 2002 remarked on the paucity of genetic information relating to AF pathophysiology and commented on an expected rapid growth in the future.104 The present review substantiates this prediction by documenting the explosion of information in this area over the past 20 years. Nevertheless, it also underlines the challenges in exploiting this vast body of information to understand the mechanistic link between many clear and important genetic factors and AF occurrence. While for many gene variants affecting cardiac functions associated with known AF mechanisms the pathophysiological links are relatively clear, this is not the case for most gene variants implicated in the arrhythmia. Clearly, major additional work needs to be done. Particularly important will be the development of robust methods through which to understand how gene variants in non-coding regions, genes encoding proteins with no known cardiac function and “gene deserts” lead to AF, which is at present not known in virtually all cases. An additional area needing major attention is the mechanistic basis for ethnic and racial variability in AF-risk, known to be important. Differences in genetic factors are already emerging as candidates to explain some of these observations, but again much additional work needs to be completed before we can truly understand why AF risk differs according to region, ethnic origin and racial factors.
Contributors
MV, DD, SN: literature search, figures, writing.
Declaration of interests
We declare no competing interests.
References
- 1.Kornej J., Benjamin E.J., Magnani J.W. Heart; 2021. Atrial fibrillation: global burdens and global opportunities. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez C.J., Soliman E.Z., Alonso A., et al. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol. 2015;25(2):71–76.e1. doi: 10.1016/j.annepidem.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewland T.A., Olgin J.E., Vittinghoff E., Marcus G.M. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation. 2013;128(23):2470–2477. doi: 10.1161/CIRCULATIONAHA.113.002449. [DOI] [PubMed] [Google Scholar]
- 4.Gillott R.G., Willan K., Kain K., Sivananthan U.M., Tayebjee M.H. South Asian ethnicity is associated with a lower prevalence of atrial fibrillation despite greater prevalence of established risk factors: a population-based study in Bradford Metropolitan District. Europace. 2017;19(3):356–363. doi: 10.1093/europace/euw010. [DOI] [PubMed] [Google Scholar]
- 5.Kowlgi G.N., Gunda S., Padala S.K., Koneru J.N., Deshmukh A.J., Ellenbogen K.A. Comparison of frequency of atrial fibrillation in blacks versus whites and the utilization of race in a novel risk score. Am J Cardiol. 2020;135:68–76. doi: 10.1016/j.amjcard.2020.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Tamirisa K.P., Al-Khatib S.M., Mohanty S., et al. Racial and ethnic differences in the management of atrial fibrillation. CJC Open. 2021;3(12 Suppl):S137–S148. doi: 10.1016/j.cjco.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gbadebo T.D., Okafor H., Darbar D. Differential impact of race and risk factors on incidence of atrial fibrillation. Am Heart J. 2011;162(1):31–37. doi: 10.1016/j.ahj.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobrev D., Heijman J., Hiram R., Li N., Nattel S. Inflammatory signalling in atrial cardiomyocytes: a novel unifying principle in atrial fibrillation pathophysiology. Nat Rev Cardiol. 2023;20(3):145–167. doi: 10.1038/s41569-022-00759-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bapat A., Anderson C.D., Ellinor P.T., Lubitz S.A. Genomic basis of atrial fibrillation. Heart. 2018;104(3):201–206. doi: 10.1136/heartjnl-2016-311027. [DOI] [PubMed] [Google Scholar]
- 10.Alzahrani Z., Ornelas-Loredo A., Darbar S.D., et al. Association between family history and early-onset atrial fibrillation across racial and ethnic groups. JAMA Netw Open. 2018;1(5) doi: 10.1001/jamanetworkopen.2018.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y.H., Xu S.J., Bendahhou S., et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299(5604):251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 12.Hodgson-Zingman D.M., Karst M.L., Zingman L.V., et al. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359(2):158–165. doi: 10.1056/NEJMoa0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Postma A.V., van de Meerakker J.B., Mathijssen I.B., et al. A gain-of-function TBX5 mutation is associated with atypical Holt-Oram syndrome and paroxysmal atrial fibrillation. Circ Res. 2008;102(11):1433–1442. doi: 10.1161/CIRCRESAHA.107.168294. [DOI] [PubMed] [Google Scholar]
- 14.Orr N., Arnaout R., Gula L.J., et al. A mutation in the atrial-specific myosin light chain gene (MYL4) causes familial atrial fibrillation. Nat Commun. 2016;7 doi: 10.1038/ncomms11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahlberg G., Refsgaard L., Lundegaard P.R., et al. Rare truncating variants in the sarcomeric protein titin associate with familial and early-onset atrial fibrillation. Nat Commun. 2018;9(1):4316. doi: 10.1038/s41467-018-06618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts J.D., Hu D., Heckbert S.R., et al. Genetic investigation into the differential risk of atrial fibrillation among black and white individuals. JAMA Cardiol. 2016;1(4):442–450. doi: 10.1001/jamacardio.2016.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorolfsdottir R.B., Sveinbjornsson G., Sulem P., et al. A missense variant in PLEC increases risk of atrial fibrillation. J Am Coll Cardiol. 2017;70(17):2157–2168. doi: 10.1016/j.jacc.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gudbjartsson D.F., Holm H., Sulem P., et al. A frameshift deletion in the sarcomere gene MYL4 causes early-onset familial atrial fibrillation. Eur Heart J. 2017;38(1):27–34. doi: 10.1093/eurheartj/ehw379. [DOI] [PubMed] [Google Scholar]
- 19.Gudbjartsson D.F., Arnar D.O., Helgadottir A., et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448(7151):353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen J.B., Thorolfsdottir R.B., Fritsche L.G., et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. 2018;50(9):1234–1239. doi: 10.1038/s41588-018-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christophersen I.E., Rienstra M., Roselli C., et al. Large-scale analyses of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat Genet. 2017;49(6):946–952. doi: 10.1038/ng.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pala M., Zappala Z., Marongiu M., et al. Population- and individual-specific regulatory variation in Sardinia. Nat Genet. 2017;49(5):700–707. doi: 10.1038/ng.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellinor P.T., Lunetta K.L., Albert C.M., et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44(6):670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy D., Ehret G.B., Rice K., et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41(6):677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holm H., Gudbjartsson D.F., Sulem P., et al. A rare variant in MYH6 is associated with high risk of sick sinus syndrome. Nat Genet. 2011;43(4):316–320. doi: 10.1038/ng.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamin E.J., Rice K.M., Arking D.E., et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. 2009;41(8):879–881. doi: 10.1038/ng.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellinor P.T., Lunetta K.L., Glazer N.L., et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010;42(3):240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roselli C., Chaffin M.D., Weng L.C., et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018;50(9):1225–1233. doi: 10.1038/s41588-018-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Man J.C.K., Bosada F.M., Scholman K.T., et al. Variant intronic enhancer controls SCN10A-short expression and heart conduction. Circulation. 2021;144(3):229–242. doi: 10.1161/CIRCULATIONAHA.121.054083. [DOI] [PubMed] [Google Scholar]
- 30.Chen S., Wang C., Wang X., et al. Significant association between CAV1 variant rs3807989 on 7p31 and atrial fibrillation in a Chinese han population. J Am Heart Assoc. 2015;4(5) doi: 10.1161/JAHA.115.001980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chalazan B., Mol D., Sridhar A., et al. Genetic modulation of atrial fibrillation risk in a Hispanic/Latino cohort. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0194480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malakootian M., Jalilian M., Kalayinia S., Hosseini Moghadam M., Heidarali M., Haghjoo M. Whole-exome sequencing reveals a rare missense variant in DTNA in an Iranian pedigree with early-onset atrial fibrillation. BMC Cardiovasc Disord. 2022;22(1):37. doi: 10.1186/s12872-022-02485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalazan B., Mol D., Darbar F.A., et al. Association of rare genetic variants and early-onset atrial fibrillation in ethnic minority individuals. JAMA Cardiol. 2021;6(7):811–819. doi: 10.1001/jamacardio.2021.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoneda Z.T., Anderson K.C., Quintana J.A., et al. Early-onset atrial fibrillation and the prevalence of rare variants in cardiomyopathy and arrhythmia genes. JAMA Cardiol. 2021;6(12):1371–1379. doi: 10.1001/jamacardio.2021.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Ouwerkerk A.F., Bosada F.M., van Duijvenboden K., et al. Identification of atrial fibrillation associated genes and functional non-coding variants. Nat Commun. 2019;10(1):4755. doi: 10.1038/s41467-019-12721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillette T.G., Hill J.A. Readers, writers, and erasers: chromatin as the whiteboard of heart disease. Circ Res. 2015;116(7):1245–1253. doi: 10.1161/CIRCRESAHA.116.303630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersen J.H., Andreasen L., Olesen M.S. Atrial fibrillation-a complex polygenetic disease. Eur J Hum Genet. 2021;29(7):1051–1060. doi: 10.1038/s41431-020-00784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jameson H.S., Hanley A., Hill M.C., et al. Loss of the atrial fibrillation-related gene, Zfhx3, results in atrial dilation and arrhythmias. Circ Res. 2023;133(4):313–329. doi: 10.1161/CIRCRESAHA.123.323029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Eldik W., den Adel B., Monshouwer-Kloots J., et al. Z-disc protein CHAPb induces cardiomyopathy and contractile dysfunction in the postnatal heart. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0189139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clausen A.G., Vad O.B., Andersen J.H., Olesen M.S. Loss-of-Function variants in the SYNPO2L gene are associated with atrial fibrillation. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.650667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gollob M.H., Jones D.L., Krahn A.D., et al. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med. 2006;354(25):2677–2688. doi: 10.1056/NEJMoa052800. [DOI] [PubMed] [Google Scholar]
- 42.Heimlich J.B., Bick A.G. Somatic mutations in cardiovascular disease. Circ Res. 2022;130(1):149–161. doi: 10.1161/CIRCRESAHA.121.319809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregers E., Ahlberg G., Christensen T., et al. Deep sequencing of atrial fibrillation patients with mitral valve regurgitation shows no evidence of mosaicism but reveals novel rare germline variants. Heart Rhythm. 2017;14(10):1531–1538. doi: 10.1016/j.hrthm.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 44.Roberts J.D., Longoria J., Poon A., et al. Targeted deep sequencing reveals no definitive evidence for somatic mosaicism in atrial fibrillation. Circ Cardiovasc Genet. 2015;8(1):50–57. doi: 10.1161/CIRCGENETICS.114.000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kertai M.D., Mosley J.D., He J., et al. Predictive accuracy of a polygenic risk score for postoperative atrial fibrillation after cardiac surgery. Circ Genom Precis Med. 2021;14(2) doi: 10.1161/CIRCGEN.120.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khurshid S., Mars N., Haggerty C.M., et al. Predictive accuracy of a clinical and genetic risk model for atrial fibrillation. Circ Genom Precis Med. 2021;14(5) doi: 10.1161/CIRCGEN.121.003355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Sullivan J.W., Raghavan S., Marquez-Luna C., et al. Polygenic risk scores for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2022;146(8):e93–e118. doi: 10.1161/CIR.0000000000001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wren G., Davies W. Sex-linked genetic mechanisms and atrial fibrillation risk. Eur J Med Genet. 2022;65(4) doi: 10.1016/j.ejmg.2022.104459. [DOI] [PubMed] [Google Scholar]
- 49.Yamashita T., Arima Y., Hoshiyama T., et al. Effect of the ALDH2 variant on the prevalence of atrial fibrillation in habitual drinkers. JACC Asia. 2022;2(1):62–70. doi: 10.1016/j.jacasi.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Biddinger K.J., Emdin C.A., Haas M.E., et al. Association of habitual alcohol intake with risk of cardiovascular disease. JAMA Netw Open. 2022;5(3) doi: 10.1001/jamanetworkopen.2022.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goette A., Kalman J.M., Aguinaga L., et al. EHRA/HRS/APHRS/SOLAECE expert consensus on Atrial cardiomyopathies: definition, characterisation, and clinical implication. J Arrhythm. 2016;32(4):247–278. doi: 10.1016/j.joa.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marcus G.M., Alonso A., Peralta C.A., et al. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010;122(20):2009–2015. doi: 10.1161/CIRCULATIONAHA.110.958306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weng L.C., Choi S.H., Klarin D., et al. Heritability of atrial fibrillation. Circ Cardiovasc Genet. 2017;10(6) doi: 10.1161/CIRCGENETICS.117.001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin H., Yin X., Xie Z., et al. Methylome-wide association study of atrial fibrillation in Framingham heart study. Sci Rep. 2017;7 doi: 10.1038/srep40377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donate Puertas R., Meugnier E., Romestaing C., et al. Atrial fibrillation is associated with hypermethylation in human left atrium, and treatment with decitabine reduces atrial tachyarrhythmias in spontaneously hypertensive rats. Transl Res. 2017;184:57–67 e5. doi: 10.1016/j.trsl.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Fischer M.A., Chapski D.J., Soehalim E., et al. Longitudinal profiling in patients undergoing cardiac surgery reveals postoperative changes in DNA methylation. Clin Epigenetics. 2022;14(1):195. doi: 10.1186/s13148-022-01414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song S., Zhang R., Mo B., et al. EZH2 as a novel therapeutic target for atrial fibrosis and atrial fibrillation. J Mol Cell Cardiol. 2019;135:119–133. doi: 10.1016/j.yjmcc.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Wang J., Bai Y., Li N., et al. Pitx2-microRNA pathway that delimits sinoatrial node development and inhibits predisposition to atrial fibrillation. Proc Natl Acad Sci U S A. 2014;111(25):9181–9186. doi: 10.1073/pnas.1405411111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall A.W., Chaffin M., Roselli C., et al. Epigenetic analyses of human left atrial tissue identifies gene networks underlying atrial fibrillation. Circ Genom Precis Med. 2020;13(6) doi: 10.1161/CIRCGEN.120.003085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen K., Tu T., Yuan Z., et al. DNA methylation dysregulations in valvular atrial fibrillation. Clin Cardiol. 2017;40(9):686–691. doi: 10.1002/clc.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu B., Shi X., Ding K., et al. The joint analysis of multi-omics data revealed the methylation-expression regulations in atrial fibrillation. Front Bioeng Biotechnol. 2020;8:187. doi: 10.3389/fbioe.2020.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scholz B., Schulte J.S., Hamer S., et al. HDAC (histone deacetylase) inhibitor valproic acid attenuates atrial remodeling and delays the onset of atrial fibrillation in mice. Circ Arrhythm Electrophysiol. 2019;12(3) doi: 10.1161/CIRCEP.118.007071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benaglio P., D'Antonio-Chronowska A., Ma W., et al. Allele-specific NKX2-5 binding underlies multiple genetic associations with human electrocardiographic traits. Nat Genet. 2019;51(10):1506–1517. doi: 10.1038/s41588-019-0499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Assum I., Krause J., Scheinhardt M.O., et al. Tissue-specific multi-omics analysis of atrial fibrillation. Nat Commun. 2022;13(1):441. doi: 10.1038/s41467-022-27953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H., Wang L., Yin D., et al. Integration of proteomic and metabolomic characterization in atrial fibrillation-induced heart failure. BMC Genom. 2022;23(1):789. doi: 10.1186/s12864-022-09044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang B., Lunetta K.L., Dupuis J., et al. Integrative omics approach to identifying genes associated with atrial fibrillation. Circ Res. 2020;126(3):350–360. doi: 10.1161/CIRCRESAHA.119.315179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hocker J.D., Poirion O.B., Zhu F., et al. Cardiac cell type-specific gene regulatory programs and disease risk association. Sci Adv. 2021;7(20) doi: 10.1126/sciadv.abf1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Ouwerkerk A.F., Bosada F.M., van Duijvenboden K., et al. Patient-specific TBX5-G125R variant induces profound transcriptional deregulation and atrial dysfunction. Circulation. 2022;145(8):606–619. doi: 10.1161/CIRCULATIONAHA.121.054347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kwon O.S., Hong M., Kim T.H., et al. Genome-wide association study-based prediction of atrial fibrillation using artificial intelligence. Open Heart. 2022;9(1) doi: 10.1136/openhrt-2021-001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Libiseller-Egger J., Phelan J.E., Attia Z.I., et al. Deep learning-derived cardiovascular age shares a genetic basis with other cardiac phenotypes. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-27254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei S., Tao J., Xu J., et al. Ten years of EWAS. Adv Sci (Weinh) 2021;8(20) doi: 10.1002/advs.202100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fischer M.A., Mahajan A., Cabaj M., et al. DNA methylation-based prediction of post-operative atrial fibrillation. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.837725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fahy G.M., Brooke R.T., Watson J.P., et al. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell. 2019;18(6) doi: 10.1111/acel.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ward-Caviness C.K. Accelerated epigenetic aging and incident atrial fibrillation: new outlook on an immutable risk factor? Circulation. 2021;144(24):1912–1914. doi: 10.1161/CIRCULATIONAHA.121.057533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang H., Sabari B.R., Garcia B.A., Allis C.D., Zhao Y. SnapShot: histone modifications. Cell. 2014;159(2):458.e1. doi: 10.1016/j.cell.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li D., Nie J., Han Y., Ni L. Epigenetic mechanism and therapeutic implications of atrial fibrillation. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.763824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tao H., Shi K.H., Yang J.J., Li J. Epigenetic mechanisms in atrial fibrillation: new insights and future directions. Trends Cardiovasc Med. 2016;26(4):306–318. doi: 10.1016/j.tcm.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 78.Rahm A.K., Wieder T., Gramlich D., et al. HDAC2-dependent remodeling of K(Ca)2.2 (KCNN2) and K(Ca)2.3 (KCNN3) K(+) channels in atrial fibrillation with concomitant heart failure. Life Sci. 2021;266 doi: 10.1016/j.lfs.2020.118892. [DOI] [PubMed] [Google Scholar]
- 79.Syren P., Rahm A.K., Schweizer P.A., et al. Histone deacetylase 2-dependent ventricular electrical remodeling in a porcine model of early heart failure. Life Sci. 2021;281 doi: 10.1016/j.lfs.2021.119769. [DOI] [PubMed] [Google Scholar]
- 80.Lugenbiel P., Govorov K., Syren P., et al. Epigenetic regulation of cardiac electrophysiology in atrial fibrillation: HDAC2 determines action potential duration and suppresses NRSF in cardiomyocytes. Basic Res Cardiol. 2021;116(1):13. doi: 10.1007/s00395-021-00855-x. [DOI] [PubMed] [Google Scholar]
- 81.Costa M.C., Cortez-Dias N., Gabriel A., et al. circRNA-miRNA cross-talk in the transition from paroxysmal to permanent atrial fibrillation. Int J Cardiol. 2019;290:134–137. doi: 10.1016/j.ijcard.2019.04.072. [DOI] [PubMed] [Google Scholar]
- 82.Donniacuo M., De Angelis A., Telesca M., et al. Atrial fibrillation: epigenetic aspects and role of sodium-glucose cotransporter 2 inhibitors. Pharmacol Res. 2023;188 doi: 10.1016/j.phrs.2022.106591. [DOI] [PubMed] [Google Scholar]
- 83.Luo X., Pan Z., Shan H., et al. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest. 2013;123(5):1939–1951. doi: 10.1172/JCI62185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adam O., Lohfelm B., Thum T., et al. Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res Cardiol. 2012;107(5):278. doi: 10.1007/s00395-012-0278-0. [DOI] [PubMed] [Google Scholar]
- 85.Cardin S., Guasch E., Luo X., et al. Role for MicroRNA-21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure. Circ Arrhythm Electrophysiol. 2012;5(5):1027–1035. doi: 10.1161/CIRCEP.112.973214. [DOI] [PubMed] [Google Scholar]
- 86.van Ouwerkerk A.F., Hall A.W., Kadow Z.A., et al. Epigenetic and transcriptional networks underlying atrial fibrillation. Circ Res. 2020;127(1):34–50. doi: 10.1161/CIRCRESAHA.120.316574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vosa U., Claringbould A., Westra H.J., et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat Genet. 2021;53(9):1300–1310. doi: 10.1038/s41588-021-00913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siontis K.C., Yao X., Pirruccello J.P., Philippakis A.A., Noseworthy P.A. How will machine learning inform the clinical care of atrial fibrillation? Circ Res. 2020;127(1):155–169. doi: 10.1161/CIRCRESAHA.120.316401. [DOI] [PubMed] [Google Scholar]
- 89.Attia Z.I., Friedman P.A., Noseworthy P.A., et al. Age and sex estimation using artificial intelligence from standard 12-lead ECGs. Circ Arrhythm Electrophysiol. 2019;12(9) doi: 10.1161/CIRCEP.119.007284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nattel S., Maguy A., Le Bouter S., Yeh Y.H. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87(2):425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 91.Nattel S., Heijman J., Zhou L., Dobrev D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ Res. 2020;127(1):51–72. doi: 10.1161/CIRCRESAHA.120.316363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kahr P.C., Piccini I., Fabritz L., et al. Systematic analysis of gene expression differences between left and right atria in different mouse strains and in human atrial tissue. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0026389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kao Y.H., Chen Y.C., Chung C.C., et al. Heart failure and angiotensin II modulate atrial Pitx2c promotor methylation. Clin Exp Pharmacol Physiol. 2013;40(6):379–384. doi: 10.1111/1440-1681.12089. [DOI] [PubMed] [Google Scholar]
- 94.Scridon A., Fouilloux-Meugnier E., Loizon E., et al. Long-standing arterial hypertension is associated with Pitx2 down-regulation in a rat model of spontaneous atrial tachyarrhythmias. Europace. 2015;17(1):160–165. doi: 10.1093/europace/euu139. [DOI] [PubMed] [Google Scholar]
- 95.Torrado M., Franco D., Lozano-Velasco E., et al. A MicroRNA-transcription factor blueprint for early atrial arrhythmogenic remodeling. BioMed Res Int. 2015;2015 doi: 10.1155/2015/263151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang J., Klysik E., Sood S., Johnson R.L., Wehrens X.H., Martin J.F. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proc Natl Acad Sci U S A. 2010;107(21):9753–9758. doi: 10.1073/pnas.0912585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chinchilla A., Daimi H., Lozano-Velasco E., et al. PITX2 insufficiency leads to atrial electrical and structural remodeling linked to arrhythmogenesis. Circ Cardiovasc Genet. 2011;4(3):269–279. doi: 10.1161/CIRCGENETICS.110.958116. [DOI] [PubMed] [Google Scholar]
- 98.Kirchhof P., Kahr P.C., Kaese S., et al. PITX2c is expressed in the adult left atrium, and reducing Pitx2c expression promotes atrial fibrillation inducibility and complex changes in gene expression. Circ Cardiovasc Genet. 2011;4(2):123–133. doi: 10.1161/CIRCGENETICS.110.958058. [DOI] [PubMed] [Google Scholar]
- 99.Tao Y., Zhang M., Li L., et al. Pitx2, an atrial fibrillation predisposition gene, directly regulates ion transport and intercalated disc genes. Circ Cardiovasc Genet. 2014;7(1):23–32. doi: 10.1161/CIRCGENETICS.113.000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nadadur R.D., Broman M.T., Boukens B., et al. Pitx2 modulates a Tbx5-dependent gene regulatory network to maintain atrial rhythm. Sci Transl Med. 2016;8(354):354ra115. doi: 10.1126/scitranslmed.aaf4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Syeda F., Holmes A.P., Yu T.Y., et al. PITX2 modulates atrial membrane potential and the antiarrhythmic effects of sodium-channel blockers. J Am Coll Cardiol. 2016;68(17):1881–1894. doi: 10.1016/j.jacc.2016.07.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kao Y.H., Chung C.C., Cheng W.L., Lkhagva B., Chen Y.J. Pitx2c inhibition increases atrial fibroblast activity: implications in atrial arrhythmogenesis. Eur J Clin Invest. 2019;49(10) doi: 10.1111/eci.13160. [DOI] [PubMed] [Google Scholar]
- 103.Collins M.M., Ahlberg G., Hansen C.V., et al. Early sarcomere and metabolic defects in a zebrafish pitx2c cardiac arrhythmia model. Proc Natl Acad Sci U S A. 2019;116(48):24115–24121. doi: 10.1073/pnas.1913905116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holmes A.P., Saxena P., Kabir S.N., et al. Atrial resting membrane potential confers sodium current sensitivity to propafenone, flecainide and dronedarone. Heart Rhythm. 2021;18(7):1212–1220. doi: 10.1016/j.hrthm.2021.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tarifa C., Serra S.A., Herraiz-Martinez A., et al. Pitx2c deficiency confers cellular electrophysiological hallmarks of atrial fibrillation to isolated atrial myocytes. Biomed Pharmacother. 2023;162 doi: 10.1016/j.biopha.2023.114577. [DOI] [PubMed] [Google Scholar]
- 106.Kim K., Blackwell D.J., Yuen S.L., et al. The selective RyR2 inhibitor ent-verticilide suppresses atrial fibrillation susceptibility caused by Pitx2 deficiency. J Mol Cell Cardiol. 2023;180:1–9. doi: 10.1016/j.yjmcc.2023.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schulz C., Lemoine M.D., Mearini G., et al. PITX2 knockout induces key findings of electrical remodeling as seen in persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2023;16(3) doi: 10.1161/CIRCEP.122.011602. [DOI] [PubMed] [Google Scholar]
- 108.Perez-Hernandez M., Matamoros M., Barana A., et al. Pitx2c increases in atrial myocytes from chronic atrial fibrillation patients enhancing IKs and decreasing ICa,L. Cardiovasc Res. 2016;109(3):431–441. doi: 10.1093/cvr/cvv280. [DOI] [PubMed] [Google Scholar]
- 109.Herraiz-Martinez A., Llach A., Tarifa C., et al. The 4q25 variant rs13143308T links risk of atrial fibrillation to defective calcium homoeostasis. Cardiovasc Res. 2019;115(3):578–589. doi: 10.1093/cvr/cvy215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ghazizadeh Z., Kiviniemi T., Olafsson S., et al. Metastable atrial state underlies the primary genetic substrate for MYL4 mutation-associated atrial fibrillation. Circulation. 2020;141(4):301–312. doi: 10.1161/CIRCULATIONAHA.119.044268. [DOI] [PubMed] [Google Scholar]
- 111.Gore-Panter S.R., Hsu J., Hanna P., et al. Atrial Fibrillation associated chromosome 4q25 variants are not associated with PITX2c expression in human adult left atrial appendages. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0086245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Degryse H.R., Van de Vyver F.L., Stevens A., De Schepper A.M. Magnetic resonance imaging of uterine cervix carcinoma. J Belge Radiol. 1988;71(3):405–410. [PubMed] [Google Scholar]
- 113.Morikawa K., Kubagawa H., Suzuki T., Cooper M.D. Recombinant interferon-alpha, -beta, and -gamma enhance the proliferative response of human B cells. J Immunol. 1987;139(3):761–766. [PubMed] [Google Scholar]
- 114.Chiang D.Y., Kongchan N., Beavers D.L., et al. Loss of microRNA-106b-25 cluster promotes atrial fibrillation by enhancing ryanodine receptor type-2 expression and calcium release. Circ Arrhythm Electrophysiol. 2014;7(6):1214–1222. doi: 10.1161/CIRCEP.114.001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Voigt N., Li N., Wang Q., et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125(17):2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garcia-Padilla C., Aranega A., Franco D. The role of long non-coding RNAs in cardiac development and disease. AIMS Genet. 2018;5(2):124–140. doi: 10.3934/genet.2018.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bai J., Lu Y., Zhu Y., et al. Understanding PITX2-dependent atrial fibrillation mechanisms through computational models. Int J Mol Sci. 2021;22(14) doi: 10.3390/ijms22147681. [DOI] [PMC free article] [PubMed] [Google Scholar]