Abstract
Background
The ALLEGRO phase 2a and 2b/3 studies demonstrated that ritlecitinib, an oral JAK3/TEC family kinase inhibitor, is efficacious at doses of ≥ 30 mg in patients aged ≥ 12 years with alopecia areata (AA).
Objective
The objective of this study was to evaluate the safety of ritlecitinib in an integrated analysis of four studies in AA.
Methods
Two cohorts were analyzed: a placebo-controlled and an all-exposure cohort. Proportions and study size–adjusted incidence rates (IRs) of adverse events (AEs) of interest and laboratory abnormalities are reported.
Results
In the placebo-controlled cohort (n = 881; median exposure: 169 days), the proportion of ritlecitinib-treated patients with AEs was 70.2–75.4% across doses versus 69.5% in the placebo group; serious AEs occurred in 0-3.2% versus 1.9% for the placebo. A total of 19 patients permanently discontinued due to AEs (5 while receiving the placebo). In the all-exposure cohort (n = 1294), median ritlecitinib exposure was 624 days [2091.7 total patient-years (PY)]. AEs were reported in 1094 patients (84.5%) and serious AEs in 57 (4.4%); 78 (6.0%) permanently discontinued due to AEs. The most common AEs were headache (17.7%; 11.9/100 PY), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive test (15.5%; 9.8/100 PY), and nasopharyngitis (12.4%; 8.2/100 PY). There were two deaths (breast cancer and acute respiratory failure/cardiorespiratory arrest). Proportions (IRs) were < 0.1% (0.05/100 PY) for opportunistic infections, 1.5% (0.9/100 PY) for herpes zoster, 0.5% (0.3/100 PY) for malignancies (excluding nonmelanoma skin cancer), and 0.2% (0.1/100 PY) for major adverse cardiovascular events.
Conclusions
Ritlecitinib is well tolerated with an acceptable safety profile up to 24 months in patients aged ≥ 12 years with AA (video abstract and graphical plain language summary available).
Trial Registries
ClinicalTrials.gov: NCT02974868 (date of registration: 11/29/2016), NCT04517864 (08/18/2020), NCT03732807 (11/07/2018), and NCT04006457 (07/05/2019).
Supplementary Information
The online version contains supplementary material available at 10.1007/s40257-024-00846-3.
| Digital Features for this article can be found at 10.6084/m9.figshare.24749436 |
Key Points
| This integrated analysis of data pooled from four clinical trials evaluated the safety of ritlecitinib, an oral JAK3/TEC family kinase inhibitor, in adults and adolescents with alopecia areata. |
| Most adverse events were mild and did not result in discontinuation of ritlecitinib; the most common adverse events included headache, SARS-CoV-2 test positive, and nasopharyngitis. |
| Ritlecitinib is well tolerated with an acceptable safety profile up to 24 months in patients aged ≥ 12 years with alopecia areata; ongoing studies will evaluate the safety of ritlecitinib in patients with alopecia areata who take it for up to 5 years. |
Introduction
Alopecia areata (AA) is an autoimmune disease with an underlying immuno-inflammatory pathogenesis that is characterized by nonscarring hair loss ranging from small bald patches to complete loss of scalp, face, and/or body hair [1]. AA has an unpredictable disease course and may result in chronic and extensive hair loss, with some patients progressing to alopecia totalis (complete loss of scalp and face hair) or alopecia universalis (complete loss of scalp, face, and body hair) [2, 3]. The global prevalence of AA has been estimated at approximately 2% [4], and patients with AA may experience psychological and psychosocial symptoms that can have a negative impact on quality of life [5–8].
Two therapies are currently approved for severe AA. These include baricitinib, an oral JAK1/2 inhibitor, approved for the treatment of adults with severe AA [9] and ritlecitinib, an oral, selective dual inhibitor of JAK3 and the TEC family of kinases, approved for the treatment of patients age ≥ 12 years with severe AA [10]. Given the often-chronic course of AA, the long-term safety of new treatments for AA is critical to understanding benefit–risk profiles and informing clinical decision making.
Based on safety findings from studies in rheumatoid arthritis (RA), regulatory agencies have issued precautions regarding increased risk of infection, malignancy, nonmelanoma skin cancer (NMSC), venous thromboembolism (VTE), major adverse cardiovascular events (MACE), increased lipid levels, and various changes in hematologic parameters with JAK inhibitors [11–16]. The safety profile of ritlecitinib may be distinct due to its mechanism of action (selective dual inhibition of JAK3 and TEC family kinases). Additionally, differences in underlying pathophysiology and associated comorbidities in the AA population versus other populations (such as RA) may impact the safety profile of drugs.
Here, we report an integrated safety analysis of ritlecitinib in patients aged ≥ 12 years with AA enrolled in the phase 2 and phase 3 studies of the ALLEGRO clinical program, including an ongoing, open-label, long-term study. The objective of this analysis was to assess the safety profile of ritlecitinib in AA, including evaluation of known safety events of interest for immunomodulators and JAK inhibitors. A graphical plain language summary is available (Electronic Supplementary Material 1).
Methods
Studies
This integrated safety analysis included data pooled from four studies: three randomized, placebo-controlled studies and an ongoing long-term, open-label, phase 3 study of ritlecitinib in AA from the ALLEGRO clinical development program: ALLEGRO phase 2a proof-of-concept study (NCT02974868; completed), ALLEGRO phase 2a safety study (NCT04517864; ongoing), pivotal ALLEGRO phase 2b/3 study (NCT03732807; completed), and long-term, open-label, phase 3 study (ALLEGRO-LT; NCT04006457; ongoing) (Table S1 in Electronic Supplementary Material 2). ALLEGRO-LT enrolled patients into two arms: (1) rollover patients who had received treatment in the ALLEGRO phase 2a proof-of-concept or phase 2b/3 study and (2) de novo patients who had not received treatment in either study. The data cutoff for this analysis was 30 May 2022.
Inclusion criteria for the ALLEGRO phase 2a safety study and phase 2b/3 study were described previously [10, 17]. Patients were aged ≥ 12 years (ALLEGRO-2b/3 and ALLEGRO-LT) or ≥ 18 years (ALLEGRO-2a and ALLEGRO-2a safety study), with ≥ 25% (ALLEGRO-2a safety study and de novo patients in ALLEGRO-LT) or ≥ 50% (ALLEGRO-2a and ALLEGRO-2b/3) scalp hair loss at baseline, including patients with alopecia totalis and alopecia universalis.
Patients with any previous use of a JAK inhibitor were excluded, except for rollover patients who had received ritlecitinib in ALLEGRO-2b/3. There were no specific exclusion criteria related to history or risk of VTE or MACE in any study. Detailed inclusion and exclusion criteria are outlined in Table S2.
Analysis Data Sets
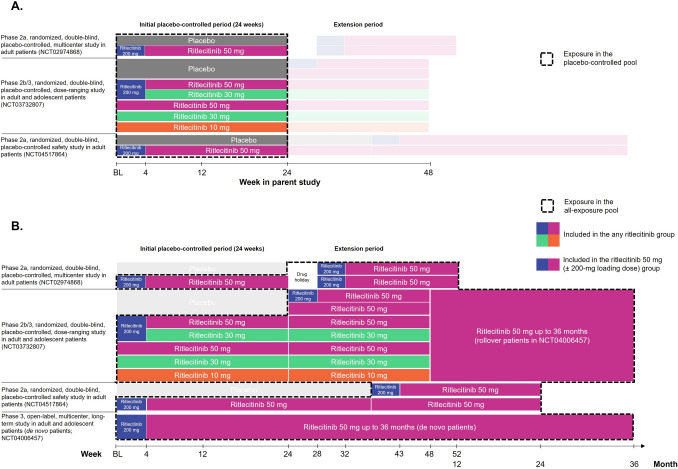
Safety data were assessed in the placebo-controlled pool (Fig. 1A) and the all-exposure pool (Fig. 1B). The placebo-controlled pool included patients from the two phase 2a studies (ALLEGRO-2a and ALLEGRO-2a safety study) and ALLEGRO-2b/3 study, who received ritlecitinib or placebo up to week 24 during the placebo-controlled period of each study. This pool included patients who were randomized and received ritlecitinib 50 mg once daily (QD) with an initial 4-week 200-mg QD loading dose (200/50 mg) from all studies, 50 mg QD (without 200-mg loading dose) from ALLEGRO-2b/3 (50/50 mg), 200/30 mg QD from ALLEGRO-2b/3, 30 mg QD from ALLEGRO-2b/3, 10 mg QD from ALLEGRO-2b/3, or placebo QD from all studies. The ritlecitinib 200/50 mg and 50/50 mg groups from all studies were combined into an “all 50 mg” group, and the 200/30 mg and 30-mg groups from ALLEGRO-2b/3 were combined into an “all 30 mg” group. This data set was used to assess ritlecitinib safety relative to placebo, dose–response relationships for frequent adverse drug reactions, and laboratory changes during the first 24 weeks of therapy.
Fig. 1.
Schematic of the A placebo-controlled pool and B the all-exposure pool. BL baseline
The all-exposure pool included all patients who received at least one dose of ritlecitinib in the ALLEGRO-2a, ALLEGRO-2a safety, ALLEGRO-2b/3, or ALLEGRO-LT study. This pool included two cohorts based on ritlecitinib dose: an “any ritlecitinib” group comprising patients who received any dose of ritlecitinib and a “ritlecitinib 50-mg” group, including just the patients who received ritlecitinib 50 mg QD with or without an initial 200-mg QD loading dose in any of the four studies (Fig. 1B). In the all-exposure pool, for the any ritlecitinib group, day 1 of exposure was the first day of ritlecitinib exposure; patients randomized to ritlecitinib in parent studies continued to receive ritlecitinib in ALLEGRO-LT. For the ritlecitinib 50-mg group, day 1 of exposure was the first day of ritlecitinib 50-mg dose (or the 200-mg loading dose), as some patients received placebo or other ritlecitinib doses in ALLEGRO-2a or -2b/3 before switching to ritlecitinib 50 mg. This data set was used to ascertain incidence rates (IRs) for adverse events (AEs) of interest and examine events of low frequency and longer latency (such as malignancies and MACE) and for subgroup analysis. Both pools included evaluation of adolescents (aged 12–17 years) and adults (aged ≥ 18 years). All patients receiving ritlecitinib in the placebo-controlled pool were included in the all-exposure pool. Consequently, any event occurring in the ritlecitinib 50-mg group or with ritlecitinib treatment in the placebo-controlled pool was also counted in the any ritlecitinib group.
Adverse Event Collection and Safety Assessments
AEs for the placebo-controlled and all-exposure pools were coded using Medical Dictionary for Regulatory Activities (MedDRA) version 24.1 and MedDRA version 25.0, respectively, and were classified as mild (defined as: does not interfere with patient’s usual function), moderate (defined as: interferes to some extent with patient’s usual function), or severe (defined as: interferes significantly with patient’s usual function), as judged by investigators. A severe AE was not necessarily a serious AE (SAE), as an SAE must have met a criterion of seriousness as defined in Table S3. AEs were summarized by MedDRA system organ class and/or preferred term. AEs of special interest categories were determined based on nonclinical and clinical experience with ritlecitinib and other immunomodulators, including JAK or TEC family kinase inhibitors. AEs of special interest were identified using standardized MedDRA queries or customized MedDRA queries; in many instances, identified events of interest were adjudicated. AEs other than SAEs were recorded from the day the patient entered the study through the last visit. SAEs were defined according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Harmonised Tripartite Guideline Clinical Safety Data Management: Definitions and Standards for Expedited Reporting E2A [18]. SAEs were reported between the time a patient provided informed consent and 35 days after the last study dose or at any time after the last dose if the SAE was suspected to be related to treatment. Only treatment-emergent SAEs reported after the first dose of study treatment are presented in this safety summary.
Adjudication of Events
Adjudication committees (external experts, blinded to treatment and independent from the study sponsor) were established to obtain objective assessment of opportunistic infections, malignancies, cardiovascular (CV) and thromboembolic events, and neurological and audiological events.
Statistical Analyses
Statistical analyses were conducted on the safety analysis set, which included all patients who were randomized and received at least one dose of treatment in the respective studies. Continuous and categorical data were analyzed using descriptive statistics. For a specific event, study size–adjusted IR was defined as the crude IR weighted by the study size; the crude IR was defined as the number of patients with events during the risk period divided by the sum of the durations of exposure of all patients during the risk period. The risk period was defined as the period from day 1 to the last dose date plus 35 days, death date, or data cutoff date. Patient exposure was censored at the time of first event. Study size–adjusted IRs with 95% confidence intervals (CIs) computed using the mid-p gamma method are reported. Using the study size–adjusted IRs, a study with a larger study size (longer exposure time and larger sample size) would have a larger weight. Safety analyses were based on the observed data with no imputation of missing data.
Results
Patient Exposure and Demographics
The placebo-controlled pool included 881 patients: 215, 130, 345, 261, 62, and 213 patients in the ritlecitinib 200/50-mg, 50/50-mg, all 50-mg, all 30-mg, 10-mg, and placebo groups, respectively. The all-exposure pool comprised a total of 1294 patients (any ritlecitinib group), of which 1228 patients received ritlecitinib 50 mg with or without a 200-mg loading dose (ritlecitinib 50-mg group). Baseline characteristics for patients in the placebo-controlled pool and the all-exposure pool were comparable (Table 1) and were well matched between treatment groups in the placebo-controlled pool. Median age was 33.0 and 32.0 years in the placebo-controlled and all-exposure pools, respectively; 11.9% (n = 105) and 14.0% (n = 181) of patients were adolescents, respectively. The majority of patients were female and white; 22% of patients in each pool were Asian.
Table 1.
Demographic and baseline characteristics in the placebo-controlled and all-exposure pools
| Placebo-controlled poola (n = 881) | All-exposure poolb (n = 1294) | ||
|---|---|---|---|
| Ritlecitinib 50 mg ± 200-mg loading dosec (n = 1228) |
Any ritlecitinib (n = 1294) | ||
| Age, mean (SD), years | 34.2 (13.9) | 33.8 (14.0) | 33.8 (14.0) |
| Age group, n (%) | |||
| 12–17 years | 105 (11.9) | 172 (14.0) | 181 (14.0) |
| ≥ 18 years | 776 (88.1) | 1056 (86.0) | 1113 (86.0) |
| ≥ 65 years | 22 (2.5) | 24 (2.0) | 27 (2.1) |
| Female, n (%) | 560 (63.6) | 780 (63.5) | 822 (63.5) |
| Race, n (%) | |||
| White | 623 (70.7) | 861 (70.1) | 904 (69.9) |
| Black | 41 (4.7) | 52 (4.2) | 55 (4.3) |
| Asian | 194 (22.0) | 270 (22.0) | 287 (22.2) |
| Other | 17 (1.9) | 28 (2.3) | 30 (2.3) |
| Not reported | 6 (0.7) | 17 (1.4) | 18 (1.4) |
| Hispanic or Latino, n (%) | 100 (11.4) | 145 (11.8) | 152 (11.7) |
| Duration of AA since diagnosis, years | |||
| Mean (SD) | 10.3 (10.6) | 10.1 (10.5) | 10.1 (10.4) |
| Median (IQR) | 6.9 (2.7–13.7) | 6.7 (2.6–13.5) | 6.7 (2.7–13.5) |
| Duration of current AA episode, years | |||
| Mean (SD) | 3.5 (3.3) | 3.3 (3.0) | 3.3 (3.0) |
| Median (IQR) | 2.5 (1.1–5.1) | 2.3 (1.0–4.8) | 2.3 (1.0–4.8) |
| Baseline SALT scored | |||
| Mean (SD) | 88.8 (17.4) | 83.5 (22.7) | 84.0 (22.3) |
| Median (IQR) | 99.0 (82.2–100.0) | 98.1 (69.6–100.0) | 98.3 (70.5–100.0) |
| Type of AA, n (%) | |||
| AT/AUe | 391 (44.4) | 502 (40.9) | 533 (41.2) |
| Non-AT/AU | 490 (55.6) | 726 (59.1) | 761 (58.8) |
AA alopecia areata, AT alopecia totalis, AU alopecia universalis, SALT Severity of Alopecia Tool
aPlacebo-controlled pool includes the placebo-controlled portion of studies ALLEGRO-2a (0–24 weeks), ALLEGRO-2b/3 (0–24 weeks), and the phase 2a safety study (0–24 weeks)
bAll-exposure pool includes all patients who received ritlecitinib in ALLEGRO-2a, the phase 2a safety study, ALLEGRO-2b/3, and ALLEGRO-LT from the start of the first dose of ritlecitinib
cPatients received ritlecitinib 50 mg once daily (QD) with or without an initial 4-week 200-mg QD loading dose
dBaseline SALT scores for all patients including those with AT or AU, who had a SALT score of 100 at baseline
ePatients in the AT/AU group had a SALT score of 100 at baseline regardless of the category in the AA history case report form
In the placebo-controlled pool, median [interquartile range (IQR)] exposure was 169 (167–173) days in each of the ritlecitinib and placebo groups. Total patient-years (PY) were 96.1, 58.3, 154.4, 114.7, 27.6, and 94.9 for the ritlecitinib 200/50-mg, 50/50-mg, all 50-mg, all 30-mg, 10-mg, and placebo groups, respectively. In total, 67 patients (7.6%) discontinued during the placebo-controlled period (Table S4). Among the 1294 patients in the all-exposure pool, median (IQR) exposure was 624 (407–792) days (2091.7 total PY); 1052 (81.3%) and 533 (41.2%) patients had ≥ 12 months and ≥ 24 months of cumulative ritlecitinib exposure, respectively (Table S5). Maximum duration of exposure was 1181 days (approximately 39 months). In the ritlecitinib 50-mg group, median (IQR) exposure was 547 (366–716) days (1813.7 total PY). At the time of data cutoff, 367 patients (28.4%) had discontinued in the all-exposure pool (Table S6).
Safety and Tolerability in the Placebo-Controlled Pool (up to 24 Weeks)
In the placebo-controlled pool, AEs occurred in 70.2%, 75.4%, 72.2%, 71.3%, and 69.4% of patients in the ritlecitinib 200/50-, 50/50-, all 50-, all 30-, and 10-mg groups, respectively, and in 69.5% of patients who received placebo (Table 2). Most treatment-emergent AEs (TEAEs) were mild to moderate in severity across treatment groups. There was no increase in overall number of TEAEs, SAEs, or severe AEs with increasing dose, and no individual SAE was reported for more than one patient in any treatment group during the placebo-controlled period. The number of temporary discontinuations due to AEs was higher in the ritlecitinib groups, occurring in 6.9–10.0% of patients compared with 3.8% of patients who received the placebo. The number of permanent discontinuations from the study or study drug due to AEs was similar across treatment groups and the placebo (1.5–3.2% for ritlecitinib groups versus 2.3% for placebo). Overall, the most common AEs (≥ 5% in any treatment group) were nasopharyngitis, upper respiratory tract infection, and headache (Table 2). The most frequent AEs (≥ 2% in any treatment group) that occurred more commonly in the ritlecitinib groups than the placebo, and in a dose-related fashion, were diarrhea, acne, urticaria, rash, and dizziness.
Table 2.
Overall safety summary and frequent treatment-emergent adverse events (all causalities) for the placebo-controlled pool
| Placebo (n = 213) | Ritlecitinib QD | |||||
|---|---|---|---|---|---|---|
| 10 mg (n = 62) | All 30 mga (n = 261) |
50/50 mg (n = 130) |
200/50 mg (n = 215) |
All 50 mgb
(n = 345) |
||
| No. of patients evaluable for AEs | 213 | 62 | 261 | 130 | 215 | 345 |
| No. of AEs | 370 | 113 | 513 | 243 | 404 | 647 |
| Patients with AEs | ||||||
| n (%) | 148 (69.5) | 43 (69.4) | 186 (71.3) | 98 (75.4) | 151 (70.2) | 249 (72.2) |
| IR (95% CI) | 323.8 (272.1, 383.0) | 250.5 (183.6, 334.3) | 275.4 (237.9, 317.2) | 310.2 (253.2, 376.3) | 342.0 (286.6, 405.4) | 353.3 (310.9, 399.9) |
| Patients with SAEs | ||||||
| n (%) | 4 (1.9) | 2 (3.2) | 1 (0.4) | 0 | 4 (1.9) | 4 (1.2) |
| IR (95% CI) | 4.5 (1.3, 11.4) | 5.8 (1.0, 19.0) | 0.7 (0.0, 3.4) | 0 | 5.3 (1.7, 12.9) | 2.7 (0.9, 6.5) |
| Patients with severe AEs | ||||||
| n (%) | 5 (2.3) | 2 (3.2) | 10 (3.8) | 2 (1.5) | 4 (1.9) | 6 (1.7) |
| IR (95% CI) | 5.0 (1.7, 12.1) | 5.9 (1.0, 19.3) | 7.0 (3.6, 12.5) | 2.7 (0.5, 9.0) | 5.4 (1.7, 12.9) | 4.1 (1.6, 8.4) |
| Discontinuations from study or study drug due to AEsc | ||||||
| n (%) | 5 (2.3) | 2 (3.2) | 4 (1.5) | 2 (1.5) | 6 (2.8) | 8 (2.3) |
| IR (95% CI) | 4.2 (1.4, 10.6) | 5.8 (1.0, 19.1) | 2.8 (0.9, 6.7) | 2.7 (0.5, 9.0) | 6.3 (2.4, 14.0) | 5.0 (2.3, 9.6) |
| Most frequent AEs occurring in ≥ 5% of patients in any treatment group by PT | ||||||
| Nasopharyngitis | ||||||
| n (%) | 15 (7.0) | 6 (9.7) | 34 (13.0) | 13 (10.0) | 21 (9.8) | 34 (9.9) |
| IR (95% CI) | 14.8 (8.2, 24.9) | 18.5 (7.5, 38.4) | 25.3 (17.8, 35.0) | 19.0 (10.5, 31.6) | 24.3 (15.0, 37.4) | 23.2 (16.3, 32.1) |
| Upper respiratory tract infection | ||||||
| n (%) | 16 (7.5) | 2 (3.2) | 21 (8.0) | 8 (6.2) | 21 (9.8) | 29 (8.4) |
| IR (95% CI) | 17.1 (9.7, 28.2) | 5.8 (1.0, 19.3) | 15.1 (9.6, 22.6) | 11.4 (5.3, 21.6) | 25.5 (15.8, 39.2) | 19.7 (13.4, 28.0) |
| Headache | ||||||
| n (%) | 17 (8.0) | 11 (17.7) | 30 (11.5) | 12 (9.2) | 20 (9.3) | 32 (9.3) |
| IR (95% CI) | 19.0 (11.1, 30.9) | 35.9 (18.9, 62.4) | 22.5 (15.5, 31.8) | 17.3 (9.4, 29.5) | 20.4 (12.3, 32.4) | 21.3 (14.7, 29.8) |
| Diarrhea | ||||||
| n (%) | 8 (3.8) | 0 | 10 (3.8) | 12 (9.2) | 14 (6.5) | 26 (7.5) |
| IR (95% CI) | 8.4 (3.7, 16.8) | 0 | 7.0 (3.6, 12.5) | 17.7 (9.6, 30.1) | 14.3 (7.7, 24.6) | 17.4 (11.6, 25.3) |
| Acne | ||||||
| n (%) | 10 (4.7) | 3 (4.8) | 14 (5.4) | 8 (6.2) | 12 (5.6) | 20 (5.8) |
| IR (95% CI) | 10.3 (5.0, 19.4) | 8.9 (2.3, 24.2) | 9.9 (5.6, 16.2) | 11.2 (5.2, 21.3) | 11.2 (5.7, 20.4) | 12.7 (8.0, 19.4) |
| Folliculitis | ||||||
| n (%) | 4 (1.9) | 2 (3.2) | 11 (4.2) | 4 (3.1) | 12 (5.6) | 16 (4.6) |
| IR (95% CI) | 4.6 (1.4, 11.6) | 5.7 (1.0, 18.9) | 7.8 (4.1, 13.5) | 5.5 (1.7, 13.2) | 13.9 (7.3, 24.4) | 10.4 (6.2, 16.6) |
| Nausea | ||||||
| n (%) | 15 (7.0) | 3 (4.8) | 12 (4.6) | 3 (2.3) | 12 (5.6) | 15 (4.3) |
| IR (95% CI) | 14.2 (7.8, 24.1) | 8.9 (2.3, 24.2) | 8.6 (4.7, 14.6) | 4.2 (1.1, 11.3) | 13.3 (6.9, 23.6) | 9.7 (5.6, 15.7) |
| Urticaria | ||||||
| n (%) | 3 (1.4) | 1 (1.6) | 10 (3.8) | 6 (4.6) | 11 (5.1) | 17 (4.9) |
| IR (95% CI) | 4.0 (1.0, 11.0) | 2.9 (0.1, 14.3) | 7.0 (3.6, 12.6) | 8.2 (3.3, 17.1) | 13.5 (6.9, 24.1) | 11.4 (6.8, 17.9) |
| Dizziness | ||||||
| n (%) | 3 (1.4) | 1 (1.6) | 10 (3.8) | 3 (2.3) | 11 (5.1) | 14 (4.1) |
| IR (95% CI) | 2.4 (0.5, 7.8) | 2.9 (0.1, 14.2) | 7.0 (3.6, 12.5) | 4.2 (1.1, 11.3) | 11.8 (5.9, 21.4) | 9.0 (5.1, 14.8) |
| Myalgia | ||||||
| n (%) | 3 (1.4) | 5 (8.1) | 6 (2.3) | 1 (0.8) | 5 (2.3) | 6 (1.7) |
| IR (95% CI) | 2.4 (0.5, 7.8) | 15.3 (5.6, 33.8) | 4.2 (1.7, 8.7) | 1.4 (0.1, 6.7) | 5.9 (2.1, 13.6) | 3.9 (1.6, 8.1) |
Study size-adjusted IRs are per 100 patient-years and are presented along with mid-p gamma CIs
AE adverse event, CI confidence interval, IR incidence rate, PT preferred term, QD once daily, SAE serious adverse event
aRitlecitinib all 30 mg includes patients from ritlecitinib 200/30 mg and 30/30 mg groups combined
bRitlecitinib all 50 mg includes patients from ritlecitinib 200/50 mg and 50/50 mg groups combined
cPatients with an AE record that indicated that the AE caused the patient to be discontinued from the study or study drug
Longer-Term Safety
The proportions of AEs, SAEs, and severe AEs across the all-exposure pool are presented in Table 3. In the any-ritlecitinib group, AEs were reported in 1094 patients (84.5%), SAEs in 57 (4.4%), and severe AEs in 83 (6.4%); 78 patients (6.0%) permanently discontinued due to AEs. The most common TEAEs in the any-ritlecitinib group were headache (17.7%), SARS-CoV-2 positive test (15.5%), nasopharyngitis (12.4%), acne (10.4%), and upper respiratory tract infection (10.2%). Except for events related to coronavirus disease 2019 (COVID-19, which predominantly occurred outside of the placebo-controlled periods), AEs were similar to those in the placebo-controlled pool. The incidence of AEs, SAEs, and severe AEs was consistent across the any-ritlecitinib and ritlecitinib 50-mg groups. Events in the infections and infestations system organ class were the most frequent SAEs (full listing of SAEs in Table S7); pregnancy (12 patients; 0.9%), headache, and urticaria (4 patients each; 0.3%) led to most of the permanent discontinuations. There were two deaths (0.2%) due to breast cancer and acute respiratory failure/cardiorespiratory arrest (described below).
Table 3.
Summary of treatment-emergent adverse events (all causalities) in the all-exposure pool
| Ritlecitinib 50 mg ± 200-mg loading dosea (n = 1228) | Any ritlecitinib (n = 1294) | |||
|---|---|---|---|---|
| n (%) | IR (95% CI)b | n (%) | IR (95% CI)b | |
| No. of patients evaluable for AEs | 1228 | 1294 | ||
| No. of AEs | 4368 | 5234 | ||
| Patients with AEs | 989 (80.5) | 160.55 (150.77, 170.80) | 1094 (84.5) | 179.76 (169.33, 190.65) |
| Patients with SAEs | 52 (4.2) | 2.79 (2.10, 3.65) | 57 (4.4) | 2.64 (2.01, 3.40) |
| Patients with severe AEs | 66 (5.4) | 3.59 (2.79, 4.55) | 83 (6.4) | 3.91 (3.13, 4.83) |
| Deaths | 2 (0.2)c | 0.10 (0.01, 0.36) | 2 (0.2)c | 0.09 (0.01, 0.31) |
| Patients discontinued from study or study drug due to AEsd | 68 (5.5) | 3.63 (2.83, 4.58) | 78 (6.0) | 3.59 (2.84, 4.45) |
| Patients with temporary discontinuation due to AEs | 259 (21.1) | 15.78 (13.93, 17.81) | 284 (21.9) | 15.10 (13.42, 16.95) |
| AEs occurring in ≥ 5% of patients in any treatment group by PT | ||||
| Headache | 186 (15.1) | 10.72 (9.25, 12.36) | 229 (17.7) | 11.90 (10.43, 13.53) |
| SARS-CoV-2 positive test | 192 (15.6) | 10.68 (9.23, 12.29) | 201 (15.5) | 9.75 (8.47, 11.18) |
| Nasopharyngitis | 117 (9.5) | 6.57 (5.45, 7.86) | 160 (12.4) | 8.17 (6.97, 9.51) |
| Acne | 111 (9.0) | 6.17 (5.09, 7.42) | 135 (10.4) | 6.75 (5.68, 7.97) |
| Upper respiratory tract infection | 104 (8.5) | 5.91 (4.84, 7.14) | 132 (10.2) | 6.48 (5.44, 7.66) |
| Pyrexia | 93 (7.6) | 5.01 (4.06, 6.13) | 98 (7.6) | 4.64 (3.79, 5.64) |
| Cough | 93 (7.6) | 5.15 (4.17, 6.29) | 96 (7.4) | 4.54 (3.70, 5.53) |
| Fatigue | 77 (6.3) | 4.15 (3.29, 5.17) | 91 (7.0) | 4.33 (3.51, 5.30) |
| Urticaria | 74 (6.0) | 4.02 (3.17, 5.03) | 88 (6.8) | 4.25 (3.43, 5.22) |
| AEs of special intereste | ||||
| Serious infections | 12 (1.0) | 0.66 (0.35, 1.13) | 14 (1.1) | 0.64 (0.36, 1.06) |
| Opportunistic infections | 1 (< 0.1) | 0.05 (0.00, 0.25) | 1 (< 0.1) | 0.05 (0.00, 0.23) |
| Herpes zoster | 18 (1.5) | 0.99 (0.60, 1.55) | 20 (1.5) | 0.92 (0.57, 1.40) |
| Herpes simplex | 25 (2.0) | 1.31 (0.86, 1.91) | 37 (2.9) | 1.72 (1.22, 2.35) |
| Malignancies (excluding NMSC) | 7 (0.6) | 0.37 (0.16, 0.75) | 7 (0.5) | 0.32 (0.14, 0.64) |
| NMSC | 3 (0.2) | 0.15 (0.03, 0.43) | 3 (0.2) | 0.14 (0.03, 0.38) |
| Breast cancerf | 4 (0.5) | 0.39 (0.12, 0.97) | 4 (0.5) | 0.35 (0.10, 0.85) |
| MACEg | 3 (0.2) | 0.15 (0.03, 0.43) | 3 (0.2) | 0.14 (0.03, 0.38) |
| Thromboembolic events (PE) | 1 (< 0.1) | 0.06 (0.00, 0.29) | 1 (< 0.1) | 0.05 (0.00, 0.23) |
| Peripheral neuropathy | 3 (0.2) | 0.16 (0.03, 0.45) | 4 (0.3) | 0.18 (0.05, 0.45) |
| Paresthesia and dysesthesia | 21 (1.7) | 1.11 (0.69, 1.67) | 26 (2.0) | 1.20 (0.80, 1.75) |
| Sensorineural hearing lossh | 12 (1.0) | 0.67 (0.36, 1.16) | 14 (1.1) | 0.64 (0.36, 1.06) |
AE adverse event, IR incidence rate, MACE major adverse cardiovascular event, NMSC nonmelanoma skin cancer, PE pulmonary embolism, PT preferred term, PY patient-years, SAE serious adverse event
aPatients received ritlecitinib 50 mg once daily (QD) with or without an initial 4-week 200-mg QD loading dose
bStudy size–adjusted IRs per 100 PY and mid-p gamma CIs
cTwo deaths were reported in the all-exposure pool: breast cancer (spindle cell carcinoma) and acute respiratory failure/cardiorespiratory arrest. Both events were determined by the investigator to be unrelated to the study treatment
dPatients with an AE record that indicated that the AE caused the patient to be discontinued from the study or study drug
eOpportunistic infections, malignancies, MACE, non-MACE, thromboembolic events, peripheral neuropathy, paresthesia and dysesthesia, and sensorineural hearing loss were adjudicated by independent clinical event committees
fDenominator and patient-years were adjusted to reflect the female patient population
gMACE was defined as a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. There was one event of percutaneous transluminal coronary angioplasty/percutaneous coronary intervention in the 50-mg group that was considered non-MACE
hSensorineural hearing loss included Medical Dictionary of Regulatory Activities (MedDRA) PTs of deafness neurosensory, deafness unilateral, and hypoacusis. PT terms defined by MedDRA do not necessarily indicate that patients developed deafness
Adverse Events of Special Interest
Infections
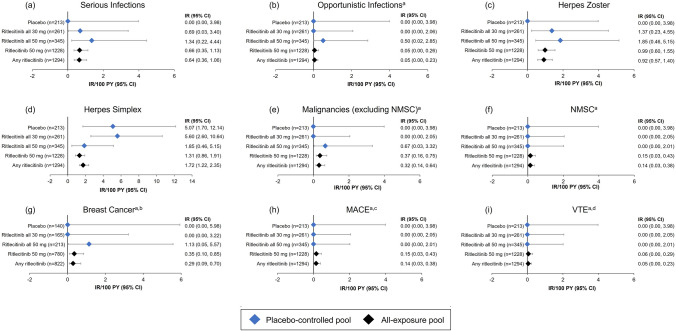
The proportions and IRs of serious infection in ritlecitinib groups were low across both the placebo-controlled and all-exposure pools. Serious infections occurred in 0.9% of patients in the 200/50-mg group, 0.6% in the all 50-mg group, 0.4% in the all 30-mg group, and none in the 50/50-mg and placebo groups. In the all-exposure pool, serious infections were reported in 12 patients (1.0%) in the ritlecitinib 50-mg group (IR: 0.66/100 PY [95% CI 0.35, 1.13]) and in 14 patients (1.1%) in the any ritlecitinib group (IR: 0.64/100 PY [95% CI 0.36, 1.06]) (Fig. 2). The most frequent serious infections (each occurring in ≤ 0.4% of patients in each group) included appendicitis, COVID-19, and COVID-19 pneumonia. Per protocol, all serious infections required discontinuation; however, all events resolved or were resolving, and none were fatal.
Fig. 2.
IRs per 100 PY for adverse events of special interest (a–i). IRs are exposure adjusted and expressed as the number of patients with events per 100 PY. IR incidence rate, MACE major adverse cardiovascular events, NMSC nonmelanoma skin cancer, PY patient-years, VTE venous thromboembolic events. Study size–adjusted IRs are per 100 PY and are shown with mid-p gamma CIs. Ritlecitinib all 30 mg includes patients who received ritlecitinib 30 mg QD with or without an initial 4-week 200-mg QD loading dose. Ritlecitinib all 50 mg and ritlecitinib 50 mg includes patients who received ritlecitinib 50 mg QD with or without an initial 4-week 200-mg QD loading dose from the placebo-controlled and all-exposure pools, respectively. aAdjudicated safety events. bIRs shown for breast cancer in female patients. cMACE was defined as a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. dVTE was defined as events of deep vein thrombosis and pulmonary embolism
In the all-exposure pool, one patient (< 0.1%) in the 200/50-mg group experienced a TEAE of varicella zoster virus infection, which was adjudicated as an opportunistic infection (OI) of multidermatomal herpes zoster (HZ) [IR: 0.05/100 PY (95% CI 0.00, 0.23)]. The event was mild in severity and resolved without treatment interruption. There were no cases of active tuberculosis in any safety pool. With a single case of OI (multidermatomal HZ) across the entire pool of data, incidence rates of OIs were low across the placebo-controlled and all-exposure pools (Fig. 2).
In the placebo-controlled pool, HZ occurred more frequently in some ritlecitinib groups (50/50 mg, 1.5%; all 50 mg, 0.9%; all 30 mg, 0.8%) than in the placebo group, in which no events were reported. All were nonserious, mild, or moderate, and all resolved; there were no events with visceral involvement. In the all-exposure pool, the proportion of patients with HZ was 1.5% [0.99/100 PY (95% CI 0.60, 1.55)] in the ritlecitinib 50-mg group and 1.5% [0.92/100 PY (95% CI 0.57, 1.40)] in the any ritlecitinib group (Fig. 2); most events were localized, with only one event affecting multiple dermatomes (the OI described above). One patient (0.1%; 71-year-old woman; 50-mg group) who experienced HZ was permanently discontinued from the study; the event was deemed moderate and related to treatment, and the patient was recovering at the time of discontinuation. Across the program, the median time to onset for HZ events was 37.4 weeks, and most cases resolved in < 6 weeks (median 15.0 days). Events of herpes simplex were not dose dependent; a higher incidence was observed with placebo than ritlecitinib 50 mg (Fig. 2).
Dermatological Events
In the placebo-controlled pool, the proportions of patients with dermatological events of interest were similar across treatment groups. The most frequently reported dermatological AEs (> 2% in any treatment group) across all treatment groups were urticaria, folliculitis, acne, dermatitis atopic, dermatitis contact, pruritus, and rash. Events that occurred more commonly in patients in the all 50-mg group than placebo included urticaria (4.9% versus 1.4%), folliculitis (4.9% versus 1.9%), atopic dermatitis (2.3% versus 0.5%), and rash (3.2% versus 1.4%). The number of dermatological events of interest in the all-exposure pool was similar to that reported in the placebo-controlled pool. The most frequently reported dermatological events in the ritlecitinib 50-mg and any ritlecitinib groups included acne (9.0% and 10.4%, respectively), urticaria (6.0% and 6.8%), and folliculitis (5.1% and 6.3%); all events were nonserious and mild to moderate in severity, and few led to interruption or discontinuation of treatment. The majority (52.8%) of urticaria events occurred during the first 12 weeks of treatment (median time to onset: 10.1 weeks; Table S8); four patients (0.3%) permanently discontinued from the study due to urticaria.
Malignancies
Seven events were adjudicated as malignancies (excluding NMSC) in the all-exposure pool [0.32/100 PY (95% CI 0.14, 0.64)]; all events were reported in the ritlecitinib 50-mg group, and 2 (both breast cancers) occurred during the placebo-controlled period (Fig. 2, Table 3). Breast cancer occurred in four female patients [0.5%, IR: 0.29/100 PY (95% CI 0.09, 0.70)], for whom one event was fatal. The SAEs of breast cancer occurred in a 64-year-old woman (50-mg group), a 66-year-old woman (50-mg group), a 58-year-old woman (50-mg group), and a 46-year-old woman (200/50-mg group); clinical details for all SAEs of breast cancer are provided in Table S9. For the four events of breast cancer, the time to onset of the event ranged from 68 to 299 days (median 156 days) after starting ritlecitinib. All four patients had identified risk factors, including supplemental estrogen therapy, family history of breast cancer, or nulliparity in two patients, current smoker or a history of smoking in three patients, and alcohol consumption in all four patients.
There was one event each of testicular cancer (21-year-old man, 50-mg group; recovered/resolved) and papillary thyroid cancer (26-year-old man, 50-mg group; ongoing at the time of discontinuation); one adjudicated event of malignant melanoma (50-year-old woman, 50-mg group; ongoing at the time of discontinuation) was reported (Table S9).
There were three patients (0.2%) with adjudicated NMSC reported in the all-exposure pool [0.14/100 PY (95% CI 0.03, 0.38)], all of which occurred in the ritlecitinib 50-mg group [0.15/100 PY (95% CI 0.03, 0.43)]; these included two patients (0.2%) with basal cell carcinoma and one patient (0.1%) with Bowen disease (Table 3).
Cardiovascular Safety
In the ritlecitinib 50-mg group of the all-exposure pool, three patients (0.2%) with SAEs adjudicated as MACE were reported [0.15/100 PY (95% CI 0.03, 0.43)] (Fig. 2). These included one event of myocardial infarction in a 49-year-old man (200/50-mg group), who was a current smoker with a history of hyperlipemia and diabetes. The patient underwent percutaneous transluminal coronary angioplasty/percutaneous coronary intervention the following day; treatment was discontinued and not resumed. The other events included one retinal artery occlusion in a 48-year-old woman (200/50-mg group) with a history of congenital carotid artery defect, patent foramen ovale, and migraine. This patient also experienced a non-SAE of antiphospholipid syndrome and was discontinued from the study. One sudden CV death (acute respiratory failure and cardiorespiratory arrest) occurred in a 51-year-old woman (50-mg group) with a history of asthma, anxiety, and smoking; ongoing concomitant medication was salbutamol for asthma (additional clinical details are provided in Table S10).
One patient (54-year-old woman) in the ritlecitinib 50-mg group of the all-exposure pool experienced a pulmonary embolism (PE) that met adjudication criteria for a VTE [0.06/100 PY (95% CI 0.00, 0.29)]. This patient had potential risk factors, including a recent positive SARS-CoV-2 test and a medical history of morbid obesity, sleep apnea, hypertension, hyperlipidemia, and monoclonal gammopathy of undetermined significance (Table S10). No AEs met the adjudication criteria for deep vein thrombosis.
Neuroaudiological Events
In the all-exposure pool, the most frequently reported adjudicated events of interest term was paresthesia and dysesthesia. In the ritlecitinib 50-mg group, 21 patients (1.7%) experienced TEAEs meeting criteria for adjudicated term paresthesia and dysesthesia [1.11/100 PY (95% CI 0.69, 1.67)] and three patients (0.2%) experienced TEAEs meeting criteria for adjudicated peripheral neuropathy [0.16/100 PY (95% CI 0.03, 0.45)]. In the any ritlecitinib group, 26 [2.0%; IR: 1.20/100 PY (95% CI 0.80, 1.75)] and four [0.3%; IR: 0.18/100 PY (95% CI 0.05, 0.45)] patients had adjudicated paresthesia and dysesthesia and peripheral neuropathy, respectively (Table 3).
In this analysis, TEAEs adjudicated by the external committee to meet criteria for an audiological event of interest reflect both the outcomes of protocol-specified audiological testing (even in the absence of spontaneously reported TEAEs related to hearing loss) and spontaneously reported TEAEs related to hearing loss. In the all-exposure pool, there were 12 patients [1.0%; 0.67/100 PY (95% CI 0.36, 1.16)] with TEAEs adjudicated to meet criteria for sensorineural hearing loss in the ritlecitinib 50-mg group and 14 patients [1.1%; 0.64/100 PY (95% CI 0.36, 1.06)] in the any ritlecitinib group; these events were all identified through protocol-specified audiological testing (Table 3), and no events were adjudicated as central hearing loss.
Laboratory Assessments
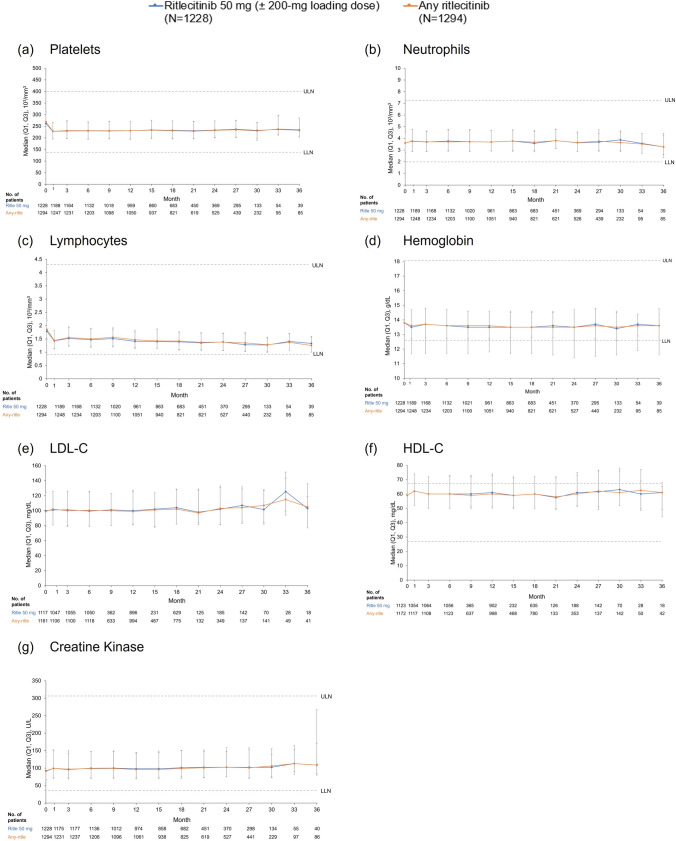
Overall, ritlecitinib was associated with small short-term changes in hematological parameters that remained stable during long-term treatment. Median platelet levels decreased to week 4 and remained stable at a lower level through month 36 (Fig. 3). In the all-exposure pool, no patients had platelet counts considered Common Terminology Criteria for Adverse Events (CTCAE) grade ≥ 2 or met protocol-specified discontinuation criteria (< 75 × 103/mm3). TEAEs of thrombocytopenia and platelet count decrease were reported in four (0.3%) and seven (0.5%) patients, respectively; one patient (0.1%) was permanently discontinued from the study due to a non-serious AE of thrombocytopenia (platelets 83 × 103/mm3).
Fig. 3.
Select laboratory parameters over time. HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, LLN lower limit of normal, ULN upper limit of normal
There was a slight decrease from baseline in median hemoglobin (Hgb) levels over the first 4 weeks, and then levels subsequently returned toward baseline and remained stable through month 36 (Fig. 3). In the all-exposure pool, TEAEs of anemia were reported for 17 patients (1.3%), and Hgb decrease was reported for 11 patients (0.9%). In the ritlecitinib 50-mg group, one patient (< 0.1%) met discontinuation criteria for change in Hgb (confirmed < 9.0 g/dL or decrease of > 30% from baseline) and one patient (< 0.1%) had Hgb levels considered CTCAE grade 3 (< 8.0 g/dL), leading to temporary treatment discontinuation (Table S11).
Absolute neutrophil count remained stable through month 36 (Fig. 3). In the ritlecitinib 50-mg group, nine patients (0.7%) had absolute neutrophil count decreases considered CTCAE grade 3 (< 1000 to 500/mm3); no patients had a decrease considered grade 4 (< 500/mm3; Table S11). No patients met protocol-specified discontinuation criteria for neutrophils (confirmed < 750/mm3). TEAEs of neutrophil count decrease, neutropenia, and abnormal neutrophil count were reported in five (0.4%), three (0.2%), and one (0.1%) patient, respectively, in the 50-mg group. Overall, two patients (0.2%) discontinued due to neutropenia, including one (0.1%) in the 50-mg group.
There was a decrease in median absolute lymphocyte count (ALC) from baseline to week 4 with ritlecitinib; ALC levels remained stable at a lower level through month 36 (Fig. 3). In the ritlecitinib 50-mg group, 25 patients (2.0%) had lymphocyte count decreases considered CTCAE grade 3 (< 500–200/mm3), and one patient (< 0.1%) had grade 4 lymphocyte count decrease (< 200/mm3; Table S11). One patient (<0.1%) met discontinuation criteria for lymphocytes (confirmed by two sequential tests ALC < 500/mm3).
Treatment with ritlecitinib was associated with concurrent small increases in median total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol from baseline to week 4 that did not progress over time and remained stable through month 36 (Fig. 3). Hyperlipidemia and hypertriglyceridemia were reported in two patients (0.2%) each in the ritlecitinib 50-mg group of the all-exposure pool; none led to discontinuation of study or study drug.
In the placebo-controlled and the all-exposure pool, there was no meaningful change over time in median alanine aminotransferase or aspartate aminotransferase levels. The proportion of patients with alanine aminotransferase or aspartate aminotransferase greater than three times the upper limit of normal (ULN) was similar in the ritlecitinib 50-mg and any ritlecitinib groups (Table S11). In the ritlecitinib 50-mg group, TEAEs of liver function test increase and abnormal liver function test were reported in four (0.3%) and two (0.2%) patients, respectively; none led to permanent discontinuation from the study or study drug. No AEs were adjudicated as Hy’s law or drug-induced liver injury.
There were changes in serum creatinine or creatine kinase (CK) levels over time with ritlecitinib through month 36 (Fig. 3). In the ritlecitinib 50-mg group, 65 patients (5.3%) had CK increases considered CTCAE grade 3 or 4 (greater than five times ULN; Table S11); TEAEs of blood creatine phosphokinase increase were reported in 40 patients (3.3%). In total, three patients (0.2%) permanently discontinued due to blood creatine phosphokinase increases; one patient (< 0.1%) met discontinuation criteria for CK (two sequential tests greater than ten times ULN). No events of rhabdomyolysis were reported, and no AEs, including myalgia, were found to be associated with increases in CK.
Discussion
This is the first report of an integrated safety analysis for ritlecitinib, an oral selective dual JAK3/TEC family kinase inhibitor, in patients age ≥ 12 years with AA. Data pooled from four trials of the ALLEGRO clinical trial program, consisting of 1294 patients with AA (representing 2092 PY of exposure), were consistent with the known safety profile of ritlecitinib. The overall pattern of reported all-causality TEAEs was consistent between the placebo-controlled and all exposure pools and demonstrated no new safety signals emerging with longer exposure. Across the ALLEGRO program, the most frequently reported AEs were headache, nasopharyngitis, and upper respiratory tract infection. Most AEs were mild and infrequently required interruption or permanent discontinuation of ritlecitinib treatment.
The pooled data reported here represent the first assessment of the potential impact of selective JAK3/TEC family kinase inhibition on adverse events of special interest that may occur at a lower frequency or have a longer latency in patients with AA. Serious and opportunistic infections and viral reactivation are associated with immunomodulators, including JAK inhibitors, and have been reported with baricitinib (JAK1/2), upadacitinib (JAK1/2), tofacitinib (JAK1/3), abrocitinib (JAK1), and filgotinib (JAK1) [11–14, 19]. An increased risk of infection has also been reported with TEC family kinase inhibitors, including the BTK inhibitor ibrutinib [20–22]. Therefore, it was deemed important to determine if ritlecitinib treatment resulted in an increase in the number of infections in the AA population. The IRs of serious infections were low across the ALLEGRO program; in this analysis, the IR was 0.66/100 PY in the ritlecitinib 50-mg group. In a real-world US claims-based study, the IR for serious infections was 1.85/100 PY (95% CI 1.65, 2.08) in patients with AA [23].
A dose-related increase in HZ infections was observed with ritlecitinib, which is consistent with previous reports for other immunomodulators, including JAK inhibitors [11, 13]. In the ritlecitinib 50-mg group of the all exposure pool of this analysis, the IR for HZ was 0.99/100 PY (95% CI 0.60, 1.55); in the US-based cohort study in patients with AA, the IR was 0.78/100 PY (95% CI 0.65, 0.93) [23]. Rates were lower in the all-exposure pool than in the placebo-controlled pool, suggesting no increased risk with longer ritlecitinib treatment. As expected, there was a trend toward increasing IR/100 PY with increasing age in adults. Most events were found to resolve within 6 weeks (median 15 days). The IR is lower than the reported IRs for HZ in patients with AA treated with the JAK1/2 inhibitor baricitinib (IR: 1.8/100 PY) [24] and in patients with atopic dermatitis (AD) treated with the JAK1/2 inhibitor upadacitinib [IRs: 3.8/100 PY (15 mg), 6.7/100 PY (30 mg)] [15] or the JAK1 inhibitor abrocitinib [IRs: 2.0/100 PY (100 mg), 4.3/100 PY (200 mg)] [14]. However, future investigations are warranted to elucidate the specific contributions of the underlying disease versus JAK inhibitor therapy in influencing HZ.
JAK inhibitors have been associated with dermatological adverse reactions including acne, folliculitis, rash, and/or urticaria [25–27], all of which occurred at higher rates with ritlecitinib 50 mg than placebo in the placebo-controlled pool of the ALLEGRO studies. All events of acne, urticaria, and folliculitis were mild to moderate in severity. Acne has been reported as a common or very common adverse drug reaction for all JAK inhibitors approved for dermatological conditions [25–30]. While no clear mechanism of action is known for this, it has been suggested that JAK inhibitors may be associated with skin dysbiosis, including microbial colonization of Demodex folliculorum. Induction of acne lesions through inhibition of immune signaling by JAK inhibitors has also been suggested. However, overexpression of JAK1 and JAK3 has also been reported in acne vulgaris lesions suggesting a complex interplay of immune modulation and the potential that acne represents a paradoxical reaction to JAK inhibition [29, 31]. For the other dermatological adverse reactions associated with JAK inhibitors, there is no clear understanding of the pathogenic mechanisms that drive or exacerbate these events during JAK inhibitor treatment.
In the ALLEGRO program, all adjudicated malignancies occurred in patients receiving ritlecitinib ≥ 50 mg, representing the largest pool of patients in this analysis (1813.7 PY for patients receiving 50 mg ± 200 mg loading dose of total 2091.7 PY). The seven reported malignancies are among the more commonly reported malignancies in the general population, and five of the patients had additional risk factors for their reported malignancies. Across the ritlecitinib program, there were four cases of breast cancer; the IR for breast cancer in female patients receiving any dose of ritlecitinib was 0.29/100 PY (95% CI 0.09, 0.70) while the background IR from the AA cohort in a US-based cohort study was 0.46/100 PY (95% CI 0.33, 0.63) [23]. The IR for malignancy (excluding NMSC) in the ritlecitinib 50-mg group in this integrated analysis was 0.37/100 PY and the IR reported for the AA cohort in US-based cohort study was 1.25/100 PY (95% CI 1.08, 1.45) [23]. Generally, AA is not known to be associated with an increased risk of cancer. Other integrated safety analyses of immunomodulatory agents have reported IRs for malignancies (excluding NMSC) in patient populations with similar demographics, including baricitinib in AD (IR: 0.22/100 PY) [32] and AA (IR: 0.2/100 PY) [24], lebrikizumab in AD (IR: 0.5/100 PY) [33], and upadacitinib in AD (IR range 0.1–0.5/100 PY) [34]. However, given the low number of events, additional long-term data are needed to further assess this risk.
Following the results of the post authorization ORAL surveillance trial of tofacitinib in RA [35], it was recognized that JAK inhibitors may increase the risk of thrombosis, MACE, malignancy, and mortality in high-risk patient populations [36, 37]. However, it is not clear that the same risks should be assumed with regard to ritlecitinib in AA. Ritlecitinib is a selective dual inhibitor of JAK3 and TEC family kinases and offers a distinct mechanism of action. Furthermore, patients with AA generally have fewer comorbidities (versus patients with RA), and events of serious infections, HZ, MACE, VTE, and malignancies have been reported to occur less frequently in patients with dermatological disorders than with rheumatological diseases [38, 39].
Three adjudicated MACE and one VTE (PE) were reported in this analysis. Overall, the IRs for MACE and VTE in the ALLEGRO clinical program were 0.14/100 PY and 0.06/100 PY, respectively; the IRs (95% CI) in the US-based cohort study for MACE and VTE were 1.6/100 PY (1.41, 1.82) and 0.43/100 PY (0.33, 0.54), respectively [23]. Notably, patients with CV risk factors were not specifically excluded from the ALLEGRO studies, and all patients with CV events had at least one CV risk factor. Other integrated safety analyses have reported similar IRs for VTE in AA and AD, including baricitinib in AD (IR: 0.09/100 PY) [32] and AA (IR: 0.1/100 PY) [24], abrocitinib in AD (IR: 0.3/100 PY) [33], and upadacitinib in AD (IRs: 0.3/100 PY [15 mg], 0.2/100 PY [30 mg]) [34]. The relationship between thromboembolic and CV events and increased lipid levels observed with some JAK inhibitors is not well understood [40]. Notably, no clinically meaningful changes in lipid levels were observed over the course of ritlecitinib treatment. While the data presented here are reassuring, additional long-term data are needed to assess the VTE risk with selective JAK3/TEC inhibition in AA.
The four JAK isoforms have complex interactions with the development and function of hematopoietic cells, and selectivity in JAK inhibition may affect the pattern of changes in laboratory values. Additionally, TEC family kinases are involved in signal transduction from antigen receptors on B cells, T cells, and platelets, and changes in hematologic parameters have been reported with TEC family kinase inhibitors [41–43]. Ritlecitinib was associated with early dose-dependent decreases in lymphocyte counts; levels partially recovered toward baseline and remained stable throughout treatment. Early decreases in platelet counts that remained stable and above the lower limit of normal throughout treatment were also observed with ritlecitinib. A small decrease in platelet counts was also reported with abrocitinib (JAK1 inhibitor) in AD [14], whereas an increase in platelet count was observed with baricitinib (JAK1/2 inhibitor) in AA [24], reinforcing the differential impact of drugs in this class of medicine on hematologic laboratory parameters.
In nonclinical, chronic toxicology studies in dogs, reversible axonal dystrophy (swelling of axons without inflammation, degeneration, or gliosis) was observed in the central nervous system (cerebellum) with ritlecitinib at exposures ≥ 7.4 times the approved human dose (50 mg), as well as in the central nervous system (superior olivary nucleus, spinal cord, and lateral lemniscus) and peripheral nervous system (branches of the vagus nerve and/or Auerbach’s and Meissner’s plexuses) at exposures ≥ 14 times the approved human dose of 50 mg [data on file]. At 33 times the approved human dose, axonal dystrophy caused reversible hearing loss and waveform deficits in brainstem auditory evoked potential (BAEP) testing, a standard measure used to assess the auditory pathway in the brainstem. No auditory threshold deficits were noted at 6 months after the end of dosing. Because of these nonclinical results, additional diligence, including standard and specialized neurological and audiological safety evaluations and event adjudication, was conducted proactively across the ALLEGRO program. Clinical data presented in this report, including evaluation of events adjudicated as potential neurological or audiological events of interest, did not demonstrate evidence of neurotoxicity with ritlecitinib treatment. Neurological events of interest did not demonstrate a pattern or features of acute or chronic, cumulative injury to axons in the central nervous system or peripheral nervous system, such as immediate or dose-dependent onset of symptoms, that worsened over time and became unrelenting. The majority of AEs associated with neurological events of interest, such as dysesthesia, hyperesthesia, hypoesthesia, and paresthesia, are commonly reported in the general population, were mild in severity, were considered unrelated to study treatment by the investigator, and resolved without change to study treatment. Of note, a phase 2a, placebo-controlled clinical safety study was conducted to specifically assess potential neurological/neuroaudiological effects of ritlecitinib in adults with AA through assessments of BAEP and intraepidermal axon histology. Results of the phase 2a study further confirmed the clinical safety of ritlecitinib, with no notable effects on nerve fiber counts or swelling of intraepidermal axons or integrity of the auditory pathway in the human brainstem, as assessed by BAEP (data on file, submitted for publication). Altogether, it provides evidence that the axonal dystrophy finding in dogs is not clinically relevant in humans.
Conclusions
This is the first report of integrated safety from multiple trials in AA of the oral, selective dual JAK3/TEC family kinase inhibitor, ritlecitinib. Ritlecitinib is well tolerated and has a safety profile appropriate for chronic clinical use. The ALLEGRO-LT long-term phase 3 study is ongoing; data from longer follow-up will provide further information on the long-term safety profile of ritlecitinib in AA.
Supplementary Information
Below is the link to the electronic supplementary material.
Video abstract: Integrated safety analysis of ritlecitinib in patients aged ≥ 12 years with alopecia areata (MP4 1.03 gb).
Acknowledgements
This study was funded by Pfizer Inc. The authors thank the study participants and their families and caregivers, as well as all investigators. Medical writing support was provided by Hannah Humphries, Ph.D., of Nucleus Global and funded by Pfizer Inc.
Declarations
Funding
This study was sponsored by Pfizer Inc.
Conflict of interest
Brett King: AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz Therapeutics, Bristol Myers Squibb, Concert Pharmaceuticals, Equillium, Horizon Therapeutics, Eli Lilly, Incyte, Janssen Pharmaceuticals, LEO Pharma, Otsuka/Visterra, Pfizer, Regeneron, Sanofi Genzyme, TWi Biotechnology, and Viela Bio. He has served on speaker bureaus for Pfizer. Jennifer Soung: speaker for Regeneron/Sanofi and Ortho Dermatologics; speaker and investigator for Amgen, AbbVie, and Pfizer; speaker, investigator, and advisor for Eli Lilly; investigator and advisor for LEO Pharma; investigator, speaker, and consultant for Novartis; investigator for UCB, Janssen, Kyowa Kirin, KoBio Labs, and Castel Biosciences; investigator and consultant for Dermavant; speaker and consultant for Bristol Myers Squibb, speaker, investigator and consultant for Arcutis. Christos Tziotzios: speaker for LEO Pharma; principal and chief investigator for Pfizer; consultant for Pfizer. Lidia Rudnicka: speaker for AbbVie, L'Oreal, LEO Pharma, Novartis, Pierre Fabre, and Pfizer; advisory board member for LEO Pharma, Janssen, L'Oreal, Novartis, Pfizer, Sanofi, and UCB. Pascal Joly: consultant for Amgen, Argenx, AstraZeneca, Innovaderm, Principia Biopharma, Roche, Sanofi-Regeneron Pharmaceuticals, Thermo Fisher Scientific, Novartis, Pfizer, Lilly, AbbVie, and Janssen Cilag. Melinda Gooderham: investigator, speaker, and/or advisor for AbbVie, Amgen, Akros, AnaptysBio, Arcutis, Bausch Health, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Dermira, Dermavant, Eli Lilly, Galderma, GSK, Incyte, Janssen, Kyowa Kirin, LEO Pharma, MedImmune, Merck, Moonlake, Nimbus, Novartis, Pfizer, Regeneron, Reistone, Roche, Sanofi Genzyme, Sun Pharma, and UCB. Rodney Sinclair: professional services to Aerotech, AbbVie, AstraZeneca, Akesobio, Amgen, Arcutis, Arena, Ascend, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Coherus Biosciences, Connect, Cutanea, Demira, Eli Lilly, Galderma, GSK, Janssen, LEO Pharma, MedImmune, Merck, MSD, Novartis, Oncobiologics, Pfizer, Regeneron, Reistone, Roche, Samson Clinical, Sanofi, Sun Pharma, and UCB. Natasha A. Mesinkovska: professional services to AbbVie, Arena Pharmaceuticals, Bristol Myers Squibb, Concert Pharmaceuticals, Eli Lilly, La Roche Posay, and Pfizer. Carle Paul: consulting fees and/or grants from AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, GSK, Janssen Cilag, LEO Pharma, Eli Lilly, Novartis, Pierre Fabre, Pfizer, Sanofi, and UCB Pharma. Yankun Gong, Helen Tran, Robert Wolk, Samuel H. Zwillich, and Alexandre Lejeune are employees of, and hold stock or stock options in, Pfizer. Susan D. Anway was an employee of Pfizer at the time of the study and holds stock or stock options in Pfizer.
Data availability
Upon request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Ethics approval
Ethics approval was obtained for individual studies included in this integrated safety analysis. The study was performed in accordance with the ethical standards as provided in the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all participants of the individual studies included in this integrated safety analysis.
Patient consent to publish
Patients signed informed consent regarding publishing their data.
Code availability
Not applicable.
Author contributions
All authors contributed to the concept and design of the study, to data analysis and interpretation, to critical revision of the publication, and all authors read and approved the final manuscript.
References
- 1.Islam N, Leung PS, Huntley AC, Gershwin ME. The autoimmune basis of alopecia areata: a comprehensive review. Autoimmun Rev. 2015;14(2):81–89. doi: 10.1016/j.autrev.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Cranwell WC, Lai VW, Photiou L, et al. Treatment of alopecia areata: an Australian expert consensus statement. Australas J Dermatol. 2019;60(2):163–170. doi: 10.1111/ajd.12941. [DOI] [PubMed] [Google Scholar]
- 3.Delamere FM, Sladden MM, Dobbins HM, Leonardi-Bee J. Interventions for alopecia areata. Cochrane Database Syst Rev. 2008; (2):CD004413. [DOI] [PubMed]
- 4.Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82(3):675–682. doi: 10.1016/j.jaad.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 5.Lauron S, Plasse C, Vaysset M, et al. Prevalence and odds of depressive and anxiety disorders and symptoms in children and adults with alopecia areata: a systematic review and meta-analysis. JAMA Dermatol. 2023;159(3):281–288. doi: 10.1001/jamadermatol.2022.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu LY, King BA, Craiglow BG. Alopecia areata is associated with impaired health-related quality of life: a survey of affected adults and children and their families. J Am Acad Dermatol. 2018;79(3):556–558.e551. doi: 10.1016/j.jaad.2018.01.048. [DOI] [PubMed] [Google Scholar]
- 7.Mesinkovska N, King B, Mirmirani P, Ko J, Cassella J. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20(1):S62–S68. doi: 10.1016/j.jisp.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Mostaghimi A, Napatalung L, Sikirica V, et al. Patient perspectives of the social, emotional and functional impact of alopecia areata: a systematic literature review. Dermatol Ther (Heidelb). 2021;11(3):867–883. doi: 10.1007/s13555-021-00512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386(18):1687–1699. doi: 10.1056/NEJMoa2110343. [DOI] [PubMed] [Google Scholar]
- 10.King B, Zhang X, Harcha WG, et al. Efficacy and safety of ritlecitinib in adults and adolescents with alopecia areata: a randomised, double-blind, multicentre, phase 2b–3 trial. Lancet. 2023;401(10387):1518–1529. doi: 10.1016/S0140-6736(23)00222-2. [DOI] [PubMed] [Google Scholar]
- 11.Harigai M. Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology (Oxford) 2019;58(suppl 1):i34–i42. doi: 10.1093/rheumatology/key287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sunzini F, McInnes I, Siebert S. JAK inhibitors and infections risk: focus on herpes zoster. Ther Adv Musculoskelet Dis. 2020;12:1759720X20936059. doi: 10.1177/1759720X20936059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13(5):320. doi: 10.1038/nrrheum.2017.51. [DOI] [PubMed] [Google Scholar]
- 14.Simpson EL, Silverberg JI, Nosbaum A, et al. Integrated safety analysis of abrocitinib for the treatment of moderate-to-severe atopic dermatitis from the phase II and phase III clinical trial program. Am J Clin Dermatol. 2021;22(5):693–707. doi: 10.1007/s40257-021-00618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burmester GR, Cohen SB, Winthrop KL, et al. Safety profile of upadacitinib over 15,000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open. 2023;9(1):e002735. doi: 10.1136/rmdopen-2022-002735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bieber T, Thyssen JP, Reich K, et al. Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. J Eur Acad Dermatol Venereol. 2021;35(2):476–485. doi: 10.1111/jdv.16948. [DOI] [PubMed] [Google Scholar]
- 17.King B, Guttman-Yassky E, Peeva E, et al. A phase 2a randomized, placebo-controlled study to evaluate the efficacy and safety of the oral Janus kinase inhibitors ritlecitinib and brepocitinib in alopecia areata: 24-week results. J Am Acad Dermatol. 2021;85(2):379–387. doi: 10.1016/j.jaad.2021.03.050. [DOI] [PubMed] [Google Scholar]
- 18.ICH Harmonised Tripartite Guideline: Clinical Safety Data Management: Definitions and Standards for Expedited Reporting E2A. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Updated October 24, 1994.
- 19.King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22(3):395–405. doi: 10.1007/s40257-021-00602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipsky A, Lamanna N. Managing toxicities of Bruton tyrosine kinase inhibitors. Hematol Am Soc Hematol Educ Program. 2020;2020(1):336–345. doi: 10.1182/hematology.2020000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Brien SM, Brown JR, Byrd JC, et al. Monitoring and managing BTK inhibitor treatment-related adverse events in clinical practice. Front Oncol. 2021;11:720704. doi: 10.3389/fonc.2021.720704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tillman BF, Pauff JM, Satyanarayana G, Talbott M, Warner JL. Systematic review of infectious events with the Bruton tyrosine kinase inhibitor ibrutinib in the treatment of hematologic malignancies. Eur J Haematol. 2018;100(4):325–334. doi: 10.1111/ejh.13020. [DOI] [PubMed] [Google Scholar]
- 23.George P, Jagun O, Liu Q, et al. Incidence rates of infections, malignancies, thromboembolism, and cardiovascular events in an alopecia areata cohort from a US claims database. Dermatol Ther (Heidelb). 2023;13(8):1733–1746. doi: 10.1007/s13555-023-00937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King B, Mostaghimi A, Shimomura Y, et al. Integrated safety analysis of baricitinib in adults with severe alopecia areata from two randomized clinical trials. Br J Dermatol. 2023;188(2):218–227. doi: 10.1093/bjd/ljac059. [DOI] [PubMed] [Google Scholar]
- 25.Olumiant (baricitinib). Prescribing Information. 2022. https://pi.lilly.com/us/olumiant-uspi.pdf. Accessed 9 Nov 2022.
- 26.Rinvoq (upadacitinib). Prescribing information. 2022. https://www.rxabbviecom/pdf/rinvoq_pipdf. Accessed 22 Mar 2022.
- 27.Sotyktu (deucravacitinib). Prescribing information. 2022. https://packageinserts.bms.com/pi/pi_sotyktu.pdf. Accessed 4 Dec 2023.
- 28.Cinbinqo (abroctinib). Prescribing information. 2023. https://labeling.pfizer.com/ShowLabeling.aspx?id=16652. Accessed 4 Dec 2023.
- 29.Sun C, Su Z, Zeng YP. Association of risk of incident acne and treatment with systemic Janus kinase inhibitors in atopic dermatitis: a systematic review and meta-analysis. Inflamm Res. 2023;72(9):1861–1871. doi: 10.1007/s00011-023-01789-x. [DOI] [PubMed] [Google Scholar]
- 30.Martinez J, Manjaly C, Manjaly P, et al. Janus kinase inhibitors and adverse events of acne: a systematic review and meta-analysis. JAMA Dermatol. 2023;159(12):1339–1345. doi: 10.1001/jamadermatol.2023.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awad SM, Tawfik YM, El-Mokhtar MA, El-Gazzar AF, Abdel Motaleb AA. Activation of Janus kinase signaling pathway in acne lesions. Dermatol Ther. 2021;34(1):e14563. doi: 10.1111/dth.14563. [DOI] [PubMed] [Google Scholar]
- 32.Bieber T, Katoh N, Simpson EL, et al. Safety of baricitinib for the treatment of atopic dermatitis over a median of 1.6 years and up to 3.9 years of treatment: an updated integrated analysis of eight clinical trials. J Dermatol Treat. 2023;34(1):2161812. doi: 10.1080/09546634.2022.2161812. [DOI] [PubMed] [Google Scholar]
- 33.Stein Gold L, Thaçi D, Thyssen JP, et al. Safety of lebrikizumab in adults and adolescents with moderate-to-severe atopic dermatitis: an integrated analysis of eight clinical trials. Am J Clin Dermatol. 2023;24(4):595–607. doi: 10.1007/s40257-023-00792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guttman-Yassky E, Thyssen JP, Silverberg JI, et al. Safety of upadacitinib in moderate-to-severe atopic dermatitis: an integrated analysis of phase 3 studies. J Allergy Clin Immunol. 2023;151(1):172–181. doi: 10.1016/j.jaci.2022.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 36.Verden A, Dimbil M, Kyle R, Overstreet B, Hoffman KB. Analysis of spontaneous postmarket case reports submitted to the FDA regarding thromboembolic adverse events and JAK inhibitors. Drug Saf. 2018;41(4):357–361. doi: 10.1007/s40264-017-0622-2. [DOI] [PubMed] [Google Scholar]
- 37.US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. 2021. https://www.fda.gov/media/151936/download.
- 38.Bieber T, Feist E, Irvine AD, et al. A review of safety outcomes from clinical trials of baricitinib in rheumatology, dermatology and COVID-19. Adv Ther. 2022;39(11):4910–4960. doi: 10.1007/s12325-022-02281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ly S, Manjaly P, Kamal K, Shields A, et al. Comorbid conditions associated with alopecia areata: a systematic review and meta-analysis. Am J Clin Dermatol. 2023;24(6):875–893. doi: 10.1007/s40257-023-00805-4. [DOI] [PubMed] [Google Scholar]
- 40.Gladman DD, Charles-Schoeman C, McInnes IB, et al. Changes in lipid levels and incidence of cardiovascular events following tofacitinib treatment in patients with psoriatic arthritis: a pooled analysis across phase III and long-term extension studies. Arthritis Care Res (Hoboken). 2019;71(10):1387–1395. doi: 10.1002/acr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andreotti AH, Schwartzberg PL, Joseph RE, Berg LJ. T-cell signaling regulated by the Tec family kinase, Itk. Cold Spring Harb Perspect Biol. 2010;2(7):a002287. doi: 10.1101/cshperspect.a002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurosaki T. Regulation of BCR signaling. Mol Immunol. 2011;48(11):1287–1291. doi: 10.1016/j.molimm.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Estupiñán HY, Berglöf A, Zain R, Smith CIE. Comparative analysis of BTK inhibitors and mechanisms underlying adverse effects. Front Cell Dev Biol. 2021;9:630942. doi: 10.3389/fcell.2021.630942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video abstract: Integrated safety analysis of ritlecitinib in patients aged ≥ 12 years with alopecia areata (MP4 1.03 gb).
Data Availability Statement
Upon request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.