Abstract
DbpA is a target for antibodies that protect mice against infection by cultured Borrelia burgdorferi. Infected mice exhibit early and sustained humoral responses to DbpA and DbpB, suggesting that these proteins are expressed in vivo. Many antigens expressed in mammals by B. burgdorferi are repressed in vitro at lower growth temperatures, and we have now extended these observations to include DbpA and DbpB. To confirm that the protective antigen DbpA is expressed in vivo and to address the question of its accessibility to antibodies during infection, we examined B. burgdorferi in blood samples from mice following cutaneous inoculation. B. burgdorferi was visualized by dark-field microscopy in plasma samples from spirochetemic mice, and an indirect immunofluorescence assay showed that these spirochetes were DbpA positive and OspA negative. We developed an ex vivo borreliacidal assay to show that hyperimmune antiserum against DbpA, but not OspA, killed these plasma-derived spirochetes, demonstrating that DbpA is accessible to antibodies during this phase of infection. Blood transferred from spirochetemic donor mice readily established B. burgdorferi infection in naive recipient mice or mice hyperimmunized with OspA, while mice hyperimmunized with DbpA showed significant protection against challenge with host-adapted spirochetes. Antiserum from persistently infected mice had borreliacidal activity against both cultured and plasma-derived spirochetes, and adsorption of this serum with DbpA substantially depleted this killing activity. Our observations show that immunization with DbpA blocks B. burgdorferi dissemination from the site of cutaneous inoculation and suggest that DbpA antibodies may contribute to control of persistent infection.
The normal life cycle of the causative agents for Lyme disease, the related spirochetes classified as Borrelia burgdorferi sensu lato, takes place in the tick vectors, primarily of the genus Ixodes, and vertebrate hosts. In Ixodes, the borreliae reside in the tick midgut and migrate through the tick to the salivary glands during feeding. In the mammalian host, the borreliae initially are deposited in the skin and then disseminate throughout the host to various distal tissues. The arthropod and mammalian environments are quite different, and it would be expected that the spirochete would adapt to these different environments by expression of various proteins useful for localization, nutrient transport, and other functions.
B. burgdorferi proteins whose differential expression has been characterized include outer surface proteins A and B (OspA and OspB), which are expressed in the tick midgut but are down-regulated in the mammalian host, and outer surface protein C (OspC), which is absent in the tick midgut but is expressed in the mammalian host (18, 23, 45). Under in vitro growth conditions, expression of either OspA or OspC may predominate, depending on the strain of B. burgdorferi sensu lato (36). Some genes that exhibit differential expression between tick and mammalian hosts are responsive to growth temperature in vitro, such as OspC, but others, such as OspA, are not (45, 49). Thus, in vitro culture conditions differ from both the tick and mammalian environments. Other proteins whose expression has been found to vary depending on whether the borreliae come from in vitro cultures or are grown in vivo include p21 (15), p35 and p37 (21), EppA (13), the OspF homologues BbK2.10 (2) and pG (51), and lp6.6 (32).
Decorin binding proteins A and B (DbpA and DbpB) are recently described B. burgdorferi lipoproteins (27, 30) which may act as spirochetal adhesins (28). We have demonstrated that immunization of mice with DbpA protected them from challenge with cultured spirochetes (12, 30), and this protection has been recently confirmed by others (19, 29). We and others have shown a high and persistent antibody response against DbpA and DbpB, but not OspA, following low-dose inoculation of cultured organisms (19, 30). In addition, passive administration of DbpA antisera, but not OspA antisera, protected mice for up to 4 days after infection (12, 30). The presence of a persistent DbpA antibody response and the efficacy of passive protection of mice by DbpA antisera after initiation of infection suggest that DbpA is expressed in vivo and, therefore, could persist as a target of antibodies during infection.
The study of the in vivo-expressed borrelial surface proteins is important from the standpoint of understanding the physiology and pathogenesis of the organism in mammals and identifying targets for immunoprophylaxis and immunotherapy. These investigations have been hampered by the low numbers of organisms in most tissues of infected mammals, and the tendency of isolated spirochetes to alter protein expression upon culture. B. burgdorferi is recoverable intact from the blood of spirochetemic animals (8, 40), but this source of host-adapted organisms has not been exploited for the characterization of surface proteins due, in part, to the low number of spirochetes in this fluid. Borreliacidal antibodies are sensitive probes of the surfaces of intact spirochetes, but the direct demonstration of borreliacidal activity of antibodies toward defined in vivo-expressed targets has been lacking. We exploited the borreliacidal activity of DbpA antibodies previously demonstrated against cultured B. burgdorferi to answer the questions of whether this protein is expressed during the early phases of disseminating infection and whether spirochetes are vulnerable to these antibodies.
MATERIALS AND METHODS
Mice.
Pathogen-free female C3H/HeJ (C3H) and male and female C3HSmn.C-PrkdcSCID/J (C3H.SCID), and BALB/cByJ (BALB) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and were used at 6 to 8 weeks of age.
B. burgdorferi and culture conditions.
B. burgdorferi sensu stricto isolates B31 (uncloned) (25) and N40 (cloned) (7) were donated by the laboratories of A. Barbour and S. Barthold, respectively. Spirochetes were propagated in tightly closed containers at 33°C in modified Barbour-Stoenner-Kelly (BSKII) medium (4) overlaid with a gas mixture of 5% O2, 5% CO2, and 90% N2. Cell densities of these cultures were determined by dark-field microscopy at ×400. Batches of BSKII were qualified for infection testing by confirming that they supported the growth of one to five cells of isolate B31. The median infectious doses of the B31 and N40 isolates by subcutaneous (s.c.) administration at the base of the tail were 6 × 101 and 3 × 102, respectively (30).
To study the effects of temperature on the expression of various proteins, 1 ml of log-phase culture of isolate B31 (in vitro passage 4) growing at 33°C was diluted into 45 ml of BSKII medium and incubated as described above at 23, 33, and 37°C. When the culture had reached log phase (cell density of approximately 7 × 107 to 1 × 108 cells/ml; 3 to 4 weeks at 23°C, 4 to 5 days at 33°C and 3 to 4 days at 37°C), the cultures were centrifuged for 15 min at 14,000 × g, washed once with phosphate-buffered saline (PBS), pH 7.4, and resuspended in 2 ml of PBS, and cell densities were determined. The resuspended cells were then frozen and stored at −20°C. The same protocol was also followed using an inoculum culture grown at 23°C prior to the temperature shift.
Expression and purification of recombinant proteins.
DbpA from isolate B31 (DbpAB31) (37), DbpA from isolate N40 (DbpAN40) (37), and OspA from N40 (OspAN40) (20) were expressed in this same host strain as chimeric lipoproteins from the vector pT7Lpp2 (30). DNA fragments encoding the entire sequence of the mature proteins after the cysteine at the site of posttranslational modification were amplified from the respective B. burgdorferi template DNAs by PCR using standard reagents and conditions. The following oligonucleotide primer pairs were used for the PCR: DbpAN40, 5′-CCGGATCCCGGATTAAAAGGAGAAACAAA-3′ (added BamHI site underlined) and 5′-CTGTCTAAGCTTAGTCGACGTTATTTTTGCATTTTTC-3′ (added HindIII and SalI sites underlined); DbpAB31, 5′-CCGGATCCCGGACTAACAGGAGCAACAAAAATC-3′ (added BamHI site underlined) and 5′-CTGTCTAAGCTTATCGACGTTATTTTTGCATTTTTC-3′ (added HindIII site underlined); OspAN40, 5′-CCGGATCCCAAGCAAAATGTTAGCAGCCTT-3′ (added BamHI site underlined) and 5′-CGATCGGTCGACCTATTTTAAAGCGTTTTTATT-3′ (added SalI site underlined). The products were digested with BamHI and HindIII (or BamHI and SalI for OspAN40) and cloned into the comparable sites of pT7Lpp2 by standard techniques (41) to yield plasmids pWCR130 expressing Lpp2:DbpAN40, pWCR134 expressing Lpp2:DbpAB31, and pWCR141 expressing Lpp2:OspAN40.
For production of Lpp2:DbpAN40 or Lpp2:DbpAB31, the appropriate E. coli clone was grown overnight in Luria-Bertani (LB) broth (41) containing 50 μg of kanamycin per ml and 25 μg of chloramphenicol per ml. The cells were diluted 1:100 into LB broth containing 50 μg of kanamycin per ml and grown to an A550 of 0.8 prior to induction of DbpA expression with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Cells were induced for 2 h prior to harvesting at 7,000 × g for 10 min. Cell pellets were suspended in a solution containing 50 mM Tris-HCl (pH 8.0), 5 mM EDTA, 1 mM benzamidine, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), and 5 μg of aprotinin per ml. The suspension was chilled on ice, and cells were lysed by passage through a French pressure cell at 10,000 lb/in2. Cellular debris was removed by centrifugation at 8,000 × g for 10 min. MgCl2 was added to 10 mM prior to the addition of 10 mg of RNase per ml and 5 mg of DNase per ml. A membrane-enriched fraction was then obtained by centrifugation at 100,000 × g for 1 h. The pellet from the centrifugation was then suspended into a solution containing 20 mM NaPO4 (pH 7.4), 100 mM NaCl, and 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) and incubated with mixing for 1 h at room temperature. The detergent-soluble fraction was obtained following a second centrifugation at 100,000 × g for 1 h. The detergent-soluble protein fraction was then added to an equal volume of MacroPrep High Q resin (Bio-Rad, Hercules, Calif.) equilibrated in 20 mM NaPO4 (pH 7.4)–100 mM NaCl. The sample was incubated in batch for 10 min after which the sample was poured into a column and was washed with 6 volumes of the equilibration buffer. The column chromatography and remaining steps were at room temperature. The flowthrough from the column was then concentrated with a PM30 membrane (Millipore, Bedford, Mass.) in a stirred cell concentrator, and the buffer was exchanged to a solution containing 10 mM NaPO4, 5 mM MgCl2, and 1% CHAPS, pH 7.4. The sample was then added to Cellufine sulfate resin (Millipore) equilibrated in the same buffer and incubated for 1 h at room temperature. The resin was poured into a column and washed with equilibration buffer, followed by a linear 0 to 500 mM NaCl gradient in the equilibration buffer. The protein eluted between 125 mM and 250 mM NaCl. The purified protein was concentrated in a stirred cell with a PM30 membrane and was buffer exchanged into PBS containing 10 mM CHAPS. The final protein concentration was determined by bicinchoninic acid (BCA) assay (Pierce Chemical Company, Rockford, Ill.).
Lpp2:OspAN40 was isolated from Escherichia coli B21(DE3)pLysS/pWCR141. The cells were grown as described above, and the lysate was prepared as described above with a change in the suspension buffer to a solution containing 20 mM NaPO4 (pH 7.4), 10 mM NaCl, 1 mM benzamidine, 0.2 mM PMSF, 4 μg of aprotinin per ml, 10 mg of RNase per ml, and 5 mg of DNase per ml. The cells were lysed, and the membrane-enriched fraction was solubilized in a solution containing 10 mM NaPO4 (pH 7.4), 10 mM NaCl, and 5% CHAPS and was isolated as described above. Following removal of insoluble material by centrifugation, the CHAPS-soluble supernatant was applied to a MacroPrep High Q column (2.5 by 4 cm) preequilibrated in a solution containing 10 mM NaPO4 (pH 7.4), 10 mM NaCl, and 15 mM CHAPS. The sample was passed over the column and was washed with 2 column volumes of the equilibration buffer. The Lpp2:OspAN40 in the flowthrough fraction was adjusted to pH 4.2 with acetic acid, concentrated in a stirred cell concentrator with a PM30 membrane, and buffer exchanged into 20 mM sodium acetate (pH 4.2)–15 mM CHAPS, and the concentrated sample was then applied to a MacroPrep High S column (Bio-Rad) (2.5 by 6 cm) column equilibrated in this same buffer. The sample was washed with equilibration buffer, and then a pH gradient from pH 4.2 to pH 5.5 was generated by using solutions containing 25 mM sodium acetate, 50 mM NaCl, and 15 mM CHAPS. Additional protein was eluted from the column by an increase in the NaCl concentration to 150 mM and an increase in the pH to 7.4 with sodium phosphate. The purified protein was concentrated with a YM10 (Millipore) ultrafiltration membrane, and the sample was dialyzed against PBS containing 8 mM CHAPS. The CHAPS concentration in the concentrated protein samples was determined by heating the samples in 75% (vol/vol) sulfuric acid at 70°C for 30 min, which results in the production of a fluorescent product (24). Measurement of the fluorescence was performed by using an excitation wavelength of 480 nm and an emission wavelength of 520 nm with a F-2000 fluorescence spectrophotometer (Hitachi, San Jose, Calif.). Purified protein samples were found to have CHAPS concentrations between 10 and 13 mM.
Recombinant OspAB31 lipoprotein was expressed in E. coli BL21(DE3)pLysS from plasmid pSO3, and recombinant Lpp1:DbpA297 and Lpp2:DbpAN40(His)6 lipoproteins were expressed in E. coli BL21(DE)3/pLysS from plasmids pMSH24 and pWCR139, respectively, and were purified as described previously (30).
A CHAPS-soluble extract of E. coli BL21(DE3)pLysS/pT7Lpp2 was made under the same conditions as those used for solubilization of the recombinant lipoproteins and was used as a negative-control immunogen in some experiments.
(His)6DbpAB31 and (His)6DbpB297 (27, 30) were expressed in E. coli M15/pREP4 from the vector pQE30 (Qiagen, Inc., Valencia, Calif.). For expression of (His)6PAL, a DNA fragment encoding the mature region of the Haemophilus influenzae peptidoglycan-associated lipoprotein (PAL) (16) was PCR amplified from H. influenzae KW20 template DNA and cloned into pQE30. (His)6PAL was expressed in E. coli M15/pREP4, extracted in 6 M guanidinium-HCl, purified on nitrilotriacetic acid (NTA)-agarose (Qiagen), and renatured by dialysis into 2 M guanidinium-HCl followed by dilution into PBS.
The various recombinant proteins were purified to >90% homogeneity as estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining.
Preparation and recovery of spirochetemic phase B. burgdorferi.
Blood-borne borreliae used in infection and in ex vivo assays were obtained from groups of 10 mice infected by s.c. inoculation at the base of the tail with 100 μl of cultured B. burgdorferi at a concentration of 107/ml. Four or six days after infection, the mice were killed by CO2 asphyxiation and blood was drawn from the mice by cardiac puncture. A 2% solution of EDTA (disodium salt) solution (Sigma Chemical, St. Louis, Mo.) was used as an anticoagulant. The blood was immediately put into a tube containing approximately 50 μl of anticoagulant/ml of blood, and the blood and anticoagulant were mixed and pooled. For infection of mice with blood-borne borreliae, 100 μl of blood from infected mice was injected s.c. at the base of the tail. To study the blood-borne borreliae in ex vivo assays, the blood was centrifuged at 1,500 × g for 20 min, and the plasma containing the borreliae was drawn off, pooled, and used immediately.
Antibody reagents.
Antisera were pooled from mice with culture-confirmed infections 16 weeks after s.c. inoculation with 102 cultured B. burgdorferi B31 (serum used for borreliacidal assays) or 4 weeks after s.c. inoculation with 103 cultured B. burgdorferi B31 (serum used for indirect immunofluorescence). Antisera against recombinant proteins were obtained from mice immunized twice intraperitoneally with 5 μg of OspAB31, 10 μg of Lpp2:OspAN40, 20 μg of (His)6DbpAB31, 20 μg of Lpp1:DbpA297, 20 μg of Lpp2:DbpAN40(His)6, or 20 μg of (His)6DbpB297 emulsified in Freund’s adjuvant (complete for priming and incomplete for boosting). The DbpA-specific monoclonal antibody (MAb) 7D2B.3F5 (immunoglobulin G2b [IgG2b]) was prepared as described previously (30). Hybridoma supernatants of the OspA-specific MAb H5332 (IgG1) and the FlaB MAb H9724 (IgG2a) were obtained from D. Denee Thomas. The OspC MAb DC1.9.25 (9.25) was prepared by standard hybridoma technology (53) by immunization of BALB mice with B. burgdorferi B31 outer membranes (prepared by the procedure described by Bledsoe et al. [10]) and fusion of splenic cells from these mice with P3X63Ag8U.1 myeloma cells and cloning of the fused cells by limiting dilution. The specificity of the 9.25 MAb was determined by screening a library of low-passage B. burgdorferi B31 total genomic DNA. To generate the library, total borrelial DNA was partially digested with Tsp509I, treated with shrimp alkaline phosphatase, and fragments larger than 6 kb were isolated from an agarose gel and ligated to EcoRI-digested pBluescript II SK+ (Stratagene, La Jolla, Calif.) by standard procedures (41). Positive clones were sequenced and found to express OspC.
Immunoblotting.
Frozen borrelia prepared from spirochetes grown at different temperatures described above were thawed and were lysed by sonication for 5 min by using a Vibra Cell Sonicator (Sonics & Materials, Danbury, Conn.) fitted with a microtip (setting of 5; 50% duty cycle). The cell lysates (at a protein level of 9 to 10 μg/lane, from approximately 5 × 107 cells) were boiled in sample buffer containing 1% SDS–2.5% 2-mercaptoethanol and then run by SDS-PAGE through 3% stacking and 12.5% acrylamide resolving gels. Gels were electroblotted onto nitrocellulose membranes, and lanes were probed with H9724 (anti-FlaB), H5332 (anti-OspA), 9.25 (anti-OspC), 7D2B.3F5 (anti-DbpA), or mouse antiserum raised against (His)6DbpB297, or a cocktail containing these five antibodies. The antibody concentrations were adjusted to give similar signal intensities when used for detection of their targets in lysates of spirochetes grown at 37°C. Bound antibodies were detected by incubation of the membrane with horseradish peroxidase-conjugated goat anti-mouse IgG followed by fluorography with ECL chemiluminescence reagents (Amersham Corp., Arlington Heights, Ill.).
Recombinant P39B31 (BmpA) protein was expressed as a fusion with maltose binding protein and was used in an immunoblot format to detect P39 antibodies in mice after challenge inoculation with B. burgdorferi B31 (30). (His)6DbpB297 was used in place of P39B31 in immunoblots for detection of antibodies form mice challenged with B. burgdorferi N40, as this protein was determined to be more sensitive than P39B31 (data not shown).
Immunofluorescence assay.
B. burgdorferi B31 were recovered from in vitro cultures or from plasma taken from C3H mice 4 or 6 days after s.c. inoculation. Plasma-derived borreliae were concentrated prior to staining by adding 500 μl of BSKII medium to 500 μl of plasma containing approximately 40 to 50 borreliae and centrifuging for 7 min at 4,000 × g, and resuspending the pellet in 100 μl of BSKII medium. The cultured borreliae were diluted to a concentration of 2 × 108/ml in 100 μl of BSKII medium. Antisera from mice immunized with OspAB31 or (His)6DbpAB31 or by infection with isolate B31, were diluted 1:25, added to an equal volume of cultured or plasma-derived spirochetes, and incubated for 1 h at 4°C. The spirochetes were then washed by adding 1 ml of a solution of PBS containing 1% bovine serum albumin (PBS/BSA) or of 20 mM N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES) and 10 mM NaCl (pH 7.6) containing 1% BSA and centrifuging for 7 min at 4,000 × g. Spirochetes were resuspended in 100 μl of PBS/BSA or HEPES-NaCl-buffered BSA, 100 μl of phycoerythrin-conjugated goat anti-mouse antibody diluted 1:100 was added, and the cells were incubated in the dark for 1 h at 4°C. After incubation, the cells were washed again with 1 ml of PBS/BSA. After the cells were washed, they were suspended in 20 to 50 μl of Hanks’ balanced salt solution containing 1% BSA (HBSS/BSA) and wet-mount slides of unfixed spirochetes were examined at ×1,000 magnification by fluorescence microscopy with a 510- to 560-nm-wavelength excitation filter and a 590-nm-wavelength barrier filter, and by dark-field microscopy with a Nikon Labophot-2 microscope and Episcopic-Fluorescence attachment EF-D (Nikon, Tokyo, Japan).
Depletion of DbpA antibodies from antisera of infected mice.
Depletion of antibodies from infected-mouse antiserum was performed by a procedure modified from that described by Gu et al. (26) by applying mouse serum to nickel-chelate-nitrilotriacetic acid (Ni-NTA) spin columns (Qiagen, Inc.) bound with (His)6DbpAB31 or (His)6PAL (an irrelevant protein). The columns were prepared by diluting 150 μg of protein in 50 mM NaH2PO4 buffer (pH 8.0) containing 300 mM NaCl and 10 mM imidizole, and applying the protein to Ni-NTA spin columns equilibrated with the same buffer and centrifuging for 2 min at 700 × g. The columns were washed once with 50 mM NaH2PO4 buffer (pH 8.0) containing 300 mM NaCl and 20 mM imidizole and once with 50 mM Tris-HCl buffer (pH 7.4) containing 150 mM NaCl. Antisera pooled from mice infected by inoculation with 102 B. burgdorferi B31 collected 16 weeks after infection were diluted 1:10 and applied to the columns, which then were centrifuged for 2 min at 700 × g. The flowthrough was collected and used in the growth inhibition assay described below. A sample of diluted, unadsorbed serum was further diluted 1:2.5 to account for the nonspecific dilution effects of the columns [as measured by the reduction of DbpA binding activity after passage through the (His)6PAL column] and also was used in the growth inhibition assay. The serum and column flowthroughs were tested for reactivity against Lpp2:DbpAB31 and OspAB31 by an enzyme-linked immunosorbent assay (ELISA) as described previously (30).
Plating and ex vivo antibody-mediated growth inhibition.
The solid medium for B. burgdorferi growth (BSKII agarose) was prepared by a procedure modified from that described by Samuels et al. (42). The BSKII agarose was made from 240 ml of P-BSK (100 g of BSA [fraction V; Miles/Bayer, Kankakee, Ill.], 7.5 g of Neopeptone [Difco Laboratories, Detroit, Mich.], 9 g of HEPES, 1.1 g of sodium citrate, 10 g of glucose, 109 ml of a 100 mM solution of sodium pyruvate, 1 g of N-acetyl-d-glucosamine, 3.3 g of sodium bicarbonate, 3.8 g of TC Yeastolate [Difco Laboratories], and NaOH to pH 7.5), 38 ml of 10× CMRL-1066 (without l-glutamine and sodium bicarbonate; Life Technologies, Rockville, Md.), 12 ml of rabbit serum (trace hemolyzed; Pel-Freeze, Rogers, Ark.), 20 ml of fresh 5% sodium bicarbonate, and 200 ml of 1.7% agarose (high-strength analytical grade; Bio-Rad Laboratories), 13.35 mg of amphotericin B per liter, 1.5 mg of phosphomycin per liter, and 14.65 mg of rifampin per liter. Before the addition of agarose, the solution was filter sterilized and warmed to 55°C. The agarose was autoclaved and held at 55°C. After the addition of agarose, the ingredients were mixed and used immediately.
Plates used for borrelial culture were prepared by pouring 10 ml of BSKII agarose into polystyrene petri dishes (15 by 100 mm) and allowing the agarose to solidify. To determine the sensitivity of in vitro-grown spirochetes and ex vivo spirochetes to killing by DbpA antiserum, B. burgdorferi B31 was obtained from log-phase cultures or recovered from plasma samples taken from C3H or C3H.SCID mice 4 or 6 days after s.c. inoculation with 106 organisms. Plasma (volume of 75 or 100 μl) was mixed with OspAB31 or (His)6DbpAB31 antiserum and BSKII medium to achieve a final serum dilution of 1:50 in a volume of 200 μl. After incubation for 60 to 90 min at 23 or 33°C (results were equivalent for both temperatures [data not shown]), 10 ml of freshly made BSKII agarose at 50°C was added to the spirochete-antiserum mixture, and overlaid on the prepared BSKII agarose plates. The plates were allowed to solidify for 2 h at 23°C, wrapped with Duraseal (Marsh Biomedical, Rochester, N.Y.), placed into a BBL Gas Pak chamber charged with a BBL CampyPak Plus Microaerophilic System Envelope with Palladium Catalyst (Becton Dickinson, Cockeysville, Md.), and incubated at 33°C. The plates were checked for growth after 10 to 12 days, and borrelia colonies were counted.
To evaluate the sensitivity of borreliae grown at low temperature to DbpA antibody-mediated killing, 100 μl of B. burgdorferi B31 or N40 obtained from log-phase cultures grown at 23°C were mixed with OspAB31, Lpp2:OspAN40, Lpp2:DbpA297, or Lpp2:DbpAN40(His)6 antiserum and BSKII medium to a final volume of 200 μl and a final antiserum concentration of 1:50, incubated for 60 to 90 min at 23°C, and plated as described above.
Antiserum from infected mice, with or without adsorption, and uninfected mouse sera were tested for their ability to kill in vitro-grown or blood-borne borreliae. Sera adsorbed with DbpA or PAL and unadsorbed sera from infected or uninfected mice were used at a final dilution of 1:25 or 1:125 in 100 μl of BSKII medium. The antiserum preparations were mixed with 100 μl of cultured B. burgdorferi B31 diluted to 103/ml or 75 μl of plasma samples from spirochetemic C3H.SCID mice, incubated at 33°C for 90 min, and plated as described above.
Immunization and challenge.
Proteins used for immunization (10 μg for DbpA and OspA and 2.5 μg for E. coli detergent extract) were emulsified 1:1 (vol:vol) with Freund’s adjuvant (Difco Laboratories) and administered intraperitoneally at a volume of 100 μl. The primary immunization was in complete Freund’s adjuvant, followed by immunization 4 weeks later with incomplete Freund’s adjuvant. The amount of E. coli detergent extract protein used as a negative control was chosen to be in excess of the amount of contaminants seen in the various protein preparations. To determine the infection status of inoculated mice, mice were killed by CO2 asphyxiation 2 weeks after inoculation, and various tissues (urinary bladders, tibiotarsal joint, ear, blood, and inoculation site skin, as indicated) were removed and placed in tubes containing BSKII medium plus 1.4% gelatin, 13 μg of amphotericin B per ml, 1.5 μg of phosphomycin per ml, and 15 μg of rifampin per ml. Borrelial outgrowth was determined 2 weeks later by examining the culture medium by dark-field microscopy. If the cultures of any of the tissue samples showed borrelial growth, the mice were scored as positive for infection. To determine significance, infection data were analyzed by Fisher’s exact test, comparing the experimental groups with the corresponding negative-control (E. coli extract) cultures. Blood taken from harvest was also analyzed for P39 or DbpB seroconversion by immunoblotting.
RESULTS
Expression of DbpA and DbpB in vitro is temperature regulated.
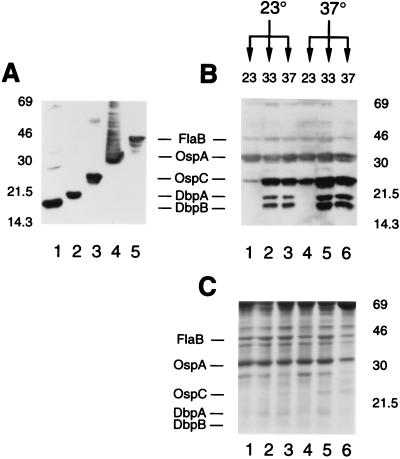
Several B. burgdorferi genes that are expressed in the mammalian host also show temperature-related differential expression in vitro. The first example of this was OspC, which was shown to be expressed more abundantly at high temperatures than at low temperatures (45, 49). We wanted to determine whether expression of DbpA and DbpB was also regulated by temperature. In this experiment, borreliae were cultured initially at 23 or 37°C and then passaged again at 23, 33, and 37°C, and protein expression was evaluated by SDS-PAGE and immunoblotting with antibodies against FlaB, OspA, OspC, DbpA, and DbpB (Fig. 1A). As shown in Fig. 1B, DbpA and DbpB both were expressed at 33 and 37°C (lanes 2, 3, 5, and 6), but expression was low or absent at 23°C (lanes 1 and 4), making their expression similar to that of OspC. Expression of OspA and FlaB was not responsive to temperature, as expected, and the comparable signal strengths for the samples confirm that equivalent levels of protein were loaded in the lanes (Fig. 1B). Borrelial proteins are slightly underrepresented in lane 6, Fig. 1B and 1C, presumably due to an excess of serum-derived protein in this sample.
FIG. 1.
In vitro expression of DbpA and DbpB is regulated by temperature. (A) Immunoblot detection of proteins expressed by B. burgdorferi grown at 37°C using antibodies against DbpB (lane 1), DbpA (lane 2), OspC (lane 3), OspA (lane 4), and FlaB (lane 5). (B and C) B. burgdorferi growing at 23°C was further passaged at this temperature (lanes 1) or at higher growth temperatures (lanes 2 and 3). Likewise, cultures growing at 37°C (lanes 6) were passaged at lower temperatures (lanes 4 and 5). Protein expression was evaluated by immunoblotting with a cocktail of the five antibodies (B) or by SDS-PAGE and Coomassie blue staining (C).
DbpA, but not OspA, is expressed by plasma-derived, uncultured borreliae.
We next examined unfixed, in vivo-adapted B. burgdorferi, recovered from plasma samples 4 or 6 days after s.c. inoculation by indirect immunofluorescence to obtain direct evidence of DbpA expression. Cultured spirochetes were also examined for comparison. Since it was unknown, at the outset, whether DbpA or OspA antiserum would label spirochetes harvested from blood samples from spirochetemic mice, we also used antisera from borrelia-infected mice which would presumably react with a variety of in vivo-expressed proteins. Cultured borreliae stained brightly with antisera from infected mice or with DbpA and OspA antisera (Fig. 2). Dark-field microscopy confirmed the presence of low levels of spirochetes in plasma samples from mice 4 or 6 days after inoculation. OspA antiserum failed to label these spirochetes, but they were detectable with DbpA antiserum and infected-mouse serum. Antiserum labeling of plasma-derived spirochetes from 4 days (data not shown) and 6 days after inoculation was similar. These data indicate that expression of DbpA in plasma-derived borreliae continued at least 6 days after initiation of infection and that OspA was absent from these borreliae.
FIG. 2.
Plasma-derived, uncultured B. burgdorferi express DbpA, but not OspA. B. burgdorferi B31 from in vitro cultures (grown in BSKII medium) or recovered from plasma samples from spirochetemic mice were incubated first with 1:50 dilutions of antisera from B31-infected mice or hyperimmune antiserum against OspAB31 or (His)6DbpAB31 and then with phycoerythrin-conjugated goat anti-mouse antibodies. Wet-mount slides of unfixed spirochetes were examined at ×1,000 magnification by fluorescence microscopy under UV light and by dark-field microscopy.
Ex vivo sensitivity of plasma-derived B. burgdorferi to killing by DbpA antisera.
Preliminary estimates of the cell density of B. burgdorferi in plasma samples from spirochetemic C3H mice were on the order of 102 spirochetes/ml at days 4 and 6 postinoculation. Thus, the indirect immunofluorescence assay may not have been sufficiently sensitive to detect a minor population of spirochetes expressing OspA, but not DbpA, during early spirochetemia, or to quantify them if they existed. Although immunofluorescence assays of unfixed spirochetes reflect primarily surface labeling (11, 14), we could not exclude the possibility of antibodies binding to subsurface proteins which had been artificially exposed on permeabilized spirochetes. For these reasons, we developed a separate assay that exploited the vulnerability of live and intact spirochetes to borreliacidal antibodies against DbpA and OspA to confirm the surface localization of these proteins on host-adapted spirochetes and to quantify the number of spirochetes expressing these proteins. Borreliae derived from plasma samples from C3H mice taken 4 or 6 days after infection were uniformly sensitive to killing by DbpA, but not OspA, antibodies (Table 1). The borreliacidal effect of DbpA antiserum on plasma-derived spirochetes was seen only when the spirochetes were preincubated with the antiserum prior to dilution into molten BSKII agarose. DbpA or OspA antiserum added directly to the BSKII agarose overlay with the spirochetes did not affect the colony counts, demonstrating that the small amounts of antisera in the overlay were insufficient to prevent colony formation (data not shown). We repeated this experiment with plasma samples harvested from spirochetemic C3H.SCID mice. C3H.SCID mice demonstrated a 10-fold-higher spirochetemia at 6 days after infection, but the borreliae derived from these mice were still killed by antiserum raised against DbpA (Table 1). These experiments confirmed that DbpA was present on the surfaces of in vivo-derived borreliae and that DbpA remained as a target in the in vivo-adapted spirochetes at this stage of infection.
TABLE 1.
Ex vivo sensitivity of plasma-derived B. burgdorferi B31 to killing by OspA and DbpA antisera
| Expt, plasma source, and day | No. of borrelia surviving antiserum treatmenta (CFU/ml of plasma)
|
||
|---|---|---|---|
| No serum | OspA antiserum | DbpA antiserum | |
| Expt 1 | |||
| C3H | |||
| Day 4 | 80 | 60 | 0 |
| Day 6 | 100 | 90 | 0 |
| Expt 2 | |||
| C3H | |||
| Day 4 | 140 | 80 | 0 |
| Day 6 | 115 | 100 | 0 |
| C3H.SCID | |||
| Day 4 | 380 | 300b | 0 |
| Day 6 | 1,400 | 1,370b | 5c |
Treatment was with 1:50 dilution of serum for 45 to 60 min at 23°C prior to plating. When used, serum was mouse OspAB31 antiserum or (His)6DbpAB31 antiserum. For experiment 1, challenge was 106 B. burgdorferi s.c. on day 0, and values are means from 0.05-ml plasma samples plated in triplicate. For experiment 2, challenge was 106 B. burgdorferi s.c. on day 0 and values are means from 0.1-ml plasma samples plated in duplicate.
Single-sample determination.
One CFU detected in two 0.1-ml plasma samples.
Borreliae grown at 23°C are killed by OspA antiserum, but not DbpA antiserum.
As shown above, DbpA is not expressed on spirochetes grown at low temperature (23°C). Since only surface-expressed proteins can act as targets for antibody-mediated killing in vitro, we would expect that antibodies to DbpA would not kill borreliae grown at 23°C. B. burgdorferi isolates B31 and N40 grown at 23°C were both sensitive to killing by OspA antisera but not DbpA antisera, indicating that DbpA was not expressed at levels sufficient for it to be a target for killing by DbpA antibodies (Table 2). In contrast, B. burgdorferi B31 and N40 grown at 33°C in liquid BSKII medium were previously shown to be vulnerable to killing by both DbpA297 antiserum and OspAB31 antiserum (30). These results provide additional evidence that the phenotype of the input spirochetes determines their vulnerability to borreliacidal antibodies, rather than the phenotype they acquire during colony formation.
TABLE 2.
Sensitivity of B. burgdorferi grown at 23°C to killing by OspA and DbpA antisera
| Isolate | No. of borrelia surviving antiserum treatmenta (CFU/ml)
|
||
|---|---|---|---|
| No serum | OspA antiserum | DbpA antiserum | |
| B31 | 98 | 0 | 86 |
| N40 | 115 | 0 | 100 |
Treatment was with 1:50 dilution of serum for 60 to 90 min at 23°C prior to plating. When used, serum was OspAB31 or Lpp2:OspAN40 antiserum or Lpp1:DbpA297 or Lpp2:DbpAN40(His)6 antiserum. For B31, the values are means from 0.25-ml samples plated in duplicate. For N40, the values are means from 0.1-ml samples plated in duplicate.
DbpA-immunized mice are protected from challenge by blood-borne borreliae.
To confirm that host-adapted spirochetes in early infection are vulnerable to DbpA antibodies in vivo, we asked whether infection of mice with blood-borne spirochetes could be prevented by prior immunization with DbpA. C3H mice immunized with Lpp2:DbpAB31 or OspAB31 lipoprotein were challenged with 100-μl blood samples from syngeneic donor mice inoculated with 106 B. burgdorferi B31. Mice immunized with DbpA showed significantly reduced incidence of infection compared to mice immunized with a control E. coli detergent-phase extract or with OspA, when challenged with blood-borne borreliae taken from mice either 4 or 6 days after infection (Table 3, P < 0.0095 and P < 0.0015, respectively). Plating a sample of the blood used as the challenge inoculum showed that the blood taken 4 days postinoculation contained 6 CFU/100 μl, and blood at 6 days postinoculation contained 8 CFU/100 μl. Others have shown that mice inoculated with live, but not killed, borreliae demonstrate a P39 antibody response (38). We saw a good correlation between the presence of infection and antibodies to P39. The reduced level of protection in the DbpA-immunized group challenged with cultured B31 borreliae was not consistent with results observed earlier (30; also data not shown) and may have reflected a change in virulence related to culture conditions of the inoculum similar to that observed by others (33).
TABLE 3.
Immunization with DbpA, but not OspA, protects mice from challenge with blood-borne B. burgdorferi B31
| Source of borrelia and day postinoculation or no. | Immunogen | Prevalence of P39 IgG | Prevalence of infectiona | P value |
|---|---|---|---|---|
| Blood, day 4 | Lpp2:DbpAB31 | 3/10 | 3/10 | 0.0095 |
| OspAB31 | 8/10 | 10/10 | 0.50 | |
| E. coli extract | 9/10 | 9/10 | ||
| Blood, day 6 | Lpp2:DbpAB31 | 3/10 | 3/10 | 0.0015 |
| OspAB31 | 8/10 | 9/10 | 0.50 | |
| E. coli extract | 10/10 | 10/10 | ||
| Culture (104) | Lpp2:DbpAB31 | 4/5 | 3/5 | 0.22 |
| OspAB31 | 0/5 | 1/5 | 0.024 | |
| E. coli extract | 5/5 | 5/5 |
Cumulative results of bladder, tibiotarsal joint, and ear tissue cultures.
To confirm and extend these protection results, this experiment was repeated with B. burgdorferi N40. C3H mice were immunized with Lpp2:DbpAN40 and Lpp2:OspAN40 and then challenged with blood-borne and cultured B. burgdorferi N40. In this experiment, the inoculation site and blood were also cultured to evaluate localized and disseminating infection. As was the case with B. burgdorferi B31, DbpA-immunized mice were protected from challenge with blood-borne B. burgdorferi N40 taken from mice either 4 or 6 days after infection (P < 0.0095 and P < 0.0027, respectively), and OspA-immunized mice were not protected (Table 4). The spirochete density in blood samples from the donor C3H mice infected with B. burgdorferi N40 was nearly identical to that of mice infected with B. burgdorferi B31. In contrast to our experiment using strain B31, both DbpA and OspA immunization conferred protection to mice with a homologous challenge of cultured borreliae. We have found previously that antisera from mice infected with strain N40 reacted only weakly with heterologous P39B31, so we did not attempt to correlate infection with P39 IgG responses. Since there is a 98% sequence identity between DbpB from strains 297 and N40 (37) and there is a vigorous immune response against DbpB in infected mice (30), we evaluated DbpB297 as a seroconversion marker for infection. In general, the presence of DbpB antibodies correlated well with the infection results, although some culture-negative mice had a weak DbpB response (Table 4).
TABLE 4.
Immunization with DbpA, but not OspA, protects mice from challenge with blood-borne B. burgdorferi N40
| Source of borrelia and day postinoculation or no. | Immunogen | Prevalence of DbpB IgG | Prevalence of infectiona | P value |
|---|---|---|---|---|
| Blood, day 4 | Lpp2:DbpAN40 | 0/10 | 1/10 | 0.0095 |
| Lpp2:OspAN40 | 9/10 | 7/10 | 0.37 | |
| E. coli extract | NDb | 7/10 | ||
| Blood, day 6 | Lpp2:DbpAN40 | 1/10 | 1/10 | 0.0027 |
| Lpp2:OspAN40 | 10/10 | 8/10 | 0.42 | |
| E. coli extract | 9/10 | 8/10 | ||
| Culture (104) | Lpp2:DbpAN40 | 0/5 | 0/5 | 0.0040 |
| Lpp2:OspAN40 | 4/5 | 1/5 | 0.024 | |
| E. coli extract | +c | 5/5 |
Cumulative results of bladder, tibiotarsal joint, ear tissue, blood, and inoculation site skin cultures.
ND, not done.
Pooled sera tested.
The majority of the antibody-mediated borreliacidal activity of sera from infected mice is due to DbpA antibodies.
Mice infected by limiting doses of cultured B. burgdorferi produce high levels of serum antibodies to DbpA and other in vivo-expressed antigens, but few or no antibodies to OspA (19, 30). Infected-mouse serum has strong biological activity against cultured B. burgdorferi (5, 6, 17) and can label plasma-derived B. burgdorferi (Fig. 2). To evaluate the contribution of these antibodies to the biological potency of infected-mouse serum, we depleted DbpA-reactive antibodies from antisera pooled from mice 16 weeks after cutaneous inoculation with 102 spirochetes by adsorption with recombinant DbpA. Infected-mouse serum at a dilution of 1:50 or 1:250, was highly potent at killing spirochetes from plasma samples from infected C3H.SCID mice and even more potent against a similar number of cultured spirochetes (Table 5). Adsorption of this antiserum with an irrelevant recombinant H. influenzae protein had little effect on DbpA antibodies or borreliacidal activity. Adsorption with DbpA reduced antibodies to this protein by 32-fold and reduced the borreliacidal activity of the infected-mouse serum toward both plasma-derived and cultured B. burgdorferi to that of uninfected-mouse serum.
TABLE 5.
The borreliacidal activity of infected-mouse serum toward blood-phase and cultured B. burgdorferi is depleted by adsorption with DbpA
| Antiserum or serumb | ELISA endpoint titer
|
Spirochetes surviving antiserum treatmenta
|
||||
|---|---|---|---|---|---|---|
| Blood-phase spirochetes plus serum diluted:
|
Cultured spirochetes plus serum diluted:
|
|||||
| DbpA IgG | OspA IgG | 1:50 | 1:250 | 1:50 | 1:250 | |
| Unadsorbed | 1,024,000 | <100 | 3 | 5 | 0 | 0 |
| H. influenzae PAL-adsorbed | 512,000 | NDc | 2 | 3.5 | 0 | 0 |
| DbpA-adsorbed | 32,000 | ND | 95 | 120 | 97 | 94 |
| Uninfected-mouse serum | <500 | ND | 128 | 112 | 104 | 104 |
0.1 ml of cultured B. burgdorferi B31 diluted to approximately 103/ml or 0.075 ml of plasma samples from spirochetemic C3H.SCID mice were incubated with antiserum at 33°C for 90 min and then plated on BSKII agarose for enumeration of viable spirochetes as CFU. Values are means of duplicate platings.
Antisera pooled from C3H mice at week 16 of B. burgdorferi B31 infection was used untreated or after adsorption with PAL or DbpA. Uninfected-mouse serum was pooled from naive, uninfected C3H mice.
ND, not done.
DISCUSSION
The ability to directly evaluate the in vivo phenotype of microbial pathogens greatly facilitates the understanding of pathogenesis, which, in turn, can lead to improved strategies for immunoprophylaxis and immunotherapy. In vivo expression of certain B. burgdorferi genes has been directly confirmed by reverse transcription-PCR (15, 21, 34) or by immunofluorescence assay with specific antibody (15, 34). Our previous work (30) suggested that the protective antigen DbpA is expressed in vivo. To obtain direct evidence of in vivo expression of DbpA and determine its location on host-adapted B. burgdorferi, we used indirect immunofluorescence and a novel assay for the ex vivo evaluation of surface protein expression on plasma-derived spirochetes based on the accessibility of these proteins to borreliacidal antibodies. We show here that DbpA, but not OspA, is expressed by B. burgdorferi in the early spirochetemic phase and that these spirochetes are vulnerable to killing by DbpA antibodies.
A key question at the outset of this study was whether B. burgdorferi remains vulnerable to DbpA antibodies later than 4 days after s.c. inoculation into mice, prompted by our earlier observations that passive administration of DbpA antiserum to mice 5 days or more after s.c. inoculation did not protect mice (12, 30). We found that B. burgdorferi in the bloodstream of mice at 4 and 6 days after dermal inoculation remains vulnerable to DbpA antibodies. Additionally, spirochetes failed to persist at the site of dermal inoculation in DbpA-immunized mice, suggesting that B. burgdorferi is vulnerable to DbpA antibodies during localized infection, as well as in the initial stages of dissemination. The fact that spirochetes in the blood of mice at days 4 and 6 of infection are vulnerable to DbpA antibodies implies that some spirochetes have left the bloodstream as early as day 5 and have reached extravascular sites where they are more resistant to killing by DbpA antibodies.
B. burgdorferi in the hematogenous phase of dissemination from a dermal inoculation site provide a source of spirochetes that are recoverable in a viable, intact, and in vivo-adapted state and are relevant to the study of pathogenesis of Lyme borreliosis. We used dermal inoculation of mice by syringe for the production of blood-phase B. burgdorferi for phenotypic characterization because spirochetemia is reliably produced in mice by this method (8) and can achieve blood densities in excess of 103 spirochetes per ml in SCID mice (40). The next challenge will be to confirm that B. burgdorferi achieves the same adaptive state in the bloodstream when dermal inoculation occurs by tick bite. Following tick bites, B. burgdorferi is recoverable from blood samples of humans in the early stages of Lyme disease (9, 48), as well as from experimentally infected mice (22), dogs (3), and monkeys (35); however, the density of spirochetemia is fairly low following dermal inoculation by tick bite and may be undetected in mice (46) or humans unless relatively large volumes of blood are analyzed (52). Thus, the lower spirochetemia following tick-borne infection may complicate this analysis.
It is generally assumed that, in human Lyme borreliosis, B. burgdorferi disseminates from the site of dermal inoculation by tick bite to distal tissues primarily by the hematogenous route (31, 47, 55). However, some studies using mouse (46) or dog (50) models of Lyme borreliosis have prompted an alternative hypothesis that spirochetes invade the bloodstream only incidentally and that dissemination occurs primarily by migration through the skin and connective tissue. Our experiments were not designed to distinguish between these two possibilities. However, local infection at the site of inoculation and spirochetemia were both absent in DbpA-immunized mice challenged with either cultured or blood-phase B. burgdorferi, suggesting that DbpA immunization may prevent dissemination by either route. Additionally, disseminated infection can be prevented in mice dermally inoculated with cultured B. burgdorferi up to 4 days postinoculation by passively administered DbpA antiserum (30).
In recent years, several studies have shown that borrelial proteins expressed in vitro are not necessarily those that are expressed in resting or feeding ticks (17, 18, 23, 45). However, persistently infected mice elicit similar immune responses to B. burgdorferi antigens whether they are inoculated by tick bite or by intradermal injections of minimal infectious doses of cultured spirochetes (43), suggesting that the spirochetes achieve similar states of host adaptation at some point during infection regardless of the phenotype of the original inoculum. It is not clear how long the host adaptation process takes or what all the host environmental signals are, but at least some B. burgdorferi genes up-regulated in mammalian hosts appear to be repressed at lower growth temperature in vitro (45, 49), as is DbpA (Fig. 1). We found that B. burgdorferi in blood samples from mice 4 days after dermal inoculation with cultured spirochetes were uniformly DbpA positive and OspA negative, despite the fact that the population of spirochetes in the original inoculum expressed high levels of OspA. This change in phenotype appears to occur within the first 2 days of localized infection (30, 44). Spirochetes in unfed ticks express OspA on their surface, but during tick engorgement, OspA expression is down-modulated and the spirochetes apparently retain this phenotype during the early stages of infection (18). At this time, we do not know whether DbpA is expressed either in feeding or resting ticks, but this protein, like OspC (18, 45), may represent an early marker for the state of host adaptation.
Propagation in dialysis membrane chambers implanted into the peritoneal cavities of mice and rats has also been used to model the host-adapted phenotype of B. burgdorferi (1, 17). Passive immunization of naive mice with immune serum transferred from persistently infected mice was unable to prevent infection from a challenge with 104 chamber-adapted spirochetes, suggesting that in vivo-adapted spirochetes are resistant to killing by specific antibodies (17). In contrast, we were successful at protecting mice from challenge with in vivo-adapted blood-phase spirochetes by active immunization with DbpA, but our study differed from that of deSilva et al. (17) in some important ways. We challenged mice with 0.1 ml of blood pooled from spirochetemic donor mice that contained less than 102 CFU per ml; thus, the challenge doses were substantially different in the two studies. We have not determined the edian infectious dose for blood-phase spirochetes, but it is apparently <10 CFU, which is below the median infectious doses we determined for cultured borreliae. The two studies also differed in their immunization procedures. We used active immunization with recombinant DbpA rather than passive immunization with immune serum to diverse in vivo-expressed B. burgdorferi antigens, and the protective antibody levels may be higher or persist longer in the active immunization.
Spirochetes propagated in dialysis membrane chambers may differ in some ways from spirochetes recovered from blood samples from infected hosts. For example, spirochetes in dialysis membrane chambers expressed several well-characterized roteins shown to be expressed during infection, including FlaB, OspE, OspF, and p21 (1), but spirochetes recovered from dialysis membrane chambers did not express the BbK2.10 protein previously shown to be preferentially expressed in vivo (2). While chamber-adapted spirochetes are replicating at host temperatures and have access to diffusible metabolites, they are barred from interaction with host cells and extracellular matrix molecules. In studies with other microorganisms, it has been shown that they can modulate their gene expression upon contact with eukaryotic cells (39, 54), but this phenomenon has not yet been documented for spirochetes. Our model may achieve an adaptive state for the spirochetes which is closer to that of a natural infection in that the spirochetes are harvested from the bloodstream after they have disseminated from the site of dermal inoculation, as would occur after a tick bite.
Like the human disease (47), Lyme borreliosis in the mouse is characterized by occasional recrudescence and resolution of disease (7), suggesting that the host’s immune response to the infecting spirochetes may act to control the infection even if it cannot completely eradicate it. Serum-mediated resolution of Lyme arthritis in the mouse model has been demonstrated (6). Antisera from infected mice have high levels of DbpA antibodies, as well as antibodies against other in vivo-expressed antigens (19, 30). When antisera from B. burgdorferi-infected mice were adsorbed with DbpA, the borreliacidal activity of this serum was substantially decreased, suggesting that DbpA antibodies may be among those that contribute to control of infection. Antisera from persistently infected mice were able to kill both cultured and blood-phase B. burgdorferi, but the blood-phase spirochetes appeared to be somewhat more resistant to this borreliacidal activity (Table 5). Antisera from DbpA-immunized mice were much more potent against these blood-phase spirochetes (Table 1), suggesting that vaccination with purified immunogens may elicit antibodies that differ in concentration, avidity, or epitope specificity from those elicited in response to persistent infection.
A central issue in Lyme borreliosis is the ability of B. burgdorferi to maintain persistent infections in immunocompetent hosts in the face of a vigorous immune response to its in vivo-expressed membrane antigens. B. burgdorferi is vulnerable to DbpA antibodies during the early phase of infection but appears to be relatively resistant at later times. The resistance of the spirochetes may be a consequence of decreased DbpA expression in certain tissues or other phenotypic changes of the spirochetes that decrease accessibility of DbpA to borreliacidal antibodies. It has recently been shown that repeated passive administrations of antibodies purified from OspC hyperimmune antiserum to SCID mice infected with B. burgdorferi can reduce the number of spirochetes in ears of these mice below the level of detection by culturing and promote a regression in arthritis (55). Experiments are in progress to address the possibility that postexposure DbpA immunization may modulate infection or disease.
The results of this study show that DbpA immunoprophylaxis may be effective against mammalian stage B. burgdorferi infections, while OspA is not. It is possible that these transmission- and dissemination-blocking antibodies may be synergistic for protective efficacy.
ACKNOWLEDGMENTS
We thank Alan Barbour and Stephen Barthold for donating B. burgdorferi strains used in this study and D. Denee Thomas for providing antibodies H9724 and H5332. We gratefully acknowledge the technical assistance of Will Roberts, Deb Couchenour, Raju Lathigra, Luis Branco, Phil Barren, Durga Paruchuri, Adiam Yohannes, Soyer Kaya, Henry Gering, David Wood, and Julie Bullock, and we thank Frank Gherardini and Thelma Welchel for assistance with B. burgdorferi membrane preparation. We thank Gil Choi for donating (His)6PAL and Magnus Höök for donating (His)6DbpB and (His)6DbpA. We also thank Scott Koenig and Solomon Langermann for thoughtful discussions and review of this manuscript and Donni Leach for assistance in manuscript preparation.
This work was supported in part by National Institutes of Health grant AI39865 to M.S.H.
REFERENCES
- 1.Akins D R, Bourell K W, Caimano M J, Norgard M V, Radolf J D. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Invest. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins D R, Porcella S F, Popova T G, Schevchenko D, Baker S I, Li M, Norgard M V, Radolf J D. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol Microbiol. 1995;18:507–520. doi: 10.1111/j.1365-2958.1995.mmi_18030507.x. [DOI] [PubMed] [Google Scholar]
- 3.Appel M J, Allan S, Jacobson R H, Lauderdale T L, Chang Y F, Todhunter R J, Summers B A. Experimental Lyme disease in dogs produces arthritis and persistent infection. J Infect Dis. 1993;167:651–664. doi: 10.1093/infdis/167.3.651. [DOI] [PubMed] [Google Scholar]
- 4.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold S W, Bockenstedt L K. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect Immun. 1993;61:4696–4702. doi: 10.1128/iai.61.11.4696-4702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold S W, deSouza M, Feng S. Serum-mediated resolution of Lyme arthritis in mice. Lab Invest. 1996;74:57–67. [PubMed] [Google Scholar]
- 7.Barthold S W, deSouza M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 8.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 9.Benach J L, Bosler E M, Hanrahan J P, Coleman J L, Habicht G S, Bast T F, Cameron D J, Ziegler J L, Barbour A G, Burgdorfer W, Edelman R, Kaslow R A. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- 10.Bledsoe H A, Carroll J A, Whelchel T R, Farmer M A, Dorward D W, Gherardini F C. Isolation and partial characterization of Borrelia burgdorferi inner and outer membranes by using isopycnic centrifugation. J Bacteriol. 1994;176:7447–7455. doi: 10.1128/jb.176.24.7447-7455.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bockenstedt L K, Hodzic E, Feng S, Bourrel K W, deSilva A M, Montgomery R R, Fikrig E, Radolf J D, Barthold S W. Borrelia burgdorferi strain-specific OspC-mediated immunity in mice. Infect Immun. 1997;65:4661–4776. doi: 10.1128/iai.65.11.4661-4667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassatt D R, Patel N K, Hanson M S, Guo B P, Höök M. Protection of Borrelia burgdorferi infection by antibodies to decorin-binding protein. In: Brown F, Burton D, Doherty P, Makalanos J, Norby E, editors. Vaccines 97. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 191–195. [Google Scholar]
- 13.Champion C I, Blanco D R, Skare J T, Haake D A, Giladi M, Foley D, Miller J N, Lovett M A. A 9.0-kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect Immun. 1994;62:2653–2661. doi: 10.1128/iai.62.7.2653-2661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox D L, Akins D R, Bourell K W, Lahdenne P, Norgard M V, Radolf J D. Limited surface exposure of Borrelia burgdorferi outer surface lipoproteins. Proc Natl Acad Sci USA. 1996;93:7973–7978. doi: 10.1073/pnas.93.15.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das S, Barthold S W, Stocker Giles S, Montgomery R R, Telford III S R, Fikrig E. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and the mammalian host. J Clin Invest. 1997;99:987–995. doi: 10.1172/JCI119264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deich R A, Metcalf B J, Finn C W, Farley J E, Green B A. Cloning of genes encoding a 15,000-dalton peptidoglycan-associated outer membrane lipoprotein and an antigenically related 15,000-dalton protein from Haemophilus influenzae. J Bacteriol. 1988;170:489–498. doi: 10.1128/jb.170.2.489-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.deSilva A M, Fikrig E, Hodzic E, Kantor F S, Telford III S R, Barthold S W. Immune evasion by tickborne and host-adapted Borrelia burgdorferi. J Infect Dis. 1998;177:395–400. doi: 10.1086/514200. [DOI] [PubMed] [Google Scholar]
- 18.deSilva A M, Telford III S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng S, Hodzic E, Stevenson B, Barthold S W. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect Immun. 1998;66:2827–2835. doi: 10.1128/iai.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fikrig E, Barthold S W, Kantor F S, Flavell R A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- 21.Fikrig E, Barthold S W, Sun W, Feng W, Telford III S R, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 22.Fikrig E, Telford III S R, Barthold S W, Kantor F S, Spielman A, Flavell R A. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proc Natl Acad Sci USA. 1992;89:5418–5421. doi: 10.1073/pnas.89.12.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fingerle V, Hauser U, Liegl G, Petko B, Preac-Mursic V, Wilske B. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus. J Clin Microbiol. 1995;33:1867–1869. doi: 10.1128/jcm.33.7.1867-1869.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fini A, Fazio G, Tonelli D, Roda A, Zuman P. Chemical properties of bile acids. V. Interaction with concentrated sulfuric acid. Farmaco. 1992;47:741–752. [PubMed] [Google Scholar]
- 25.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J-F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 26.Gu J, Stephenson C G, Idarola M J. Recombinant proteins attached to a nickel-NTA column: use in affinity purification of antibodies. BioTechniques. 1994;17:257–262. [PubMed] [Google Scholar]
- 27.Guo, B. P., E. L. Brown, D. W. Dorward, L. C. Rosenberg, and M. Höök. Decorin binding adhesins from Borrelia burgdorferi. Mol. Microbiol., in press. [DOI] [PubMed]
- 28.Guo B P, Norris S J, Rosenberg L C, Höök M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagman K E, Lahdenne P, Popova T G, Porcella S F, Akins D R, Radolf J D, Norgard M V. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect Immun. 1998;66:2674–2683. doi: 10.1128/iai.66.6.2674-2683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanson M S, Cassatt D R, Guo B P, Patel N K, McCarthy M, Dorward D W, Höök M. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66:2143–2153. doi: 10.1128/iai.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer M D, Wallich R, Simon M M. The outer surface protein A (OspA) of Borrelia burgdorferi: a vaccine candidate and bioactive mediator. Infection. 1996;24:190–194. doi: 10.1007/BF01713338. [DOI] [PubMed] [Google Scholar]
- 32.Lahdenne P, Porcella S F, Hagman K E, Akins D R, Popova T G, Cox D L, Katona L I, Radolf J D, Norgard M V. Molecular characterization of a 6.6-kilodalton Borrelia burgdorferi outer membrane-associated lipoprotein (lp6.6) which appears to be downregulated during mammalian infection. Infect Immun. 1997;65:412–421. doi: 10.1128/iai.65.2.412-421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma Y, Seiler K P, Eichwald E J, Weis J H, Teuscher C, Weis J J. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect Immun. 1998;66:161–168. doi: 10.1128/iai.66.1.161-168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montgomery R R, Malawista S E, Feen K J M, Bockenstedt L K. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Philipp M T, Aydintug M K, Jr, Bohm R P, Cogswell F B, Dennis V A, Lanners H N, Jr, Lowrie R C, Roberts E D, Conway M D, Karacorlu M, Peyman G A, Gubler D J, Johnson B J B, Piesman J, Gu Y. Early and early disseminated phases of Lyme disease in the rhesus monkey: a model for infection in humans. Infect Immun. 1993;61:3047–3059. doi: 10.1128/iai.61.7.3047-3059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preac-Mursic V, Wilske B, Patsouris E, Jauris S, Will G, Rainhardt S, Lehnert G, Klockmann U, Mehraein P. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against B. burgdorferi infection. Infection. 1992;20:342–349. doi: 10.1007/BF01710681. [DOI] [PubMed] [Google Scholar]
- 37.Roberts W C, Mullikin B A, Lathigra R, Hanson M S. Molecular analysis of sequence heterogeneity among genes encoding decorin binding proteins A and B of Borrelia burgdorferi sensu lato. Infect Immun. 1998;66:5275–5285. doi: 10.1128/iai.66.11.5275-5285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roessler D, Hauser U, Wilske B. Heterogeneity of BmpA (P39) among European isolates of Borrelia burgdorferi sensu lato and influence of interspecies variability on serodiagnosis. J Clin Microbiol. 1997;35:2752–2758. doi: 10.1128/jcm.35.11.2752-2758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadziene A, Barbour A G, Rosa P A, Thomas D D. An OspB mutant of Borrelia burgdorferi has reduced invasiveness in vitro and reduced infectivity in vivo. Infect Immun. 1993;61:3590–3596. doi: 10.1128/iai.61.9.3590-3596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Samuels D S, Marconi R T, Huang W M, Garon C F. gyrB mutations in coumermycin A1-resistant Borrelia burgdorferi. J Bacteriol. 1994;176:3072–3075. doi: 10.1128/jb.176.10.3072-3075.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiable U E, Gern L, Wallich R, Kramer M D, Prester M, Simon M M. Distinct patterns of protective antibodies are generated against Borrelia burgdorferi in mice experimentally inoculated with high and low doses of antigen. Immunol Lett. 1993;36:219–226. doi: 10.1016/0165-2478(93)90056-8. [DOI] [PubMed] [Google Scholar]
- 44.Schiable U E, Kramer M D, Eichman K, Modelell M, Museteanu C, Simon M M. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (SCID) mice. Proc Natl Acad Sci USA. 1990;87:3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shih C M, Telford III S R, Pollack R J, Spielman A. Rapid dissemination by the agent of Lyme disease in hosts that permit fulminating infection. Infect Immun. 1993;61:2396–2399. doi: 10.1128/iai.61.6.2396-2399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 48.Steere A C, Grodzicki R L, Kornblatt A N, Craft J E, Barbour A G, Burgdorfer W, Schmid G P, Johnson E, Malawista S E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 49.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Straubinger R, Straubinger A F, Harter L, Jacobson R H, Chang Y-F, Summers B A, Erb H N. Borrelia burgdorferi migrates into joint capsules and causes an up-regulation of interleukin-8 in synovial membranes of dogs experimentally infected with ticks. Infect Immun. 1998;65:1273–1285. doi: 10.1128/iai.65.4.1273-1285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wormser G P, Nakowski J, Nadelman R B, Bittker S, Cooper D. Improving the yield of blood cultures for patients with early Lyme disease. J Clin Microbiol. 1998;36:296–298. doi: 10.1128/jcm.36.1.296-298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yokoyama W M. Production of monoclonal antibodies. In: Coligan J E, Kruisbeek A M, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons; 1991. pp. 2.5.1–2.5.17. [Google Scholar]
- 54.Zhang J P, Normark S. Induction of gene expression in Escherichia coli after pilus-mediated adherence. Science. 1996;273:1234–1236. doi: 10.1126/science.273.5279.1234. [DOI] [PubMed] [Google Scholar]
- 55.Zhong W, Stehle T, Museteanu C, Seibers A, Gern L, Kramer M D, Wallich R, Simon M M. Therapeutic passive vaccination against chronic Lyme disease in mice. Proc Natl Acad Sci USA. 1997;94:12533–12538. doi: 10.1073/pnas.94.23.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]