Abstract
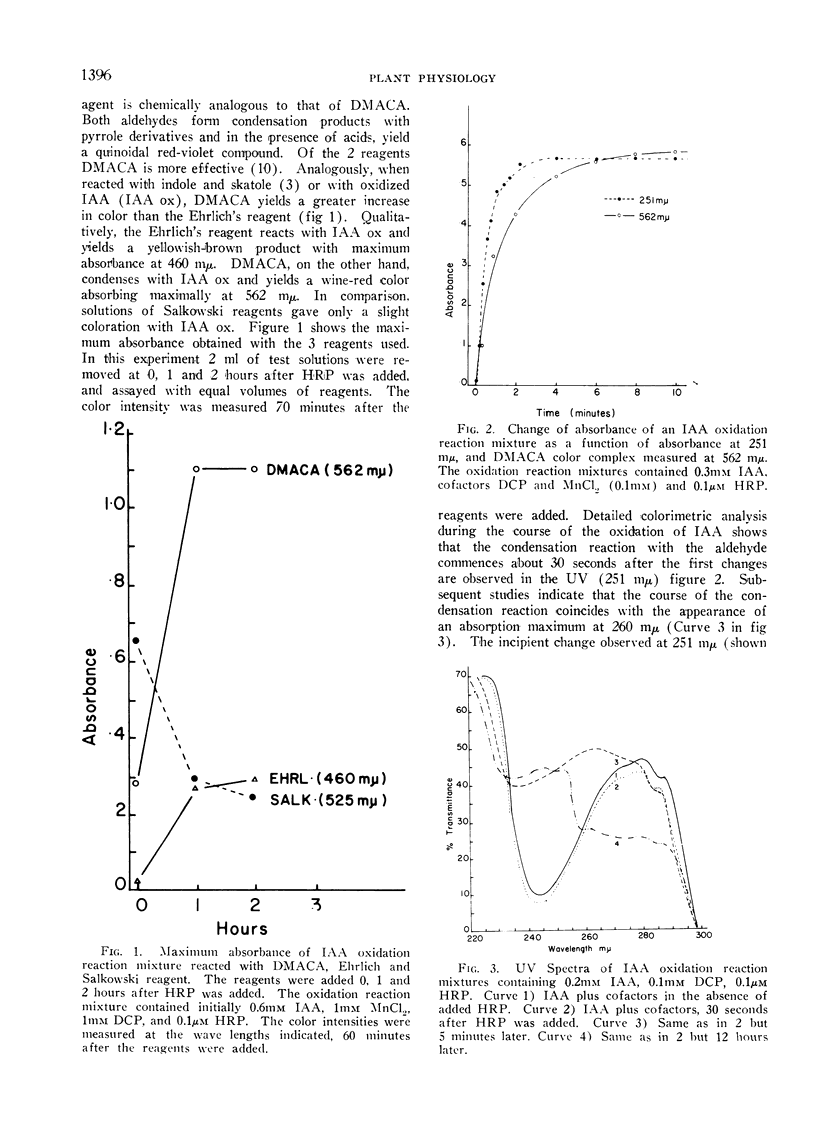
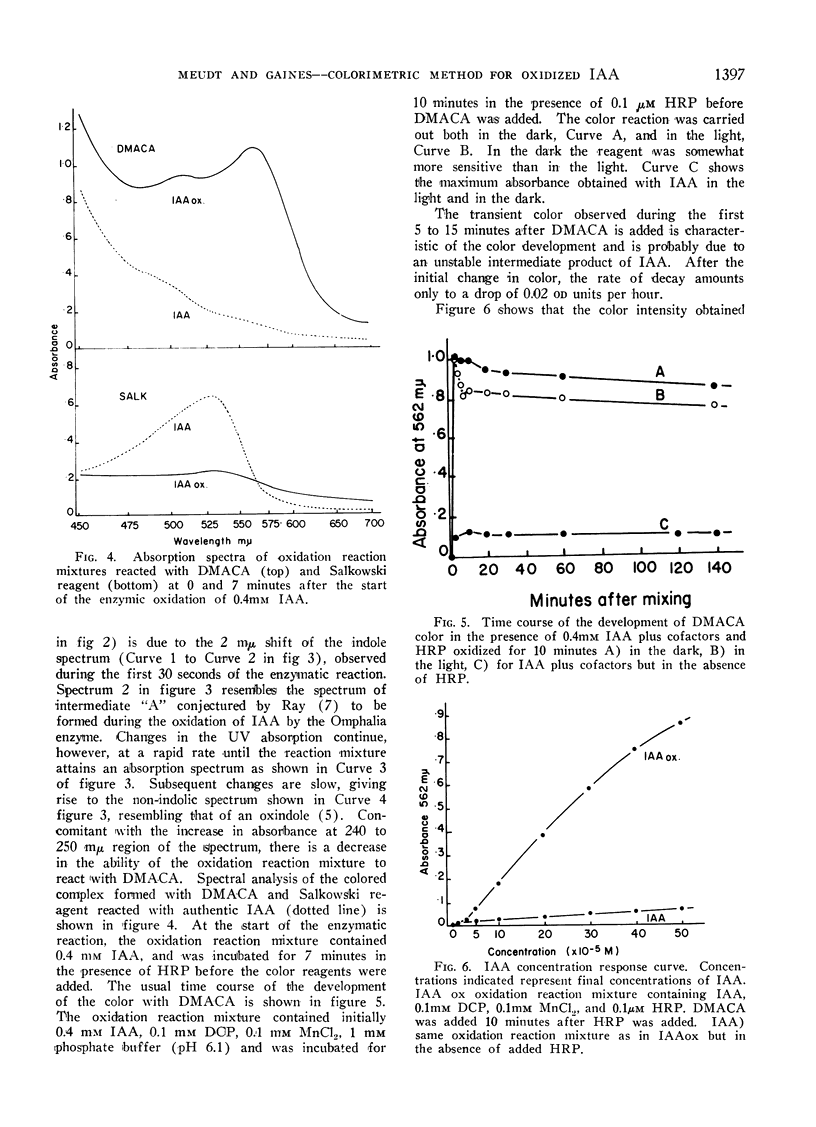
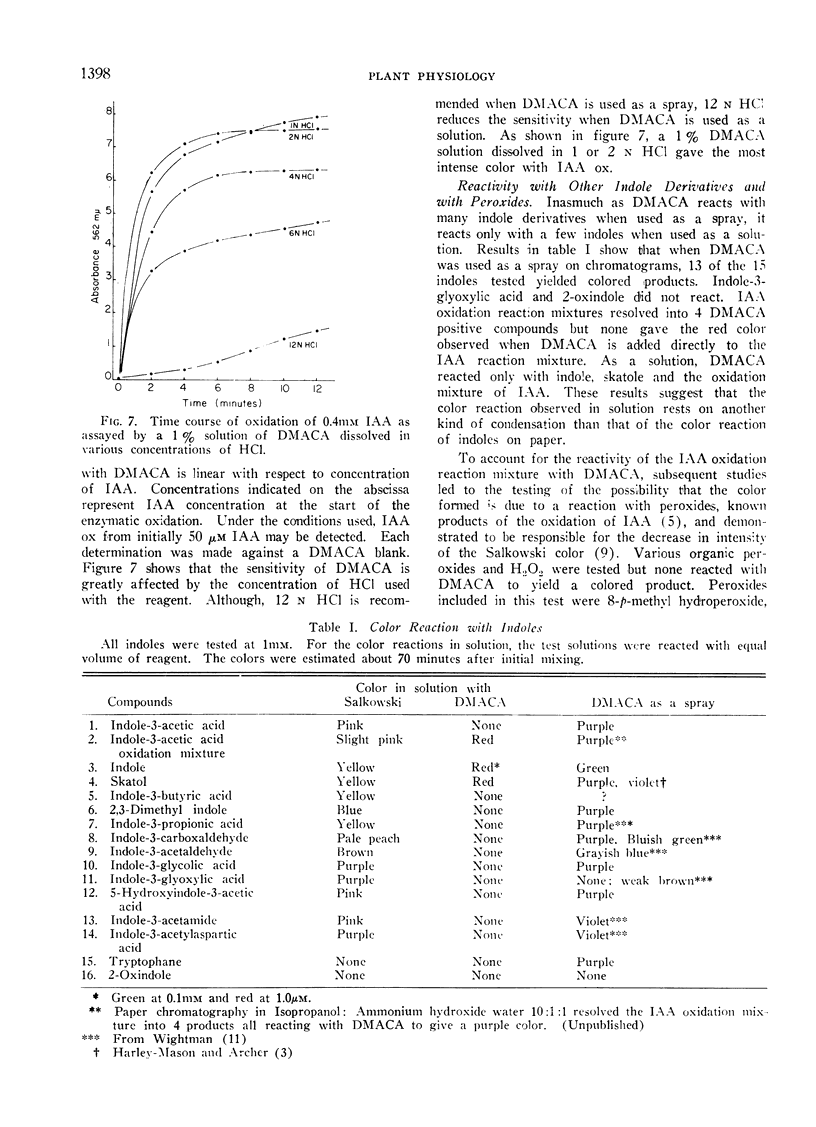
The method described here is based on a brief report by Harley-Mason and Archer. It involves the use of p-dimethylaminocinnamaldehyde (DMACA), a vinylogue of Ehrlich's reagent, as a color reagent for indoles. Colorimetric analyses of indoleacetic acid (IAA) oxidation reaction mixtures were made with the DMACA reagent as a solution rather than a spray. DMACA reagent will yield a wine-red color with IAA oxidation products in solution. Under similar conditions DMACA reacts with authentic IAA to yield only slight coloration at best. In comparison with other indoles, DMACA is more relative with IAA oxidation reaction products than either Salkowski or Ehrlich's reagents. Data discussed support a concept that the color produced with DMACA is due to the presence of tautomeric oxidation product(s) of IAA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HINMAN R. L., LANG J. PEROXIDASE-CATALYZED OXIDATION OF INDOLE-3-ACETIC ACID. Biochemistry. 1965 Jan;4:144–158. doi: 10.1021/bi00877a023. [DOI] [PubMed] [Google Scholar]
- RAY P. M., THIMANN K. V. Steps in the oxidation of indoleacetic acid. Science. 1955 Jul 29;122(3161):187–188. doi: 10.1126/science.122.3161.187. [DOI] [PubMed] [Google Scholar]
- RAY P. M. The destruction of indoleacetic acid. II. Spectrophotometric study of the enzymatic reaction. Arch Biochem Biophys. 1956 Sep;64(1):193–216. doi: 10.1016/0003-9861(56)90254-5. [DOI] [PubMed] [Google Scholar]


