Abstract
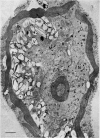
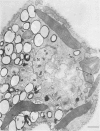
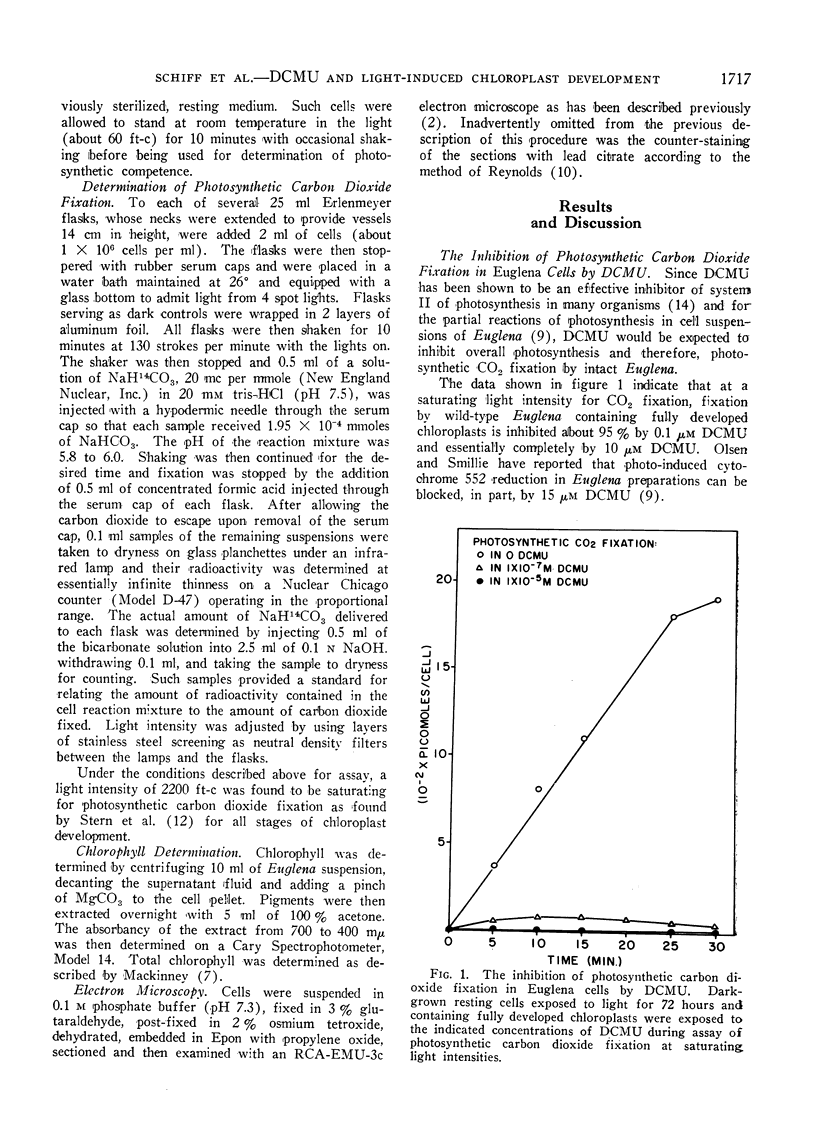
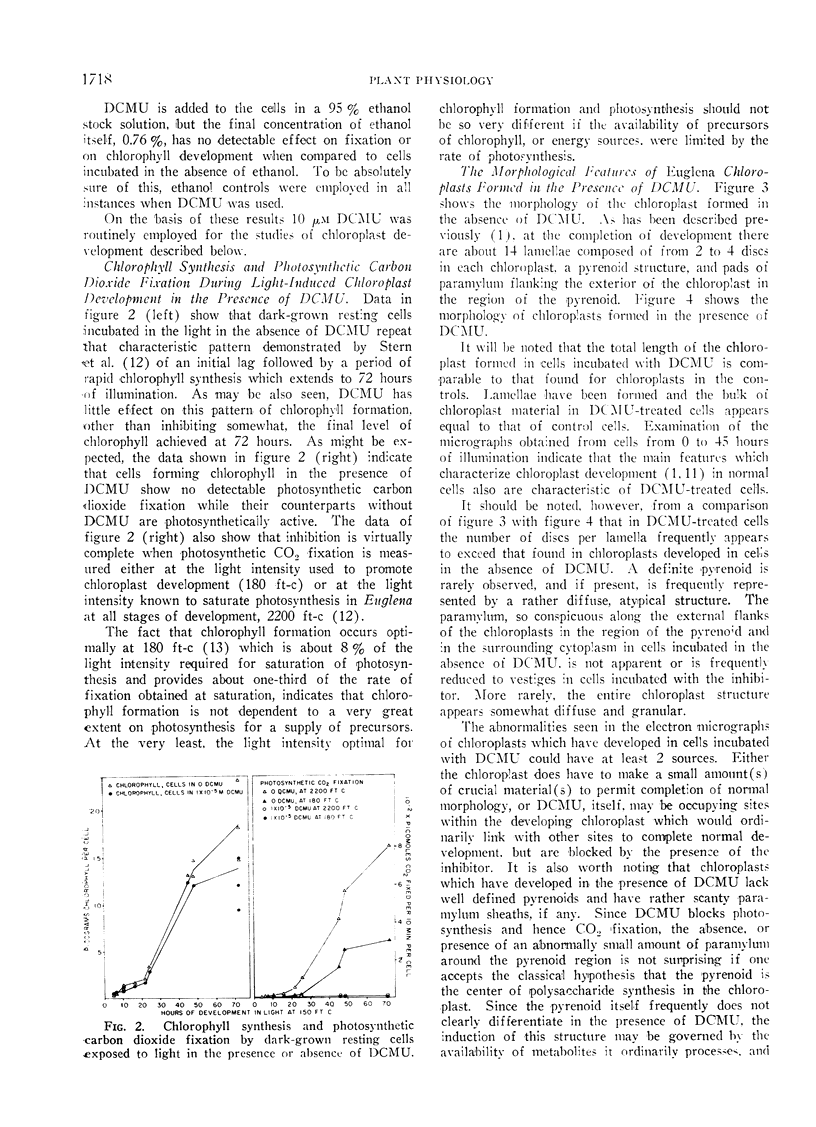
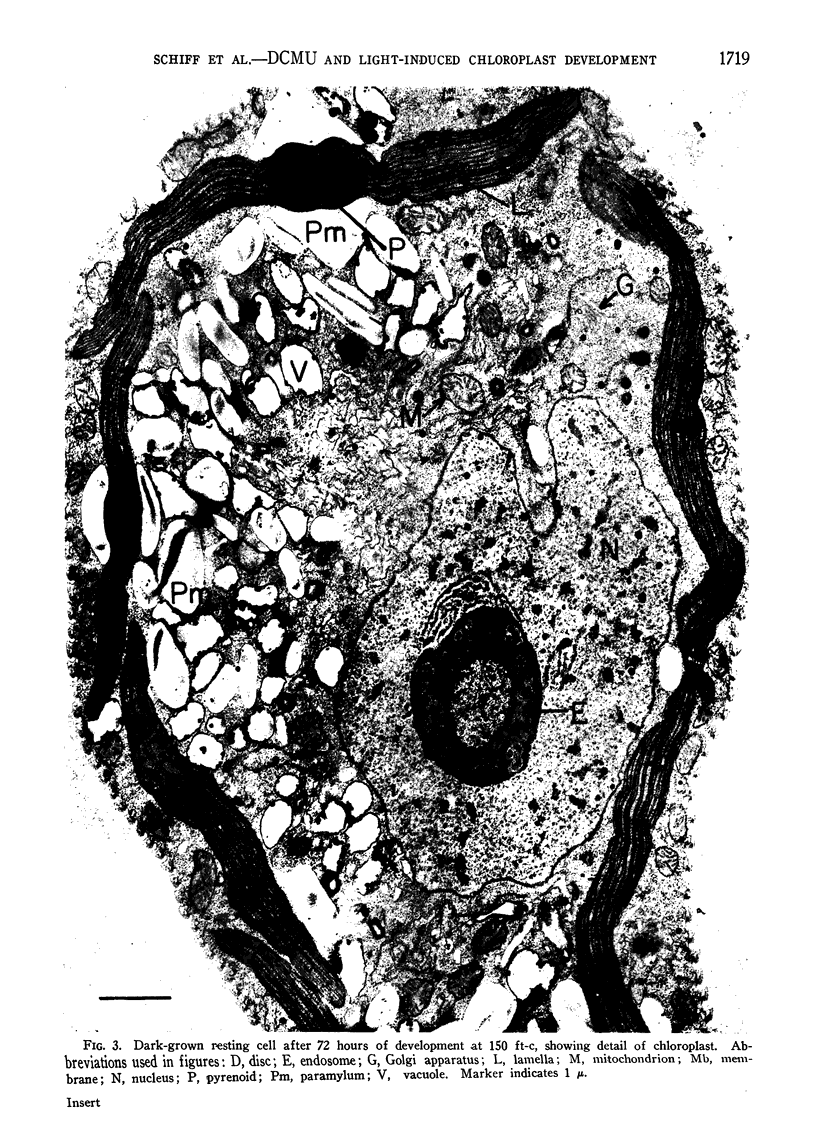
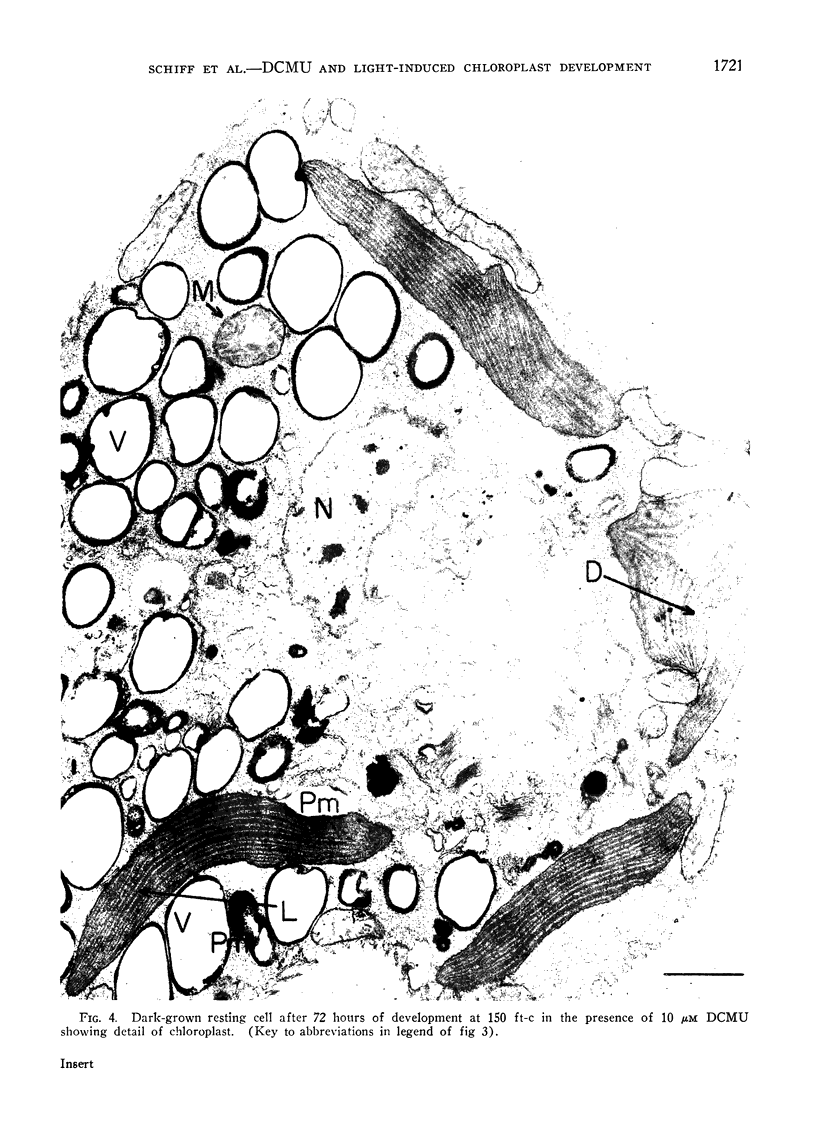
The possibility that photosynthetic competence is gratuitous for light-induced chloroplast development in Euglena gracilis var. bacillaris was examined by incubating dark-grown resting cells in the light with DCMU, an inhibitor of photosynthesis. Under these conditions photosynthetic carbon dioxide fixation was inhibited essentially completely at all times during chloroplast development, but about 70% of the chlorophyll was formed with essentially the same pattern of accumulation found for cells incubated in the absence of the inhibitor. Electron microscopy of cells incubated with DCMU in the light revealed the formation of morphologically recognizable chloroplasts having comparable overall dimensions and structural elements to those found in normally developed chloroplasts, but frequently lacking a readily detectable pyrenoid with paramylum sheaths, and often containing increased numbers of discs per lamella. Such abnormalities are considered minor since upon removal of DCMU by centrifugation, the cells usually regained almost full photosynthetic competence on a chlorophyll basis.
It is concluded that photosynthetic competence is not necessary for chloroplast development in Euglena and supports the hypothesis, already suggested from other evidence, that light induction results in activation of synthetic machinery external to the developing chloroplast.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Shaul Y., Schiff J. A., Epstein H. T. Studies of Chloroplast Development in Euglena. VII. Fine Structure of the Developing Plastid. Plant Physiol. 1964 Mar;39(2):231–240. doi: 10.1104/pp.39.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman M., Epstein H. T., Schiff J. A. Isolation and characterization of DNA from the mitochondrial fraction of Euglena. J Mol Biol. 1966 Jun;17(2):463–469. doi: 10.1016/s0022-2836(66)80156-0. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Photosynthetic Electron Transport Chain of Chlamydomonas reinhardi VI. Electron Transport in Mutant Strains Lacking Either Cytochrome 553 or Plastocyanin. Plant Physiol. 1966 Dec;41(10):1648–1656. doi: 10.1104/pp.41.10.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A. I., Epstein H. T., Schiff J. A. Studies of Chloroplast Development in Euglena. VI. Light Intensity as a Controlling Factor in Development. Plant Physiol. 1964 Mar;39(2):226–231. doi: 10.1104/pp.39.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A. I., Schiff J. A., Epstein H. T. Studies of Chloroplast Development in Euglena. V. Pigment Biosynthesis, Photosynthetic Oxygen Evolution and Carbon Dioxide Fixation during Chloroplast Development. Plant Physiol. 1964 Mar;39(2):220–226. doi: 10.1104/pp.39.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERNON L. P., AVRON M. PHOTOSYNTHESIS. Annu Rev Biochem. 1965;34:269–296. doi: 10.1146/annurev.bi.34.070165.001413. [DOI] [PubMed] [Google Scholar]