Abstract
Primary and secondary metabolites of inorganic nitrogen metabolism were evaluated as inhibitors of nitrate reductase (EC 1.6.6.1) induction in green leaf tissue of corn seedlings. Nitrite, nitropropionic acid, ammonium ions, and amino acids were not effective as inhibitors of nitrate reductase activity or synthesis. Increasing α-amino nitrogen and protein content of intact corn seedlings by culture techniques significantly enhanced rather than decreased the potential for induction of nitrate reductase activity in excised seedlings.
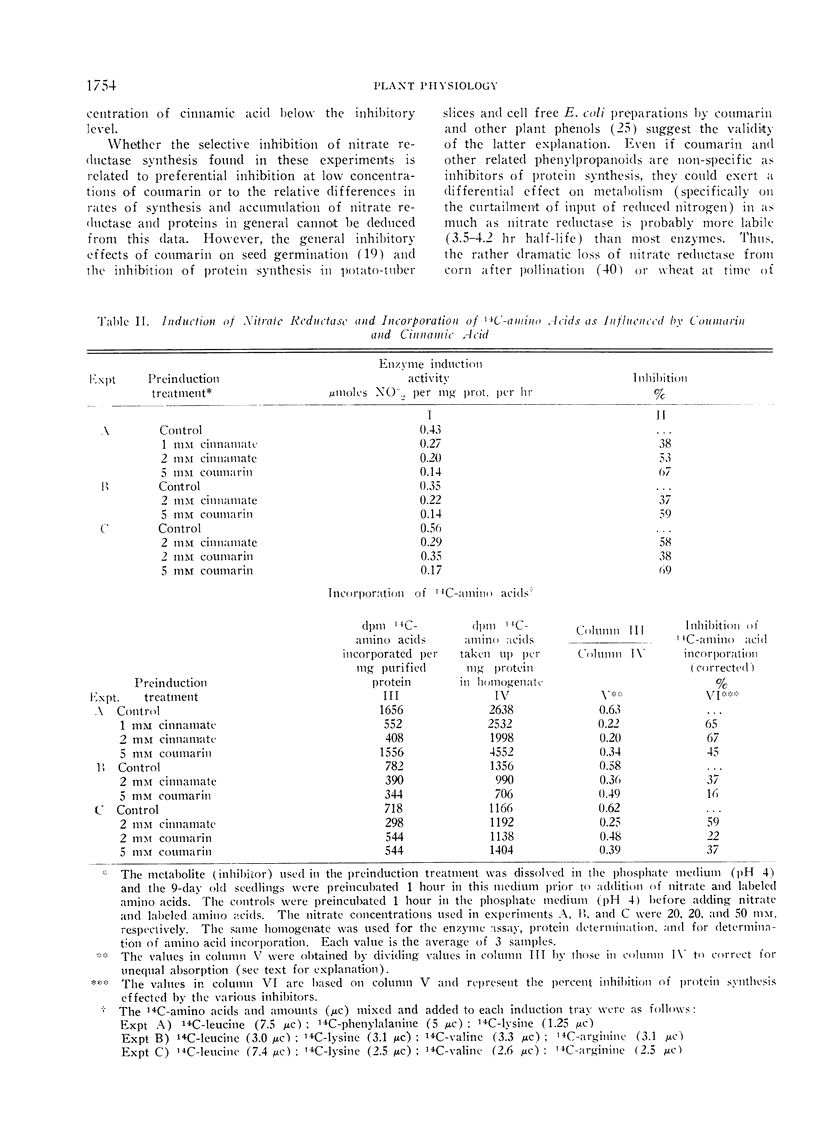
Secondary metabolites, derived from phenylalanine and tyrosine, were tested as inhibitors of induction of nitrate reductase. Of the 9 different phenylpropanoid compounds tested, only coumarin, trans-cinnamic and trans-o-hydroxycinnamic acids inhibited induction of nitrate reductase.
While coumarin alone exhibited a relatively greater inhibitory effect on enzyme induction than on general protein synthesis (the latter measured by incorporation of labeled amino acids), this differential effect may have been dependent upon unequal rates of synthesis and accumulation with respect to the initial levels of nitrate reductase and general proteins. Because of the short half-life of nitrate reductase, inhibitors of protein synthesis in general could still achieve differential regulation of nitrogen metabolism. Coumarin did not inhibit nitrate reductase activity when added directly to the assay mixture at 5 mm.
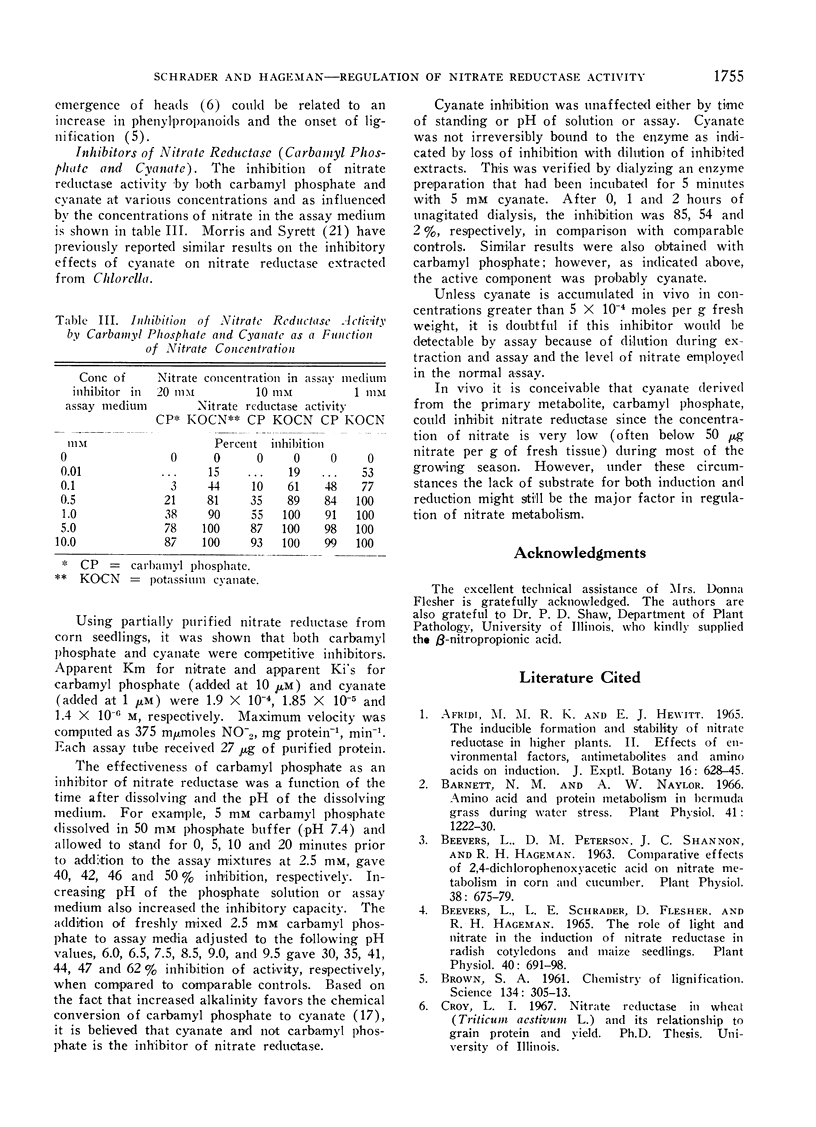
Carbamyl phosphate and its chemical derivative, cyanate, were found to be competitive (with nitrate) inhibitors of nitrate reductase. The data suggest that cyanate is the active inhibitor in the carbamyl phosphate preparations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett N. M., Naylor A. W. Amino Acid and protein metabolism in bermuda grass during water stress. Plant Physiol. 1966 Sep;41(7):1222–1230. doi: 10.1104/pp.41.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers L., Peterson D. M., Shannon J. C., Hageman R. H. Comparative Effects of 2,4-Dichlorophenoxyacetic Acid on Nitrate Metabolism in Corn and Cucumber. Plant Physiol. 1963 Nov;38(6):675–679. doi: 10.1104/pp.38.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers L., Schrader L. E., Flesher D., Hageman R. H. The Role of Light and Nitrate in the Induction of Nitrate Reductase in Radish Cotyledons and Maize Seedlings. Plant Physiol. 1965 Jul;40(4):691–698. doi: 10.1104/pp.40.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. A. Chemistry of Lignification: Biochemical research on lignins is yielding clues to the structure and formation of these complex polymers. Science. 1961 Aug 4;134(3475):305–313. doi: 10.1126/science.134.3475.305. [DOI] [PubMed] [Google Scholar]
- Filner P. Regulation of nitrate reductase in cultured tobacco cells. Biochim Biophys Acta. 1966 May 5;118(2):299–310. doi: 10.1016/s0926-6593(66)80038-3. [DOI] [PubMed] [Google Scholar]
- GOODWIN B. C., SIZER I. W. HISTONE REGULATION OF LACTIC DEHYDROGENASE IN EMBRYONIC CHICK BRAIN TISSUE. Science. 1965 Apr 9;148(3667):242–244. doi: 10.1126/science.148.3667.242. [DOI] [PubMed] [Google Scholar]
- GORTNER W. A., KENT M. J. The coenzyme requirement and enzyme inhibitors of pineapple indoleacetic acid oxidase. J Biol Chem. 1958 Sep;233(3):731–735. [PubMed] [Google Scholar]
- HUANG R. C., BONNER J. Histone, a suppressor of chromosomal RNA synthesis. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1216–1222. doi: 10.1073/pnas.48.7.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman R. H., Flesher D. Nitrate Reductase Activity in Corn Seedlings as Affected by Light and Nitrate Content of Nutrient Media. Plant Physiol. 1960 Sep;35(5):700–708. doi: 10.1104/pp.35.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parups E. V. Effect of various plant phenols on protein synthesis in excised plant tissues. Can J Biochem. 1967 Apr;45(4):427–434. doi: 10.1139/o67-051. [DOI] [PubMed] [Google Scholar]
- Rabson R., Novelli G. D. THE INCORPORATION OF LEUCINE-C INTO PROTEIN BY A CELL-FREE PREPARATION FROM MAIZE KERNELS. Proc Natl Acad Sci U S A. 1960 Apr;46(4):484–488. doi: 10.1073/pnas.46.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer E., Griffin D. H. Activation and Inhibition of Indoleacetic Acid Oxidase Activity from Peas. Science. 1960 Mar 4;131(3401):672–672. doi: 10.1126/science.131.3401.672. [DOI] [PubMed] [Google Scholar]
- Tweedy J. A., Ries S. K. Effect of simazine on nitrate reductase activity in corn. Plant Physiol. 1967 Feb;42(2):280–282. doi: 10.1104/pp.42.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]


