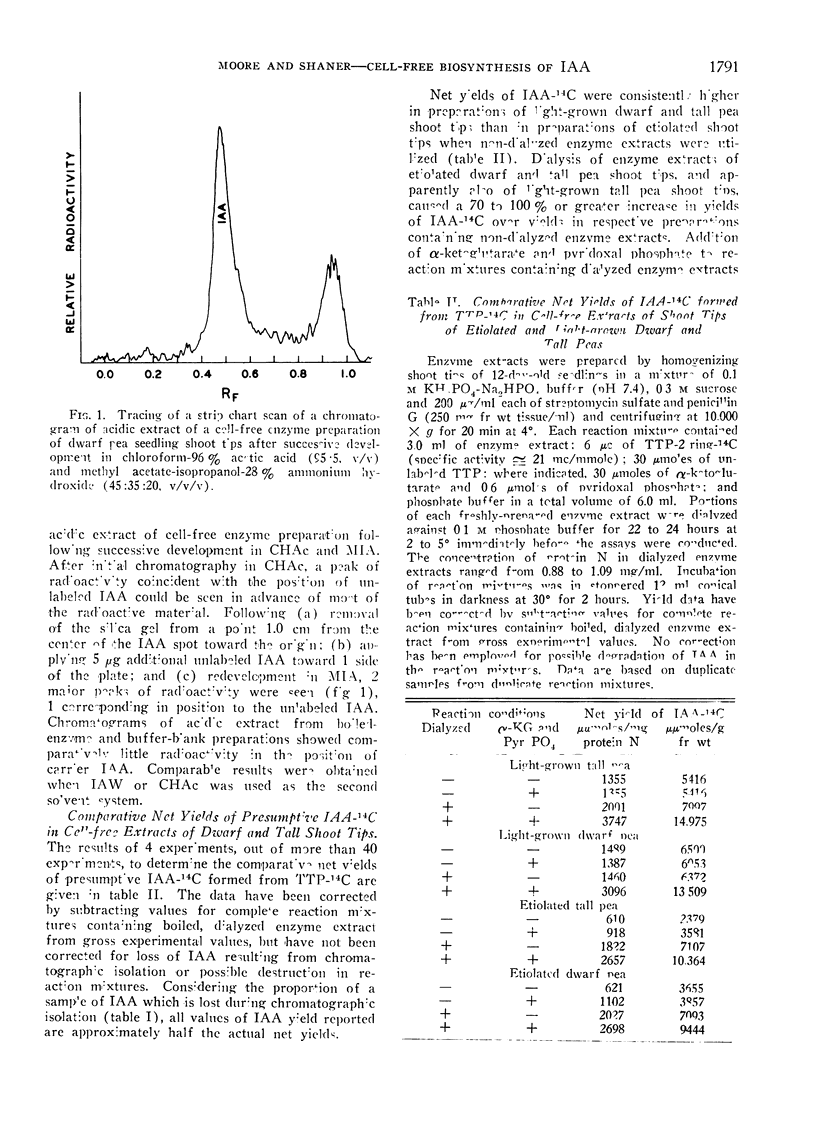
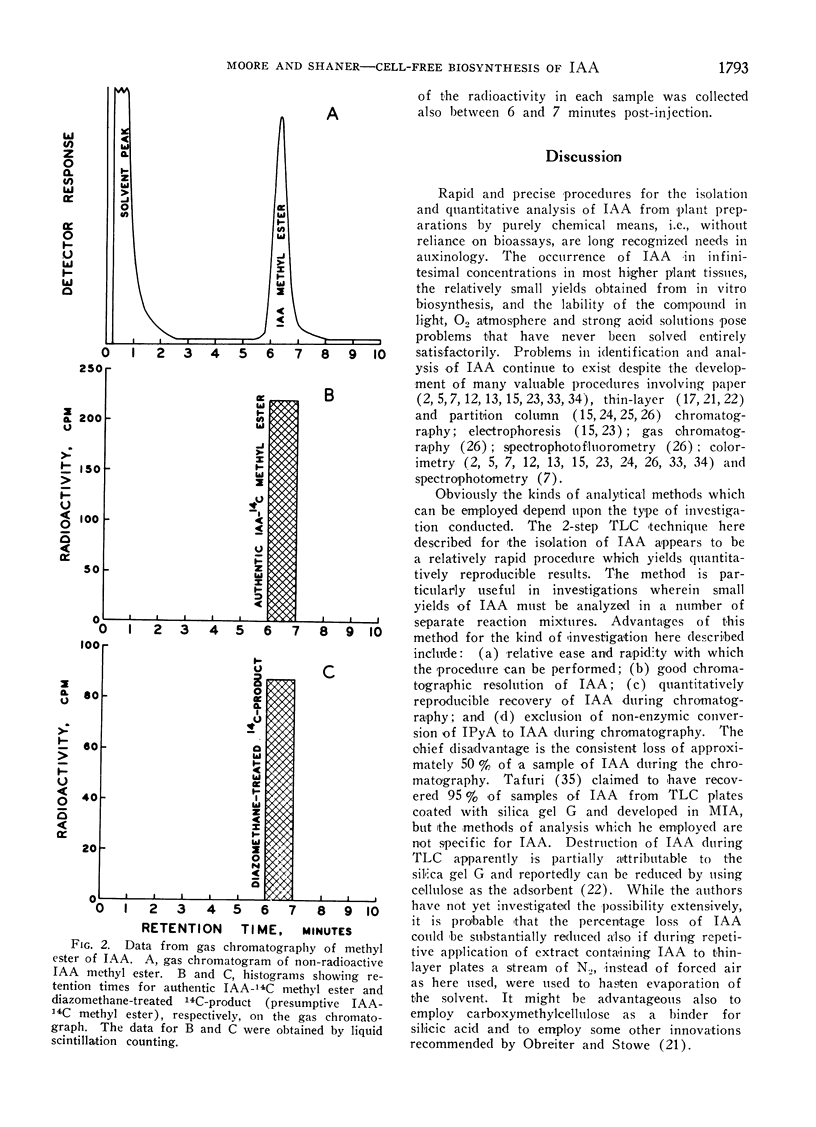
Abstract
A 2-step, 1-dimensional thin-layer chromatographic procedure for isolating indoleacetic acid (IAA) was developed and utilized in investigations of the biosynthesis of IAA from tryptophan-14C in cell-free extracts of pea (Pisum sativum L.) shoot tips. Identification of a 14C-product as IAA was by (a) co-chromatography of authentic IAA and 14C-product on thin-layer chromatography, and (b) gas-liquid and thin-layer chromatography of authentic and presumptive IAA methyl esters. Dialysis of enzyme extracts and addition of α-ketoglutaric acid and pyridoxal phosphate to reaction mixtures resulted in approximately 2- to 3-fold increases in net yields of IAA over yields in non-dialyzed reaction mixtures which did not contain additives essential to a transaminase reaction of tryptophan. Addition of thiamine pyrophosphate to reaction mixtures further enhanced net biosynthesis of IAA. It is concluded that the formation of indolepyruvic acid and its subsequent decarboxylation probably are sequential reactions in the major pathway of IAA biosynthesis from tryptophan in cell-free extracts of Pisum shoot tips. Comparison of maximum net IAA biosynthesis in extracts of shoot tips of etiolated and light-grown dwarf and tall pea seedlings revealed an order, on a unit protein N basis, of: light-grown tall > light-grown dwarf > etiolated tall ≅ etiolated dwarf. It is concluded that the different rates of stem elongation among etiolated and light-grown dwarf and tall pea seedlings are correlated, in general, with differences in net IAA biosynthesis and sensitivity of the tissues to IAA.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams G. J. Auxin Relations of a Dwarf Pea. Plant Physiol. 1953 Jul;28(3):443–456. doi: 10.1104/pp.28.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. D., Moore T. C. Biosynthesis of (-)-Kaurene in Cell-free Extracts of Immature Pea Seeds. Plant Physiol. 1967 Nov;42(11):1527–1534. doi: 10.1104/pp.42.11.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENTLEY J. A., FARRAR K. R., HOUSLEY S., SMITH G. F., TAYLOR W. C. Some chemical and physiological properties of 3-indolylpyruvic acid. Biochem J. 1956 Sep;64(1):44–49. doi: 10.1042/bj0640044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICZFALUSY E., TILLINGER K. G., ESSER R. J., HOUTMAN A. C. METABOLISM OF SOME PROGESTATIONALLY ACTIVE 9-BETA,10-ALPHA-STEROIDS IN MAN. Nature. 1963 Oct 5;200:79–80. doi: 10.1038/200079b0. [DOI] [PubMed] [Google Scholar]
- Dannenburg W. N., Liverman J. L. Conversion of Tryptophan-2-C to Indoleacetic Acid by Watermelon Tissue Slices. Plant Physiol. 1957 Jul;32(4):263–269. doi: 10.1104/pp.32.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedio W., Zalik S. Gas chromatography of indole auxins. Anal Biochem. 1966 Jul;16(1):36–52. doi: 10.1016/0003-2697(66)90078-9. [DOI] [PubMed] [Google Scholar]
- FLETCHER R. A., ZALIK S. QUANTITATIVE SPECTROPHOTOMETRIC DETERMINATION OF INDOLYL-3-ACETIC ACID. Nature. 1963 Aug 31;199:903–904. doi: 10.1038/199903b0. [DOI] [PubMed] [Google Scholar]
- FURUYA M., GALSTON A. W., STOWE B. B. Isolation from peas of co-factors and inhibitors of indolyl-3-acetic acid oxidase. Nature. 1962 Feb 3;193:456–457. doi: 10.1038/193456a0. [DOI] [PubMed] [Google Scholar]
- GRAEBE J. E., DENNIS D. T., UPPER C. D., WEST C. A. BIOSYNTHESIS OF GIBBERELLINS. I. THE BIOSYNTHESIS OF (-)-KAUREN-19-OL, AND TRANS-GERANYLGERANIOL IN ENDOSPERM NUCELLUS OF ECHINOCYSTIS MACROCARPA GREENE. J Biol Chem. 1965 Apr;240:1847–1854. [PubMed] [Google Scholar]
- Galston A. W., Baker R. S. STUDIES ON THE PHYSIOLOGY OF LIGHT ACTION. IV. LIGHT ENHANCEMENT OF AUXIN-INDUCED GROWTH IN GREEN PEAS. Plant Physiol. 1951 Apr;26(2):311–317. doi: 10.1104/pp.26.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. A. Intracellular Localization of the Tryptophan-indoleacetate Enzyme System. Plant Physiol. 1958 Jan;33(1):23–27. doi: 10.1104/pp.33.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. A., Paleg L. G. Formation of auxin from tryptophan through action of polyphenols. Plant Physiol. 1961 Nov;36(6):838–845. doi: 10.1104/pp.36.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. Tracking cobalt project. Nature. 1965 Sep 18;207(5003):1311–1311. doi: 10.1038/2071311a0. [DOI] [PubMed] [Google Scholar]
- Hamilton R. H. Isolation of indole-3-acetic acid from corn kernels & etiolated corn seedlings. Plant Physiol. 1961 May;36(3):354–359. doi: 10.1104/pp.36.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantican B. P., Muir R. M. Isolation and properties of the enzyme system forming indoleacetic Acid. Plant Physiol. 1967 Aug;42(8):1158–1160. doi: 10.1104/pp.42.8.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune D. C., Galston A. W. Inverse Effects of Gibberellin on Peroxidase Activity and Growth in Dwarf Strains of Peas and Corn. Plant Physiol. 1959 Jul;34(4):416–418. doi: 10.1104/pp.34.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OBREITER J. B., STOWE B. B. CARBOXMETHYLCELLULOSE: A BINDER FOR THIN-LAYER CHROMATOGRAPHY OF LIPIDS AND INDOLES. J Chromatogr. 1964 Oct;16:226–228. doi: 10.1016/s0021-9673(01)82464-x. [DOI] [PubMed] [Google Scholar]
- Perley J. E., Stowe B. B. On the ability of Taphrina deformans to produce indoleacetic acid from tryptophan by way of tryptamine. Plant Physiol. 1966 Feb;41(2):234–237. doi: 10.1104/pp.41.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell L. E. Preparation of Indole Extracts from Plants for Gas Chromatography and Spectrophotofluorometry. Plant Physiol. 1964 Sep;39(5):836–842. doi: 10.1104/pp.39.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell L. E. Separation of Plant Growth Regulating Substances on Silica Gel Columns. Plant Physiol. 1960 Mar;35(2):256–261. doi: 10.1104/pp.35.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdovinos J. G., Ernest L. C. Gibberellin-Enhanced CO(2) Release from Tryptophan-1-C-14 in Plant Apical Tissue. Plant Physiol. 1966 Nov;41(9):1551–1552. doi: 10.1104/pp.41.9.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdovinos J. G., Perley J. E. Metabolism of tryptophan in petioles of coleus. Plant Physiol. 1966 Dec;41(10):1632–1636. doi: 10.1104/pp.41.10.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]


