Abstract
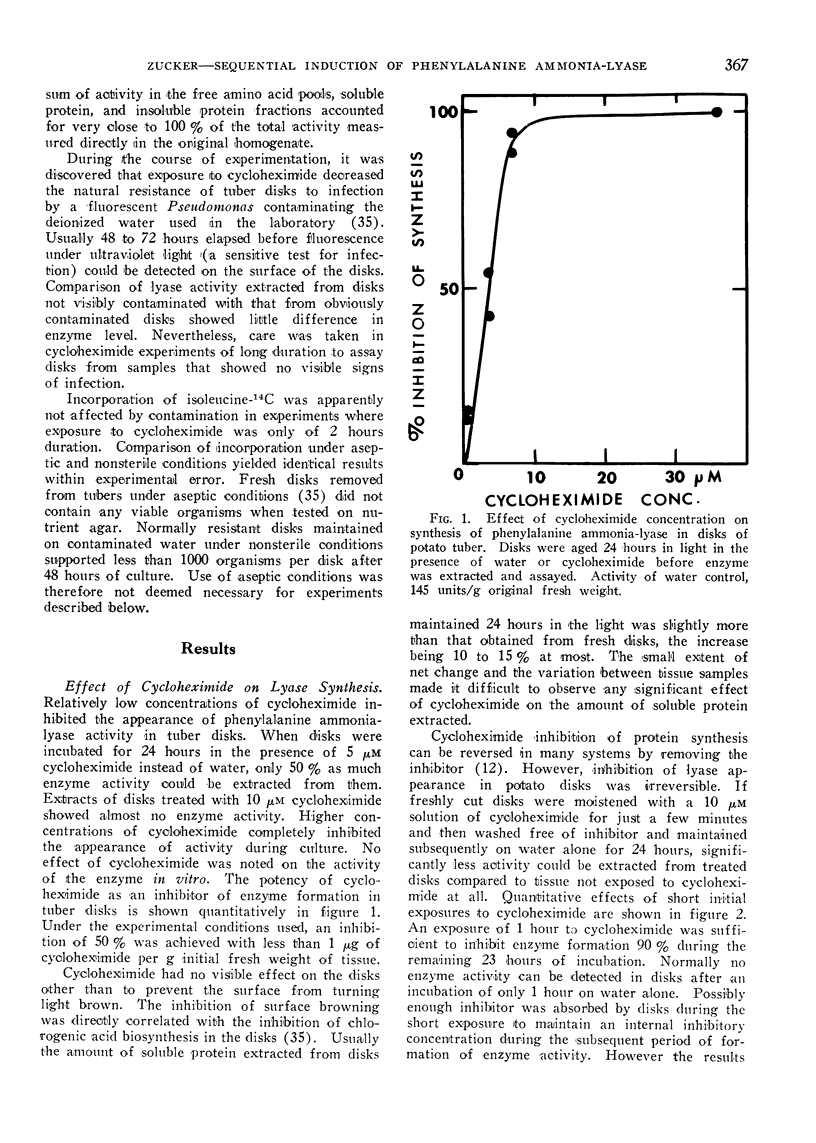
The light induced synthesis of phenylalanine ammonia-lyase in disks cut from potato tubers is very sensitive to cycloheximide. Synthesis is inhibited 50% in disks cultured on 5 μm cycloheximide instead of water and almost completely in disks aged in the presence of 10 μm inhibitor. Inhibition is irreversible. Fresh disks exposed only 1 hour to 10 μm cycloheximide do not synthesize enzyme during the subsequent 24 hours.
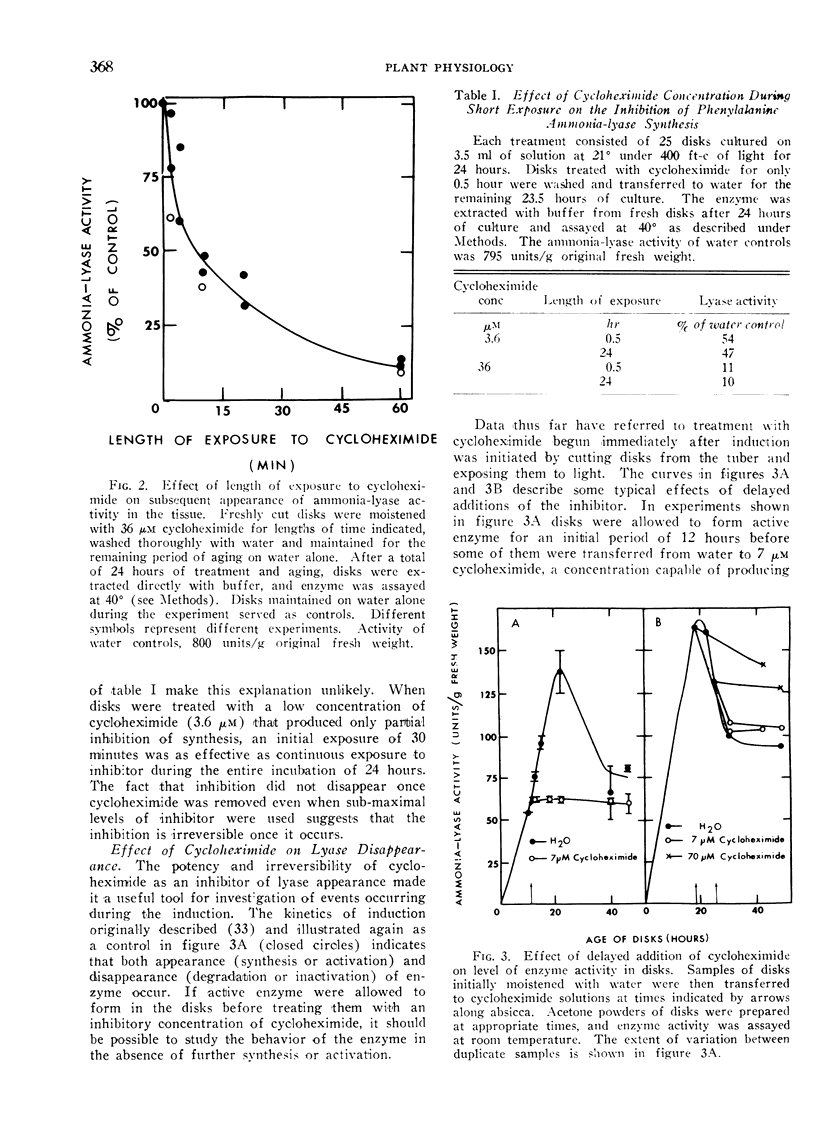
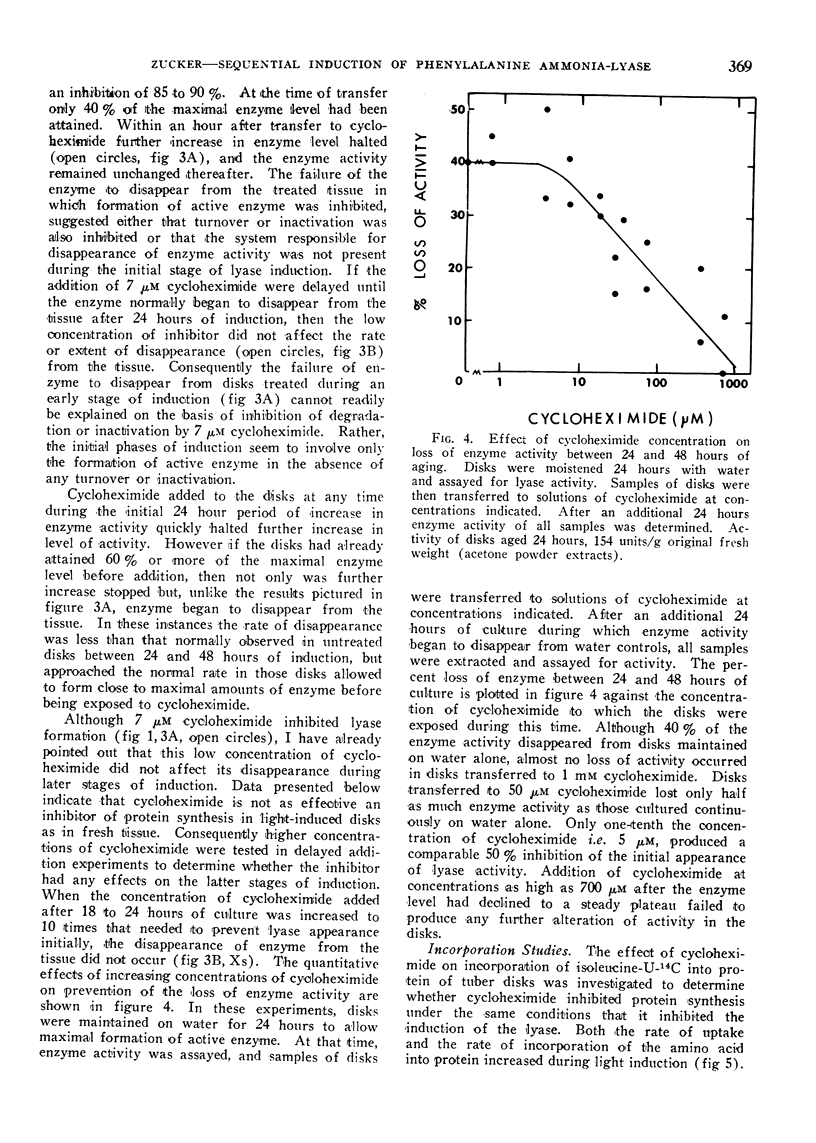
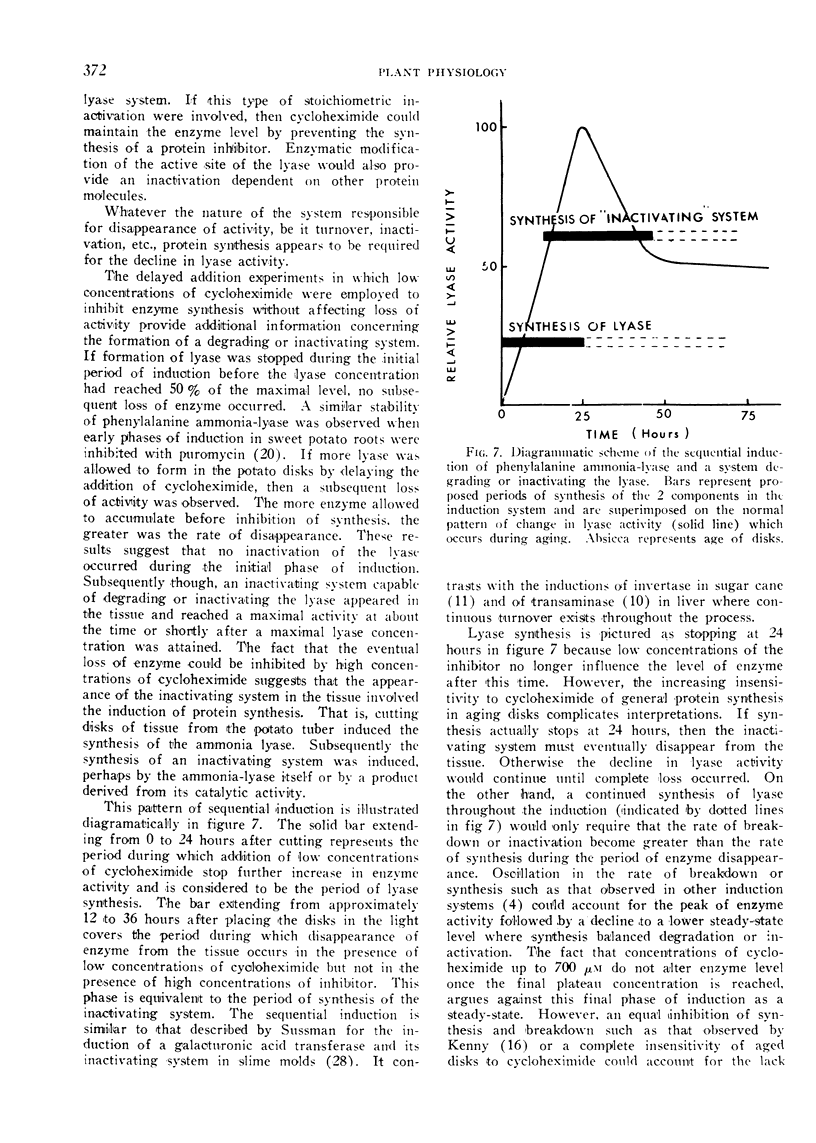
Normally a maximal enzyme activity develops in disks about 24 hours after being cut from the tuber. Thereafter enzyme activity declines. The disappearance of enzyme is not affected by concentrations of cycloheximide sufficient to inhibit the synthesis of enzyme initially. No disappearance of enzyme is noted during the initial phase of induction if enzyme synthesis is inhibited by cycloheximide. However, enzyme does disappear from the tissue if more than half the maximal enzyme content is allowed to form before synthesis is inhibited. If cycloheximide at a concentration 10-fold that needed to inhibit synthesis completely is added to disks after they have attained a maximal enzyme level, then subsequent loss of enzyme activity from the tissue is prevented. The initial stability of the enzyme in the absence of further synthesis and the inhibition of enzyme disappearance by high concentrations of cycloheximide suggest A) that early phases of induction involve synthesis of enzyme protein in the absence of turnover, B) that a system capable of degrading or inactivating the lyase subsequently forms in the tissue, and C) that the formation of the degrading or inactivating system requires protein synthesis.
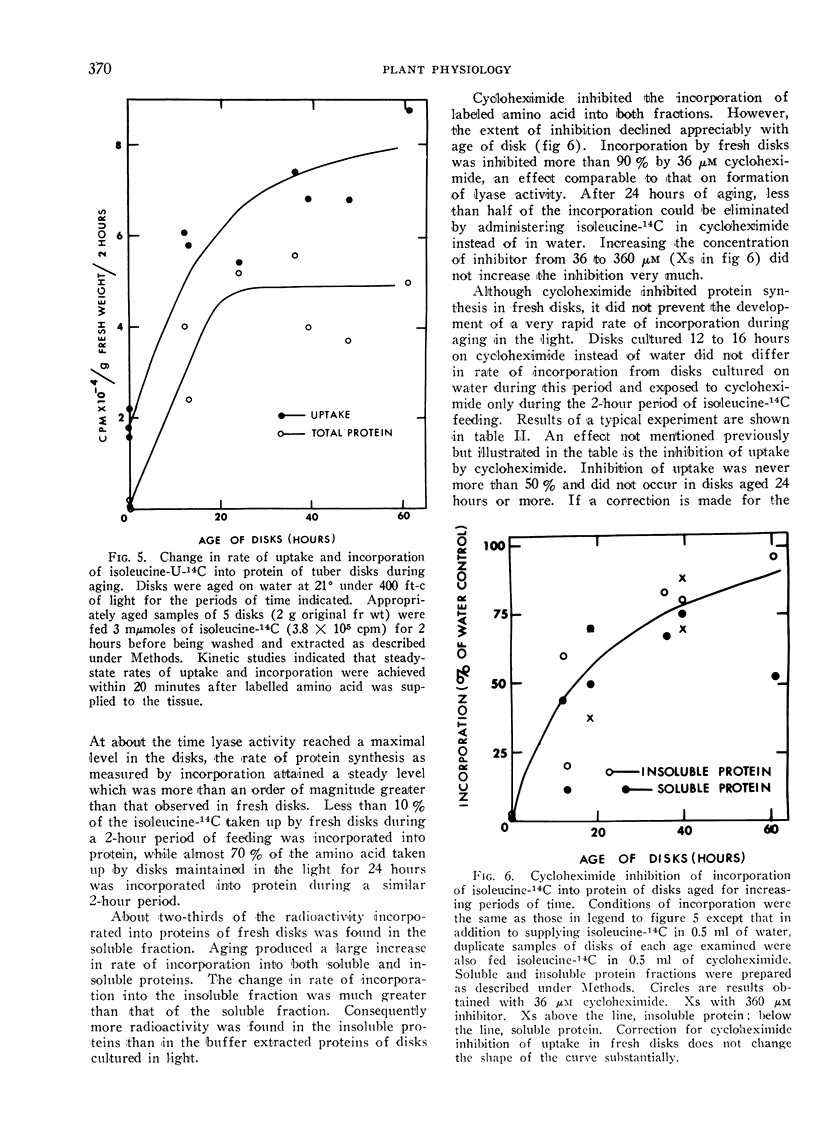
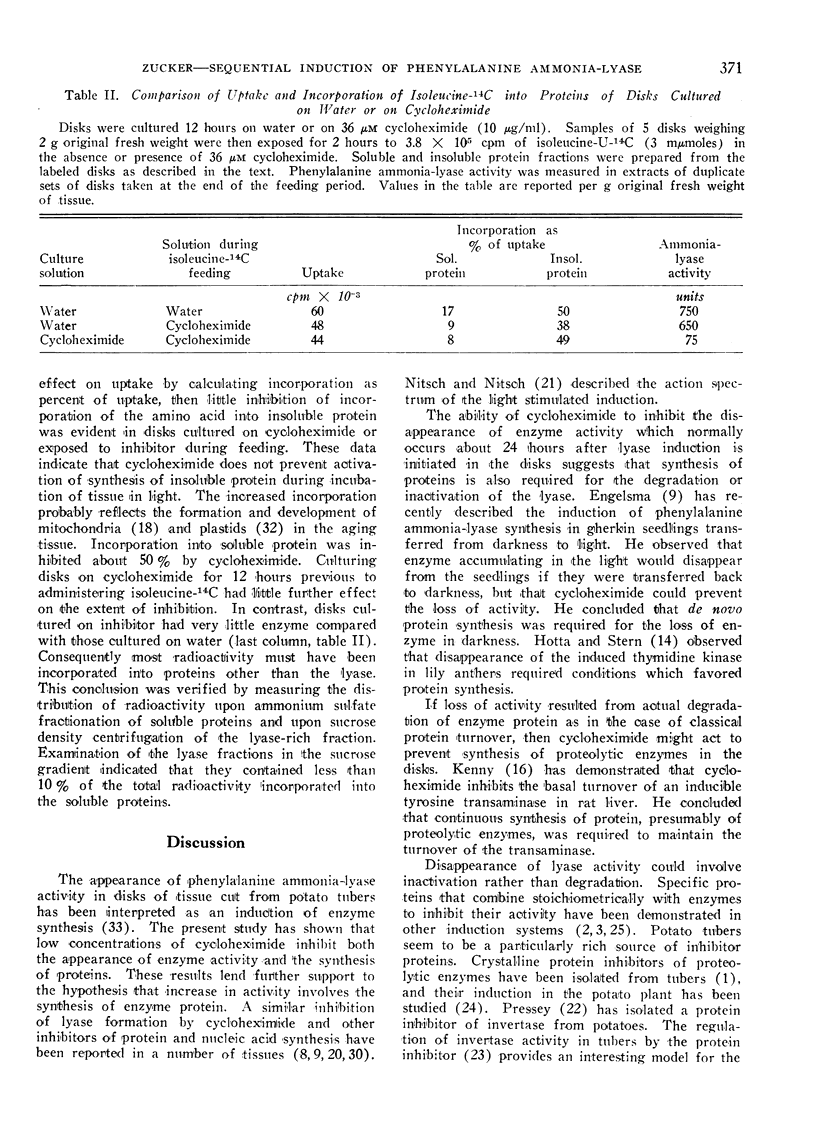
The effect of cycloheximide on uptake and incorporation of l-isoleucine-U-14C into soluble and insoluble proteins of tuber disks was also examined. During induction the rate of uptake increased 3 to 4-fold, and the rate of incorporation into protein, corrected for change in uptake, increased 25-fold. Cycloheximide inhibited incorporation of isoleucine-14C into proteins of fresh disks more than 80%. It did not prevent activation of general protein synthesis during induction and inhibited incorporation in induced disks only 20%. At all times incorporation of amino acid into the soluble, lyase-rich, protein fraction was more sensitive to cycloheximide than the insoluble fraction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALLS A. K., RYAN C. A. CONCERNING A CRYSTALLINE CHYMOTRYPTIC INHIBITOR FROM POTATOES, AND ITS BINDING CAPACITY FOR THE ENZYME. J Biol Chem. 1963 Sep;238:2976–2982. [PubMed] [Google Scholar]
- BEEVERS H., WALKER D. A. The oxidative activity of particulate fractions from germinating castor beans. Biochem J. 1956 Jan;62(1):114–120. doi: 10.1042/bj0620114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechet J., Wiame J. M. Indication of a specific regulatory binding protein for ornithinetranscarbamylase in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1965 Nov 8;21(3):226–234. doi: 10.1016/0006-291x(65)90276-7. [DOI] [PubMed] [Google Scholar]
- Bernhardt W., Panten K., Holzer H. Gedämpftes Oscillieren der Synthesegeschwindigkeit von DPN-Abhängiger Glutamatdehydrogenase in Hefezellen. Biochim Biophys Acta. 1965 Jun 22;99(3):531–539. [PubMed] [Google Scholar]
- CLICK R. E., HACKETT D. P. THE ROLE OF PROTEIN AND NUCLEIC ACID SYNTHESIS IN THE DEVELOPMENT OF RESPIRATION IN POTATO TUBER SLICES. Proc Natl Acad Sci U S A. 1963 Aug;50:243–250. doi: 10.1073/pnas.50.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst F., Mohr H. Phytochrome-mediated induction of enzyme synthesis in mustard seedlings(Sinapis alba L.). Naturwissenschaften. 1966 Oct;53(20):531–532. doi: 10.1007/BF00600655. [DOI] [PubMed] [Google Scholar]
- Engelsma G. Effect of cycloheximide on the inactivation of phenylalanine deaminase in gherkin seedlings. Naturwissenschaften. 1967 Jun;54(12):319–320. doi: 10.1007/BF00640619. [DOI] [PubMed] [Google Scholar]
- GARREN L. D., HOWELL R. R., TOMKINS G. M., CROCCO R. M. A PARADOXICAL EFFECT OF ACTINOMYCIN D: THE MECHANISM OF REGULATION OF ENZYME SYNTHESIS BY HYDROCORTISONE. Proc Natl Acad Sci U S A. 1964 Oct;52:1121–1129. doi: 10.1073/pnas.52.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasziou K. T., Waldron J. C., Bull T. A. Control of invertase synthesis in sugar cane. Loci of auxin and glucose effects. Plant Physiol. 1966 Feb;41(2):282–288. doi: 10.1104/pp.41.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman A. P. Structural basis for inhibition of protein synthesis by emetine and cycloheximide based on an analogy between ipecac alkaloids and glutarimide antibiotics. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1867–1874. doi: 10.1073/pnas.56.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOTTA Y., STERN H. Molecular facets of mitotic regulation. II. Factors underlying the removal of thymidine kinase. Proc Natl Acad Sci U S A. 1963 Jun;49:861–865. doi: 10.1073/pnas.49.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleman J. M., Key J. L. Inactive and protein precursor pools of amino acids in the soybean hypocotyl. Plant Physiol. 1967 Jan;42(1):29–36. doi: 10.1104/pp.42.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jick H., Shuster L. The turnover of microsomal reduced nicotinamide adenine dinucleotide phosphate-cytochrome c reductase in the livers of mice treated with phenobarbital. J Biol Chem. 1966 Nov 25;241(22):5366–5369. [PubMed] [Google Scholar]
- Kenney F. T. Turnover of rat liver tyrosine transaminase: stabilization after inhibition of protein synthesis. Science. 1967 Apr 28;156(3774):525–528. doi: 10.1126/science.156.3774.525. [DOI] [PubMed] [Google Scholar]
- Leaver C. J., Key J. L. Polyribosome formation and RNA synthesis during aging of carrot-root tissue. Proc Natl Acad Sci U S A. 1967 May;57(5):1338–1344. doi: 10.1073/pnas.57.5.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A., Feeley J. Ribosome activation and polysome formation in vitro: requirement for ATP. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1770–1777. doi: 10.1073/pnas.56.6.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R., Shaw R. Effect of temperature on invertase, invertase inhibitor, and sugars in potato tubers. Plant Physiol. 1966 Dec;41(10):1657–1661. doi: 10.1104/pp.41.10.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A., Huisman O. C. Chymotrypsin inhibitor I from potatoes: a transient protein component in leaves of young potato plants. Nature. 1967 Jun 3;214(5092):1047–1049. doi: 10.1038/2141047a0. [DOI] [PubMed] [Google Scholar]
- SWARTZ M. N., KAPLAN N. O., LAMBORG M. F. A heat-activated diphosphopyridine nucleotide pyrophosphatase from Proteus vulgaris. J Biol Chem. 1958 Jun;232(2):1051–1063. [PubMed] [Google Scholar]
- Zucker M. The Influence of Light on Synthesis of Protein and of Chlorogenic Acid in Potato Tuber Tissue. Plant Physiol. 1963 Sep;38(5):575–580. doi: 10.1104/pp.38.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]


