Abstract
Hepatitis, proliferative typhlitis, and colitis were characterized in young adult and older SCID/NCr mice naturally infected with Helicobacter hepaticus. Liver lesions consisted of Kupffer, Ito, and oval cell hyperplasia along with multifocal to coalescing coagulative hepatocyte necrosis. Numerous Warthin-Starry-positive bacteria were observed in the parenchyma, and there were minimal to mild accumulations of monocytic cells and neutrophils. Proliferative typhlitis was characterized by moderate to marked mucosal epithelial cell hyperplasia with mild monocytic and neutrophilic infiltration. Minimal to mild colitis with mucosal epithelial cell hyperplasia of the colon was most marked in older mice. Comparable gastrointestinal lesions were not observed in uninfected control SCID/NCr mice. H. hepaticus was cultured from fetal viscera of 2 of 11 pups sampled late in gestation from infected SCID/NCr females, suggesting transplacental infection of H. hepaticus. As expected, most of the naturally infected SCID/NCr mice had no serum immunoglobulin G response against H. hepaticus. These findings contrast with those in infected immunocompetent A/JCr mice, which develop a significant immune response to H. hepaticus associated with prominent multifocal mononuclear cell infiltrates in the liver, with only rare bacteria observable at the periphery of inflammatory foci or in the biliary canaliculi. The results demonstrate that chronic inflammatory and proliferative lesions simultaneously affecting the liver, cecum, and colon are associated with natural infection of SCID/NCr mice with H. hepaticus and that lesions are progressive with age. Concurrent infection with H. hepaticus may confound studies that have been attributed to similar lesions due to other experimental manipulations of SCID/NCr mice.
Since the discovery of Helicobacter pylori as the infectious cause of chronic, active gastritis in humans (19, 24), additional Helicobacter species have been isolated from humans and animals. Considerable research has focused on helicobacter-associated diseases such as gastritis, peptic ulcers, and gastric cancers in humans and animals (11, 12, 21, 22, 32, 44), cholecystitis in humans (13), bacteremia in immunodeficient humans (33), and hepatitis, hepatocellular carcinoma, and inflammatory bowel disease in mice (4, 15, 17, 29, 38–41). Mouse models of helicobacter infection are proving to be valuable for investigating the complex etiopathogenesis of several human idiopathic chronic inflammatory diseases that are hypothesized to be the result of an exaggerated host response to either the normal enteric flora or unidentified pathogens. Mouse models have allowed comparison of the host response to persistent helicobacter infection against different inbred genetic backgrounds as well as between various strains of mice that differ in immunocompetence.
Helicobacter hepaticus is associated with a chronic, active hepatitis in susceptible mouse strains including A/JCr, BALB/CAnNCr, SJL/NCr, B6C3F1, SCID/NCr, and C3H/HeNCr mice (14, 16, 17, 41). The A/JCr mouse, an immunocompetent strain, and SCID/NCr, an immunodeficient strain, appear to be among the most disease-susceptible mouse strains identified to date, and in addition to hepatitis, H. hepaticus-infected mice develop some features of inflammatory bowel disease. H. hepaticus infection in A/JCr mice has been well characterized on a longitudinal basis through 18 months of age (15, 41). In this strain of mouse, H. hepaticus persists in the large bowel and liver and is associated with a chronic proliferative hepatitis and liver cancer and, in some mice, chronic typhlitis (15, 41). Severe transmural typhlitis and transmural enterocolitis have also been found in experimentally infected male A/JCr mice (43) and female germ-free outbred mice (17), respectively. Infected A/JCr mice develop sustained serum immunoglobulin G (IgG) antibody responses to H. hepaticus and elevations in enzyme levels in serum indicative of hepatocellular injury. H. hepaticus-associated hepatitis and progression to hepatocellular carcinoma is most prevalent after 1 year postinfection. A clinical syndrome of inflammatory bowel disease and rectal prolapse, with and without concomitant hepatitis, has been described in several strains of genetically manipulated immunocompromised mice, as well as young immunodeficient SCID/NCr and nude mice infected with H. hepaticus (10, 29, 39) and with H. bilis (31).
The importance of the immune response in either limiting colonization or contributing to the intensity of the inflammatory response to helicobacter infections has been widely debated. In A/JCr mice, the development of helicobacter-associated lesions, particularly hepatitis and typhlitis, has been associated with the concomitant development of a significant serum IgG and Th1 cell-mediated immune response to H. hepaticus antigens that intensifies over the course of infection (15, 38, 41, 43). Antibody responses to H. hepaticus infection tend to be low in strains of mice that are persistently colonized in the gut but do not develop hepatitis, whereas significant serum antibody to H. hepaticus antigens is demonstrable in strains of mice that do develop hepatitis (42). Ward et al. reported that mice infected with H. hepaticus develop antibodies to hsp70 expressed both by H. hepaticus and diseased hepatocytes and suggested that such cross-reactive host responses may contribute to lesion development (40). SCID C.B-17 mice reconstituted with memory CD45RBhigh CD4+ T cells developed severe inflammatory bowel disease when concurrently infected experimentally with H. hepaticus, in contrast to a lower incidence in nonreconstituted but infected immunodeficient controls (4). However, when SCID C.B-17 and congenic immunocompetent mice were experimentally infected with H. felis to model H. pylori gastritis, similar grades of gastritis and colonization developed in mice incapable and capable, respectively, of mounting an immune response to H. felis, suggesting that the antibody response to helicobacters is not necessary to induce disease (2).
In the present study, we assessed the extent of hepatitis, proliferative typhlitis, and colitis that developed in SCID/NCr mice naturally infected with H. hepaticus up to the age of 10 months. The objective was to evaluate if the severity of H. hepaticus-associated lesions is progressive with age in an immunodeficient host genotype, as has been established in older H. hepaticus-infected A/JCr mice (15, 41, 43). In addition, because H. hepaticus-infected SCID/NCr mice have many more bacteria observable within the liver than do the infected but immunocompetent A/JCr mice, we determined if transplacental infection of fetuses with H. hepaticus occurred in these mice.
MATERIALS AND METHODS
Animals.
In the first part of this retrospective study, SCID/NCr mice naturally infected with H. hepaticus were kindly supplied by the National Cancer Institute (Frederick, Md.) (14, 41). These included 9 young adult males (4 to 6 months old), 14 older males (9 to 10 months old), and 8 pregnant females (6 to 8 months old). After the colony had been rederived to eliminate H. hepaticus, 6 older (9 to 10 months old) male SCID/NCr mice that were confirmed to be H. hepaticus free were evaluated as negative controls. In addition, 11 fetuses from 8 pregnant SCID/NCr mice and 14 fetuses from 4 pregnant A/JCr mice naturally infected with H. hepaticus were sampled for evidence of transplacental transmission of the infection. Upon arrival, the mice were either necropsied immediately or briefly housed under isolated, quarantine conditions in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Necropsy sampling.
At necropsy, samples of the cecum, colon, and liver were collected aseptically for culture and PCR to identify H. hepaticus in tissues. In pregnant female mice, fetuses were aseptically removed from the uterus and the fetal viscera were ground and then cultured for H. hepaticus. Liver samples were processed in Karnovsky’s fixative for transmission electron microscopy. The remaining tissues from each mouse were fixed in neutral buffered 10% formalin for histopathological and immunofluorescence evaluation.
Histopathology.
After 24 h of fixation in formalin, tissue samples were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin-eosin and by the Warthin-Starry method. Samples were taken from each liver lobe longitudinally from the apical or free margin to the hilus. Separate sections of liver, cecum, and colon were mounted on Probe-On Plus slides (Fisher, Pittsburgh, Pa.) to demonstrate the bacterium in tissues by immunofluorescence.
Liver lesions were graded for hepatocyte necrosis, inflammatory cell infiltrates in the parenchyma, periportal and perivenous areas, oval cell and bile duct hyperplasia, Kupffer and Ito cell hyperplasia, and hepatocyte pleomorphism. Each of these parameters was graded semiquantitatively as 0 (normal), 1 (minimal; <5% involvement), 2 (mild; 5 to 25% involvement), 3 (moderate; 25 to 50% involvement) or 4 (severe; >50% involvement) in the liver. The score from each parameter was averaged for each mouse and then compared for each group. The cecum and colon were examined for the extent of inflammatory cell infiltrates in the wall, especially the mucosa and submucosa, and for epithelial cell hyperplasia by using the same grading system. Lesion scores were compared for significant differences by the Mann-Whitney test for nonparametric statistics.
Immunofluorescence.
Liver, cecum, and colon samples were processed for immunofluorescence by using polyclonal rabbit anti-H. hepaticus sera (15). Briefly, tissue sections were deparaffinized in xylene and rehydrated through ethanol to water. The sections were preincubated with normal rabbit serum (Sigma, St. Louis, Mo.) for 5 min to block nonspecific binding and then incubated with anti-H. hepaticus serum at 1:100 dilution. Fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Sigma) at a 1:50 dilution was then applied. The slides were rinsed with phosphate-buffered saline (PBS) between staining steps, and incubations were done in a humid chamber at room temperature for 1 h. After a final wash with PBS, the slides were coverslipped with buffered glycerol and examined under a Zeiss fluorescent microscope. Control staining was performed in the same manner except that normal rabbit serum was substituted for the polyclonal antibody.
Transmission electron microscopy.
Liver samples were fixed in Karnovsky’s fixative, postfixed with 1% osmium tetroxide, dehydrated through graded acetone, and embedded in Epon-Araldite 6005 mixture (23). The plastic blocks were semi-thin sectioned at 1 μm and stained with methylene blue/azure II. The areas of interest were identified under a light microscope, cut from the plastic block, and mounted to a plastic bean capsule. A 50- to 70-nm-thick section was then cut, stained with uranyl acetate-lead citrate, and visualized with a transmission electron microscope.
Bacterial isolation.
Samples of the liver, cecum, and colon were collected aseptically from each mouse and from the viscera of 20- to 21-day-old SCID/NCr (n = 11) and A/JCr (n = 14) pups aseptically harvested in utero. The tissues were homogenized in 1 ml of PBS, filtered through a 0.45-μm-pore-size nylon filter (Millipore, Bedford, Mass.), and cultured for H. hepaticus on blood agar supplemented with 1% trimethoprim, vancomycin, and polymyxin (Remel Labs, Lenexa, Kans.). The plates were incubated at 37°C under microaerobic conditions in vented jars (90% N2, 5% H2, 5% CO2). Cultured organisms were evaluated biochemically by measuring urease production and morphologically by Gram stain and phase-contrast microscopy.
PCR.
PCR evaluation of liver, cecum, and colon samples was performed to supplement previously described (30) culture techniques to confirm infection with H. hepaticus. Briefly, bacterial DNA was isolated from tissue samples by the alkali lysis method. The DNA pellet was then redissolved in sterile distilled water. Two oligonucleotides, 5′ GCA TTT GAA ACT GTT ACT CTG 3′ and 5′ CTG TTT TCA AGC TCC CC 3′, were used as primers for amplification. These primers recognize an H. hepaticus-specific region of the 16S rDNA and produce an amplified product of 417 bp. A total of 35 cycles were performed at 94, 61, and 72°C for 1, 2 1/4, and 2 1/2 min, respectively, followed by an elongation step for 7 min at 72°C. The PCR products were separated by electrophoresis on a 6% Visigel separation matrix gel (Stratagene, La Jolla, Calif.), stained with ethidium bromide, and visualized with UV illumination.
ELISA for serum IgG.
Serum was collected at necropsy and screened for serum IgG specific for H. hepaticus antigens by using a standard enzyme-linked immunosorbent assay (ELISA) method described previously (43). Positive and negative controls for detection of serum IgG specific for H. hepaticus antigens were A/JCr male mice that had been experimentally infected with H. hepaticus for 1 year or were known to be H. hepaticus free, respectively.
RESULTS
Pathologic findings.
Grossly, two 4-month-old and one 6 month-old young adult males had single to multiple yellow to gray necrotic foci 0.5 to 2 mm in diameter in one or more liver lobes. Older males and pregnant females had a variably prominent reticular pattern in one or more liver lobes. The cecal wall and/or mucosa was mildly to moderately thickened, and the mucosa appeared to be rough or granular in selected older males and two pregnant females. The severity of the histologic changes in the liver, cecum, and colon is shown in Fig. 1. The major findings in the liver are illustrated in Fig. 2 to 7, and those in the cecum and colon are illustrated in Fig. 8 to 10.
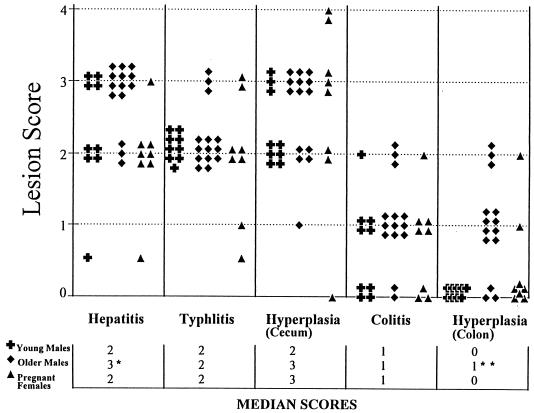
FIG. 1.
Severity of liver, cecum, and colon lesions in H. hepaticus-infected young adult males, older males, and pregnant female SCID/NCr mice. Individual histologic scores and medians are shown. Older males had more severe hepatitis than pregnant females (P < 0.0032) but there was only a trend for older males to have more severe lesions than younger males. The extents of typhlitis, cecal epithelial hyperplasia, and colitis were similar among groups. Older males had greater colonic epithelial hyperplasia than did younger males or pregnant females (P < 0.05).
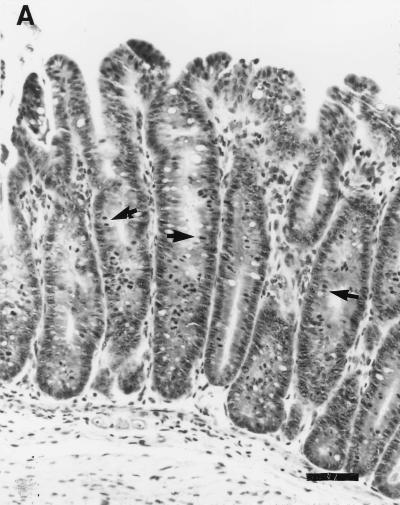
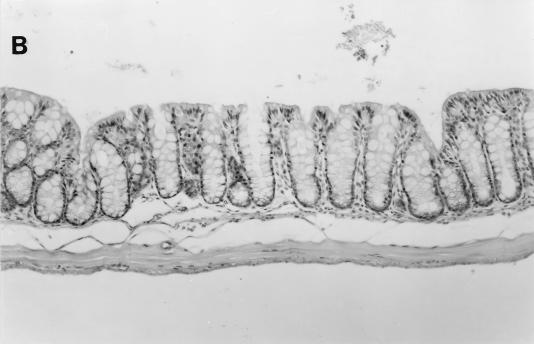
FIG. 8.
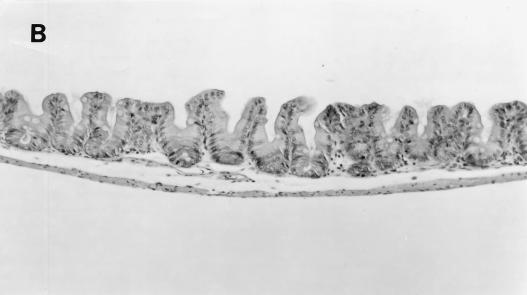
(A) Cecum section from a pregnant female SCID/NCr mouse. Marked epithelial cell hyperplasia in the mucosa villous folding of the mucosa into the cecal lumen, with many mitotic figures (arrows), and mild inflammatory cell infiltration in the mucosa are visible. Bar, 63 μm. (B) Cecum section from a control uninfected older male SCID/NCr mouse. In comparison to the cecum from an infected mouse (A), note the absence of mononuclear cells and mitotic figures and the normal, thin epithelial cell layer. Magnification, ×100.
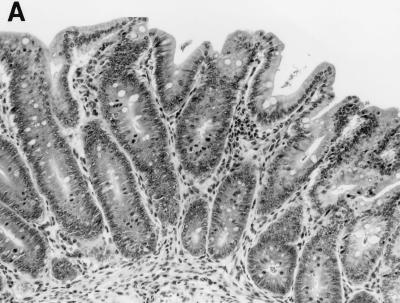
FIG. 10.
(A) Colon section from an older infected male SCID/NCr mouse. Note the mild inflammatory infiltrate of histiocytes and hyperplasia of mucosal epithelial cells. Magnificaiton, ×100. (B) Colon section from a control older uninfected male SCID/NCr mouse. In comparison to the colon from an infected mouse (A), note the absence of mononuclear cells and the normal, thin epithelial cell layer. Magnification, ×100.
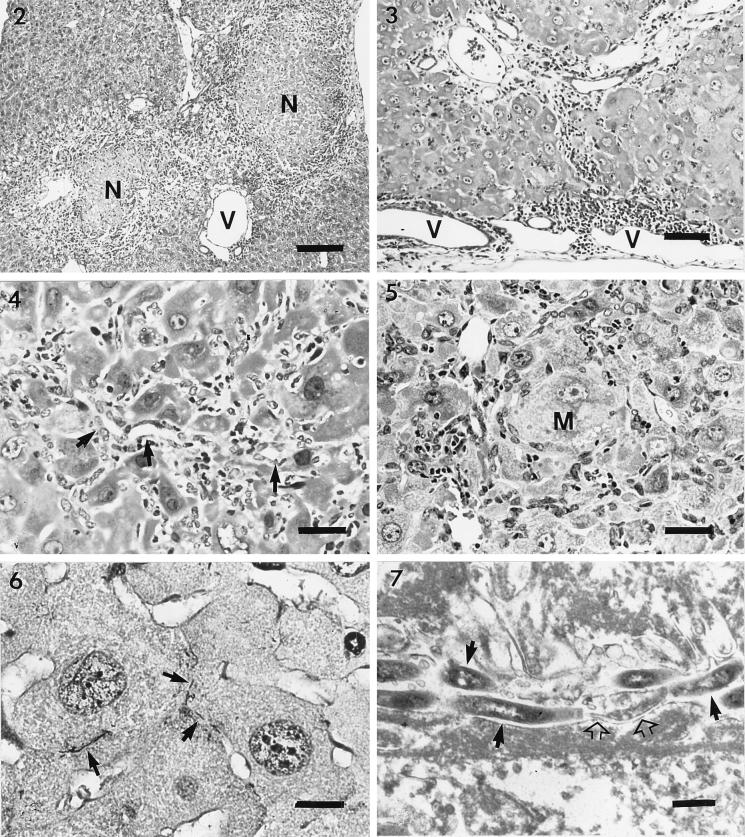
The predominant lesions in the livers of infected SCID/NCr mice were multifocal to coalescing coagulative necrosis of the hepatocyte parenchyma with minimal to mild infiltration of macrophages and neutrophils at the periphery (Fig. 2). The size of the necrotic foci varied from a few to numerous hepatocytes. Inflammatory cell infiltration was minimal to mild in the remaining parenchyma and periportal areas. In the livers of pregnant female SCID/NCr mice, mild infiltration of mononuclear cells and neutrophils was observed in the periportal and perivenous areas, with marked variation in hepatocellular size and shape (Fig. 3). In older males, inflammatory cell infiltration was also mild, but Kupffer cell, Ito cell, and oval cell hyperplasia or hypertrophy and hepatocyte pleomorphism were prominent in the affected livers (Fig. 4). A small number of macrophages and neutrophils accumulated multifocally in the parenchyma and periportal and perivenous areas. Some of the macrophages and Kupffer cells contained intracytoplasmic yellow to brown pigment. Hyperplastic oval cells radiated from the inflamed foci to adjacent hepatic parenchyma and sometimes bridged to adjacent portal areas, with occasional formation of bile ductules. Hepatocyte necrosis became less prominent in the parenchyma, but cytomegaly and karyomegaly of the hepatocytes were marked, with frequent intranuclear pseudoinclusions (Fig. 5). Collapse of hepatic plates and capsular surfaces due to the loss of hepatocytes was observed frequently. Hepatic changes in the younger males were similar but tended to be less severe than those in the older males; pregnant females had significantly less severe changes than did older males (P < 0.0032) (Fig. 1). Altered or adenomatous hepatocellular foci were not observed in the livers of any mice. All six of the older male H. hepaticus-free control SCID/NCr mice were free of comparable lesions in the liver (except for focal, peribiliary accumulations of histiocytic cells, not shown), cecum (see Fig. 8B), or colon (see Fig. 10B).
Warthin-Starry stain revealed that numerous H. hepaticus organisms were present in the liver parenchyma and the cecal and colonic mucosa of all infected mice but were absent from the liver sections of control mice. In the livers of infected mice, bacteria appeared localized between hepatocytes (Fig. 6). The severity of the hepatic lesions appeared to be associated with a relative abundance of bacteria. By transmission electron microscopy, spiral bacteria with morphology consistent with H. hepaticus were observed within bile canaliculi of affected livers (Fig. 7). The bile canaliculi were variably dilated, with swelling, blunting, and decreased numbers of canalicular microvilli. The hypertrophic hepatocytes contained abundant mitochondria and/or endoplasmic reticulum. Cytoplasmic invagination into nuclei was frequently seen (intranuclear pseudoinclusions were seen histologically). Hyperplastic or hypertrophic Ito cells often had markedly vacuolated cytoplasm and peripherally displaced nuclei. Hyperplastic or hypertrophic oval cells often formed small ductules, with variably patent lumina and intercellular junctional structures.
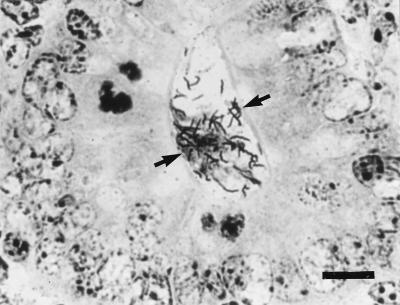
Proliferative typhlitis was observed in all infected mice except for one pregnant female (Fig. 1). Inflammatory cell infiltration, consisting mainly of macrophages and neutrophils, was mild, but mucosal epithelial cell hyperplasia was moderate to marked in all affected mice, particularly in two pregnant females (Fig. 8). The mucosa was markedly thickened, with frequent villous to papillomatous folds extending into the lumen. Epithelial cells were markedly basophilic and densely piled up and had a high mitotic index. Goblet cells were variably decreased in number or were completely absent in the proliferative mucosa. In the cecum and colon, bacteria morphologically consistent with H. hepaticus were located within the crypts or glandular lumen of the mucosa (Fig. 9). Minimal to mild colitis was seen in most mice in each group, whereas mucosal epithelial cell hyperplasia was observed in 11 of 14 older males and in 2 of 7 pregnant females and was absent in younger males (0 of 9) (Fig. 10).
FIG. 9.
Colon section from a pregnant female SCID/NCr mouse. Warthin-Starry stain reveals many spiral H. hepaticus-like organisms within a crypt (arrows). Bar, 9 μm.
Two infected SCID/NCr mice had a measurable serum IgG response to H. hepaticus by ELISA (see “ELISA for serum IgG”). The lesion severity scores for one of the two mice, an older male SCID/NCr mouse, were hepatitis [3], typhlitis [2] with epithelial cell hyperplasia in the cecum (2) and colitis [1] with epithelial cell hyperplasia in the colon [1]; scores for the second mouse, a pregnant female, were hepatitis [2], typhlitis [3] with epithelial cell hyperplasia in the cecum [4], and colitis [2] with epithelial cell hyperplasia in the colon [2].
Immunofluorescence.
As with the Warthin-Starry stain, immunofluorescence with anti-H. hepaticus serum revealed numerous organisms in the liver parenchyma. Some uninfected mice had very weakly fluorescent, scattered positive granules in the cytoplasm of a few hepatocytes, which may be due to cross-reactivity of the antisera with intracytoplasmic components. However, the pattern and intensity of the positive fluorescence in uninfected mice were clearly of a much lower magnitude from that observed in infected mice. The number of positive-staining helical bacteria appeared to correlate with the severity of hepatitis in the infected SCID/NCr mice. There were no positive-staining bacteria without evidence of hepatitis, nor were any bacteria noted in the livers of control mice.
Bacterial culture.
Bacterial cultures of selected liver, cecal, and colonic samples were performed for the control group of six older male SCID/NCr mice and for the naturally infected mice. The older male control SCID/NCr mice were free of H. hepaticus by culture of liver and cecal tissue. Samples of viscera from 2 of the 11 SCID/NCr fetuses were culture positive for H. hepaticus, whereas no recovery of H. hepaticus was obtained from any of the 14 A/JCr fetuses. Colonic cultures were 100% positive for recovery of H. hepaticus from SCID/NCr young adult males and pregnant females, and cecal cultures were all positive for recovery of H. hepaticus from young adult males and from five of seven pregnant females. Colonic and cecal cultures were contaminated in six of six older males, and the culture results were not interpretable. Liver cultures were positive for H. hepaticus in five of eight young adult males, nine of nine older males, and six of eight pregnant females. Bacterial cultures of the liver, cecal, and colonic samples were positive for H. hepaticus in at least one sample per mouse; therefore, on a cumulative basis, each mouse was H. hepaticus positive.
PCR.
The results of PCR analysis of H. hepaticus in tissue samples correlated well with those of bacterial culture. Liver, cecal, and colonic samples from pregnant females and older males were all positive for H. hepaticus. An amplified product of 417 bp was produced and visualized for each sample. For four older males for which the bacterial culture was contaminated, PCR analysis of specimens were all positive for H. hepaticus. Control mice were negative for all Helicobacter spp. by PCR.
ELISA for serum IgG.
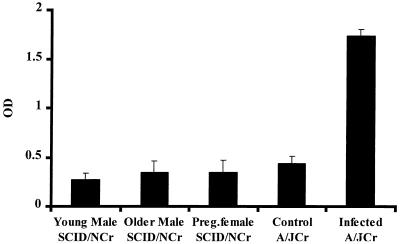
Serum samples from the SCID/NCr mice were compared by an H. hepaticus-specific ELISA to sera obtained from male A/JCr mice that had been experimentally infected with H. hepaticus for 1 year (positive controls) and to negative control sera obtained from H. hepaticus-free male A/JCr mice. The mean ELISA values measured for SCID/NCr mice of all age groups were lower than the mean value measured for the negative control A/JCr mice, indicating the absence of serum IgG to H. hepaticus in the SCID/NCr mice (Fig. 11). The positive control A/JCr mice had a high IgG response to H. hepaticus antigens. Serum from one older male and one pregnant female SCID/NCr mice yielded ELISA optical density values (0.94 and 0.78, respectively) that exceeded the mean plus 3 standard deviations of the ELISA values measured for the negative A/JCr mice (0.67), suggesting that these SCID/NCr mice were “leaky” and had developed some serum antibody to H. hepaticus.
FIG. 11.
Mean and standard deviation (error bars) of an ELISA measurement of serum IgG specific for H. hepaticus antigens. Only the positive control A/JCr mice infected with H. hepaticus for 1 year had a significant antibody response. OD, optical density.
DISCUSSION
Hepatitis, proliferative typhlitis, and colitis with progressive mucosal hyperplasia were characterized in this study of young adult male, pregnant female, and older male SCID/NCr mice naturally infected with H. hepaticus. Consistent with the results of previous experiments examining the effects of age and sex on the pathogenesis of H. hepaticus infection in A/JCr mice (15, 43), older males had significantly more severe hepatitis than did pregnant females and there was a trend for more severe lesions in the older males than in the younger males. All the infected mice had similar grades of mild to moderate typhlitis and minimal to mild colitis, but colonic mucosal hyperplasia was most severe in older males and notably absent in younger males and most pregnant females. This study is the first to show that colonic mucosal hyperplasia associated with persistent H. hepaticus infection in male SCID/NCr mice becomes more severe with age. Although control groups were not evaluated because uninfected SCID/NCr mice were not available at the time, subsequent analysis of older, uninfected SCID/NCr male mice and previous studies of control, uninfected SCID C.B-17 mice (4, 31) indicated that these findings are consistent with a hypothesis that a chronic inflammatory response to bacterial infection may be a cofactor for some forms of idiopathic hepatic disease (13) and inflammatory bowel disease (4, 28, 36). In addition, recovery of H. hepaticus from a limited number of near-term fetuses carried by infected SCID/NCr dams raises new concerns that transplacental infection of H. hepaticus may occur in naturally infected immunodeficient mice.
As expected, most SCID/NCr mice infected with H. hepaticus did not have detectable serum IgG against H. hepaticus antigens. The two SCID/NCr mice with a measurable antibody response were apparently leaky, a term used to describe the emergence of functional B cells in older SCID mice (1). These two mice had mild to severe lesions that varied by tissue (see Results). Because only two mice were leaky and their lesion scores were not significantly different from those of other mice within the same cohort, conclusive statements about the significance of their antibody response to H. hepaticus cannot be made. By light and electron microscopy, numerous bacteria with a morphology consistent with H. hepaticus were observed in the crypts of the cecum and colon of all infected mice and between hepatocytes or within bile canaliculi of the majority of the infected SCID/NCr mice. Persistence of infection was also supported by both bacterial culture and PCR analysis of liver, cecal, and colonic samples that were positive for H. hepaticus in at least one tissue per mouse. Recovery of H. hepaticus by culture from the livers of these naturally infected SCID/NCr mice was higher than in previous attempts to culture H. hepaticus from immunocompetent, naturally infected A/JCr mice (14, 15). Additionally, hepatic inflammation was less severe in the SCID/NCr mice than in the infected A/JCr mice, which have been shown to respond to H. hepaticus with a proinflammatory Th1-cell-mediated immune response (43). Taken together, the more moderate inflammatory response and larger number of organisms in SCID/NCr mice than in immunocompetent A/JCr mice support the role of an adaptive immune response in limiting colonization of the liver with H. hepaticus. This suggests that the immune response inhibits colonization and/or clearance of the bacteria from the liver by potentially preventing bacterial adherence to the mucosa by IgA, by antibody-mediated killing of bacteria by Fc-receptor positive phagocytic cells, and by complement-mediated bacterial cell lysis. SCID/NCr mice also are deficient in important T-cell-mediated responses that have been shown to be proinflammatory in H. hepaticus-infected AJ/Cr mice (43).
There also is evidence the host immune response has limited ability to restrict helicobacter replication and spread, particularly during mucosal surface infection. Not only can some intestinal helicobacters colonize the liver, but also vertical transmission of a helicobacter was first proposed when “H. rappini” was suspected to have crossed the placenta and caused hepatic necrosis in fetuses during natural infection in presumably immunocompetent sheep (3). The same isolate reproduced liver necrosis in the fetuses when given as an experimental challenge to pregnant guinea pigs (3). The apparent transplacental H. hepaticus infection in the SCID/NCr mice in our study suggests that immunodeficiency is a contributing factor; more studies are needed before definitive conclusions can be made. A role for the adaptive immune response in limiting helicobacter colonization or influencing the extent of inflammation was not supported by studies of H. felis-induced gastritis in SCID C.B-17 mice (2). The number of H. felis organisms was not statistically different between immunodeficient and congenic immunocompetent mice, and the two groups of mice had similar grades of gastritis. There are important differences between the H. felis and H. hepaticus mouse models that may explain the disparity between the immune status and the ability or inability to limit bacterial colonization. The H. felis mouse model involves a gastric mucosal infection produced by an acute experimental challenge with a large number of organisms that are not naturally acquired mouse pathogens. In contrast, natural infection of the mouse with H. hepaticus results in colonization of the lower bowel in virtually all mouse strains examined, but colonization of the liver occurs only in select inbred strains, indicating that genetic factors such as those that regulate the immune response are critical to host defense. Lesions observed to develop in the infected SCID/NCr mice in this study may have been promoted by other host and bacterial virulence factors suspected to be involved in the pathogenesis of helicobacter infection and disease. In brief, these include chemotactic factors released from monocytes and neutrophils, as well as bacterial release of cytotoxin, urease, and lipopolysaccharide (5–8, 20, 25–27, 34, 35, 37, 43).
Our results with younger SCID/NCr mice are similar to other descriptions of H. hepaticus-associated hepatitis, typhlitis with or without associated colitis, and proctitis with rectal prolapse in young SCID and other immunodeficient mice (10, 29, 38, 39). Interestingly, our data demonstrates that colonic mucosal proliferation was more severe in older than in younger male H. hepaticus-infected SCID/NCr mice. Mild to severe typhlitis has been observed occasionally in infected A/JCr mice (15, 34, 39, 43). In contrast, proliferative typhlitis was marked and consistent in the SCID/NCr mice. The lesions of H. hepaticus-associated hepatitis and proliferative typhlitis found in a small number of male SCID/NCr mice from the age of 7 weeks through 5 months (29) were very similar to the lesions noted in the 4- to 6-month-old male mice in our study. We recently described inflammatory large bowel disease with rectal prolapse in several strains of immunodeficient mice infected with H. hepaticus, including six female SCID/NCr mice that were 32 to 42 weeks old (39). All of the mice had variable degrees of proliferative typhlitis, colitis, and proctitis with rectal prolapse, but concomitant hepatitis was rarely observed. This low incidence of hepatitis may be related to the fact that only 9 of 64 mice examined were male and these male mice were on a C57BL/6 background, which is resistant to the development of helicobacter-associated hepatitis (40). The H. hepaticus-infected SCID/NCr mice examined in this study showed consistent development of hepatitis and proliferative typhlitis and variable colitis, without concomitant proctitis and rectal prolapse. The difference in the extent and distribution of lesions between mice of similar genetic backgrounds is currently unexplained, but environmental factors (18) and the interaction of H. hepaticus with other resident enteric bacteria (9) have received the most emphasis. Thus, the mechanisms that govern the spectrum of disease manifestation in H. hepaticus-infected mice cannot be attributed solely to the host immune response and appear to be multifactorial.
FIG. 2-7.
Liver section from an adult male SCID/NCr mouse. Multifocal coagulative necrosis (N) of hepatocytes and inflammatory cell infiltration at the periphery and in the periportal and perivenous areas (V) are visible. Bar, 170 μm.
Fig. 2 Liver section from an adult male SCID/NCr mouse. Multifocal coagulative necrosis (N) of hepatocytes and inflammatory cell infiltration at the periphery and in the periportal and perivenous areas (V) are visible. Bar, 170 μm.
Fig. 3 Liver section from a pregnant female SCID/NCr mouse. Mild infiltration of mononuclear cells and neutrophils in the periportal and perivenous areas (V) and variation in hepatocellular size and shape are visible. Bar, 70 μm.
Fig. 4 Liver section from an older male SCID/NCr mouse. Oval cell hyperplasia with formation of bile ductules (arrows), hepatocytomegaly and karyomegaly, and inflammatory cell infiltration are visible. Bar, 48 μm.
Fig. 5 Liver section from an older male SCID/NCr mouse. Hepatocytomegaly and karyomegaly (M) surrounded by inflammatory cells and hyperplasia or hypertrophy of oval and Kupffer cells (arrows) are visible. Bar, 46 μm.
Fig. 6 Liver section from an older male SCID/NCr mouse. Warthin-Starry stain reveals many spiral H. hepaticus cells with the hepatic parenchyma or between the hepatocytes (arrows). Bar, 10 μm.
Fig. 7 Liver section from an older male SCID/NCr mouse. Electron microscopy reveals several spiral-shaped H. hepaticus organisms in a bile canaliculus (arrows), some with a flagellum (open arrows). Bar, 0.7 μm.
ACKNOWLEDGMENTS
This research was supported in part by NIH grants RO1 CA 67529, RO1 DK 52413, and RR 07036.
REFERENCES
- 1.Ansell J D, Bancroft G J. The biology of the SCID mutation. Immunol Today. 1989;10:322–325. doi: 10.1016/0167-5699(89)90181-3. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard T G, Czinn S J, Nedrud J G, Redline R W. Helicobacter-associated gastritis in SCID mice. Infect Immun. 1995;63:1113–1115. doi: 10.1128/iai.63.3.1113-1115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryner J H, Ritchie A E, Pollet L, Kirkbride C A, Collins J E. Experimental infection and abortion of pregnant guinea pigs with a unique spirillum-like bacterium isolated from aborted ovine fetuses. Am J Vet Res. 1987;48:91–97. [PubMed] [Google Scholar]
- 4.Cahill R J, Foltz C J, Fox J G, Dangler C A, Powrie F, Schauer D B. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;65:3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Censini S, Lange C, Xiang Z Y, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cover T L, Cao P, Lind C D, Tham K T, Blaser M J. Correlation between vacuolating cytotoxin production by Helicobacter pylori isolates in vitro and in vivo. Infect Immun. 1993;61:5008–5012. doi: 10.1128/iai.61.12.5008-5012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craig P M, Territo M C, Karnes W E, Walsh J H. Helicobacter pylori secretes a chemotactic factor for monocytes and neutrophils. Gut. 1992;33:1020–1023. doi: 10.1136/gut.33.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies G R, Banatvala N, Collins C E, Sheaff M T, Abdi Y, Clements L, Rampton D S. Relationship between infective load of Helicobacter pylori and reactive oxygen metabolite production in antral mucosa. Scand J Gastroenterol. 1994;29:419–424. doi: 10.3109/00365529409096832. [DOI] [PubMed] [Google Scholar]
- 9.Dianda L, Hanby A M, Wright N A, Sebesteny A, Hayday A C, Owen M J. T cell receptor-α β-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997;150:91–97. [PMC free article] [PubMed] [Google Scholar]
- 10.Foltz C J, Fox J G, Cahill R, Murphy J C, Yan L, Shames B, Schauer D. Spontaneous inflammatory bowel disease in multiple mutant mouse lines: association with colonization by Helicobacter hepaticus. Helicobacter. 1998;3:69–78. doi: 10.1046/j.1523-5378.1998.08006.x. [DOI] [PubMed] [Google Scholar]
- 11.Forman D. Helicobacter pylori and gastric cancer. Scand J Gastroenterol. 1996;214:31–33. [PubMed] [Google Scholar]
- 12.Fox J G, Dangler C A, Whary M T, Edelman W, Kucherlapati R, Wang T C. Mice carrying a truncated Apc gene have diminished epithelial proliferation, gastric inflammation, and humoral immunity in response to Helicobacter felis infection. Cancer Res. 1997;57:3972–3978. [PubMed] [Google Scholar]
- 13.Fox J G, Dewhirst F E, Shen Z, Taylor N S, Paster B J, Ericson R L, Lau C N, Correa P, Araya J C, Roa I. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 1998;114:755–763. doi: 10.1016/s0016-5085(98)70589-x. [DOI] [PubMed] [Google Scholar]
- 14.Fox J G, Dewhirst F E, Tully J G, Paster B J, Yan L, Taylor N S, Collins M J, Jr, Gorelick P L, Ward J M. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox J G, Li X, Yan L, Cahill R J, Hurley R, Lewis R, Murphy J C. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of Helicobacter-induced carcinogenesis. Infect Immun. 1996;64:1548–1558. doi: 10.1128/iai.64.5.1548-1558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox J G, MacGregor J, Shen Z, Li X, Lewis R, Dangler C A. Comparison of methods of identifying Helicobacter hepaticus in B6C3F1 mice used in a carcinogenesis bioassay. J Clin Microbiol. 1998;36:1382–1387. doi: 10.1128/jcm.36.5.1382-1387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox J G, Yan L, Shames B, Campbell J, Murphy J C, Li X. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect Immun. 1996;64:3673–3681. doi: 10.1128/iai.64.9.3673-3681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaskins H R, Vondrak-Juergens G L, McCracken B A, Woolsey J H. Specific-pathogen-free conditions enhance inflammatory bowel disease in T-cell receptor knockout, but not C3H/HeJBir mice. Lab Anim Sci. 1997;47:650–655. [PubMed] [Google Scholar]
- 19.Goodwin C S, Worsley B W. The helicobacter genus: the history of H. pylori and taxonomy of current species. In: Goodwin C S, Worsley B W, editors. Helicobacter pylori: biology and clinical practice. Boca Raton, Fla: CRC Press; 1993. pp. 1–13. [Google Scholar]
- 20.Hewett J A, Roth R A. Hepatic and extrahepatic pathobiology of bacterial lipopolysaccharides. Pharmacol Rev. 1993;45:381–411. [PubMed] [Google Scholar]
- 21.Lee, A. 1994. The use of a mouse model in the study of Helicobacter sp.-associated gastric cancer. Eur. J. Gastroenterol. Hepatol. 6(Suppl. 1):S67–S71. [PubMed]
- 22.Lee A, Fox J, Hazell S. Pathogenicity of Helicobacter pylori: a perspective. Infect Immun. 1993;61:1601–1610. doi: 10.1128/iai.61.5.1601-1610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Castleman W L. Ultrastructural morphogenesis of 4-ipomeanol-induced bronchiolitis and interstitial pneumonia in calves. Vet Pathol. 1990;27:141–149. doi: 10.1177/030098589002700301. [DOI] [PubMed] [Google Scholar]
- 24.Marshall B J, Royce H, Annear D I, Goodwin C S, Pearman J W, Warren J R, Armstrong J A. Original isolation of Campylobacter pyloridis from human gastric mucosa. Microbios Lett. 1984;25:83–86. [Google Scholar]
- 25.McCathey S, Whary M T, Taylor N S, Dangler C A, Fox J G. In vivo administration of Helicobacter hepaticus cytotoxin is associated with hepatic inflammation and necrosis. Lab Anim Sci. 1997;47:440. . (Abstract.) [Google Scholar]
- 26.Moran A P. The role of lipopolysaccharide in Helicobacter pylori pathogenesis. Aliment Pharmacol Ther. 1996;10:39–50. doi: 10.1046/j.1365-2036.1996.22164004.x. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen H, Birkholz S, Andersen L P, Moran A P. Neutrophil activation by Helicobacter pylori lipopolysaccharides. J Infect Dis. 1994;170:135–139. doi: 10.1093/infdis/170.1.135. [DOI] [PubMed] [Google Scholar]
- 28.Powrie F, Leach M W. Genetic and spontaneous models of inflammatory bowel disease in rodents: evidence for abnormalities in mucosal immune regulation. Ther Immunol. 1995;2:115–123. [PubMed] [Google Scholar]
- 29.Russell R J, Haines D C, Anver M R, Battles J K, Gorelick P L, Blumenauer L L, Gonda M A, Ward J M. Use of antibiotics to prevent hepatitis and typhlitis in male scid mice spontaneously infected with Helicobacter hepaticus. Lab Anim Sci. 1995;45:373–378. [PubMed] [Google Scholar]
- 30.Shames B, Fox J G, Dewhurst F, Yan L L, Shen Z L, Taylor N S. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J Clin Microbiol. 1995;33:2968–2972. doi: 10.1128/jcm.33.11.2968-2972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shomer N H, Dangler C A, Schrenzel M D, Fox J G. Helicobacter bilis-induced inflammatory bowel disease in scid mice with defined flora. Infect Immun. 1997;65:4858–4864. doi: 10.1128/iai.65.11.4858-4864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sipponen, P., and H. Hyvarinen. 1993. Role of Helicobacter pylori in the pathogenesis of gastritis, peptic ulcer and gastric cancer. Scand. J. Gastroenterol. 196(Suppl.):3–6. [DOI] [PubMed]
- 33.Trivettmoore N L, Rawlinson W D, Yuen M, Gilbert G L. Helicobacter westmeadii sp. nov, a new species isolated from blood cultures of two AIDS patients. J Clin Microbiol. 1997;35:1144–1150. doi: 10.1128/jcm.35.5.1144-1150.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tummuru M K R, Sharma S A, Blaser M J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995;18:867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- 35.Valkonen K H, Wadstrom T, Moran A P. Interaction of lipopolysaccharides of Helicobacter pylori with basement membrane protein laminin. Infect Immun. 1994;62:3640–3648. doi: 10.1128/iai.62.9.3640-3648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viney J L. Altering cytokine soups: a recipe for inflammatory bowel disease? Gut. 1998;42:607–608. doi: 10.1136/gut.42.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wadstrom T, Rydberg J, Rozalska B, Lelwala-Guruge J. Intravenous Helicobacter pylori induces low levels of TNF-α and IL-1 α in a murine model. APMIS. 1994;102:49–52. [PubMed] [Google Scholar]
- 38.Ward J M, Anver M R, Haines D C, Benveniste R E. Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am J Pathol. 1994;145:959–968. [PMC free article] [PubMed] [Google Scholar]
- 39.Ward J M, Anver M R, Haines D C, Melhorn J M, Gorelick P, Yan L, Fox J G. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci. 1996;46:15–20. [PubMed] [Google Scholar]
- 40.Ward J M, Benveniste R E, Fox C H, Battles J K, Gonda M A, Tully J G. Autoimmunity in chronic active Helicobacter hepatitis of mice. Serum antibodies and expression of heat shock protein 70 in liver. Am J Pathol. 1996;148:509–517. [PMC free article] [PubMed] [Google Scholar]
- 41.Ward J M, Fox J G, Anver M R, Haines D C, George C V, Collins M J, Jr, Gorelick P L, Nagashima K, Gonda M A, Gilden R V. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86:1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- 42.Whary M T, Fox J G. Mouse strains resistant and susceptible to Helicobacter hepaticus-related hepatitis differ in systemic immune response to experimental infection. Gastroenterology. 1998;114:A1113. [Google Scholar]
- 43.Whary M T, Morgan T J, Dangler C A, Gaudes K J, Taylor N S, Fox J G. Chronic active hepatitis induced by Helicobacter hepaticus in the A/JCr mouse is associated with Th1 cell-mediated immune response. Infect Immun. 1998;66:3142–3148. doi: 10.1128/iai.66.7.3142-3148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whary M T, Palley L S, Batchelder M, Murphy J C, Yan L, Taylor N S, Fox J G. Promotion of ulcerative duodenitis in young ferrets by oral immunization with Helicobacter mustelae and muramyl dipeptide. Helicobacter. 1997;2:65–77. doi: 10.1111/j.1523-5378.1997.tb00061.x. [DOI] [PubMed] [Google Scholar]