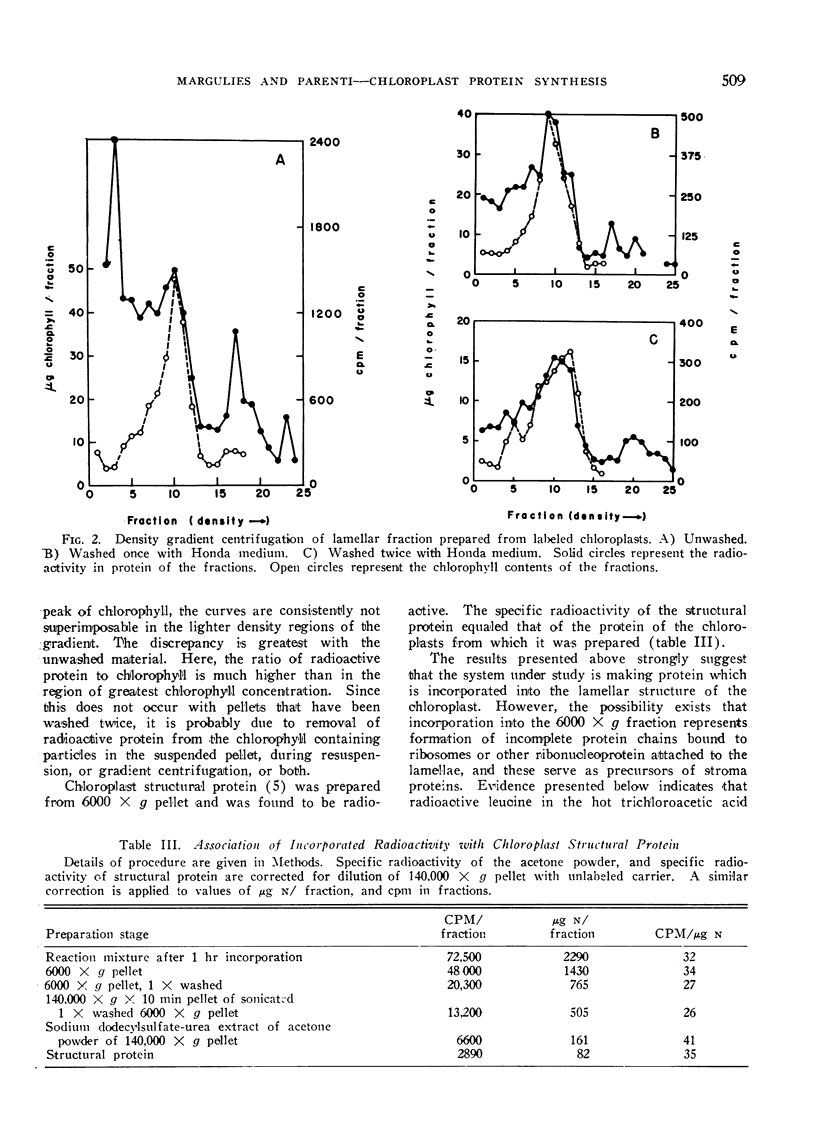
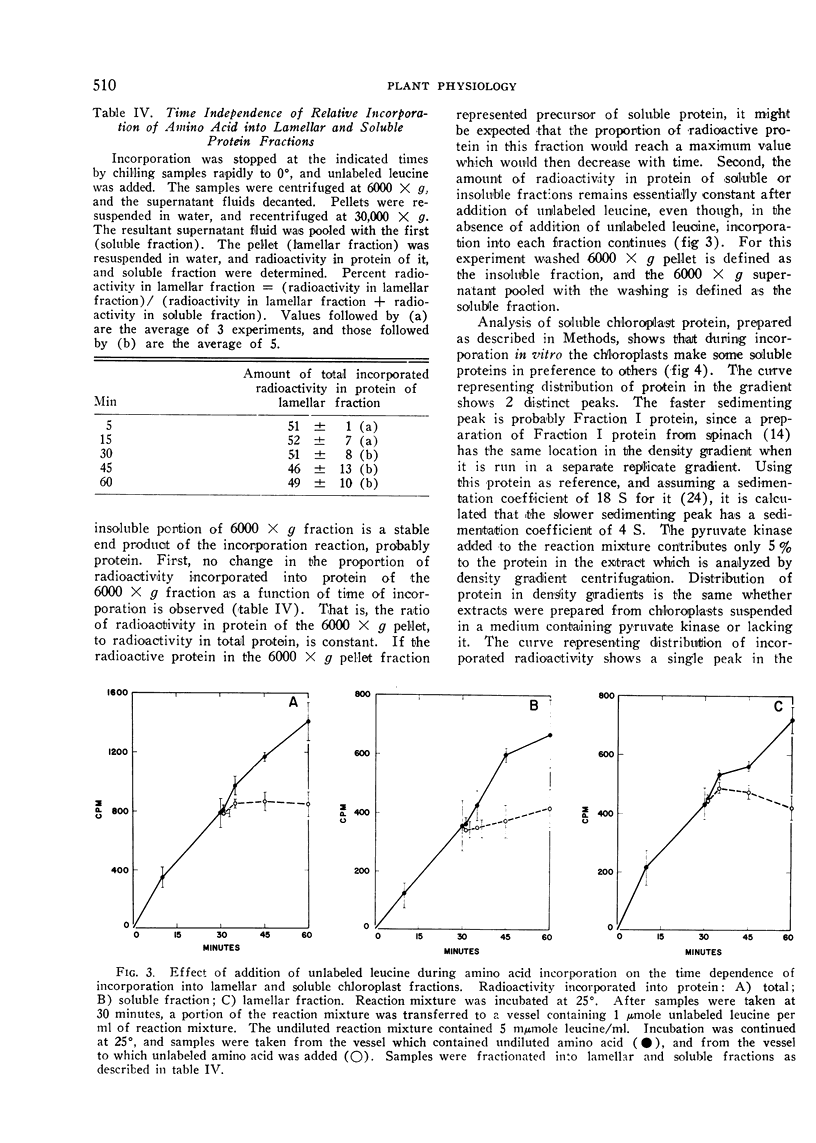
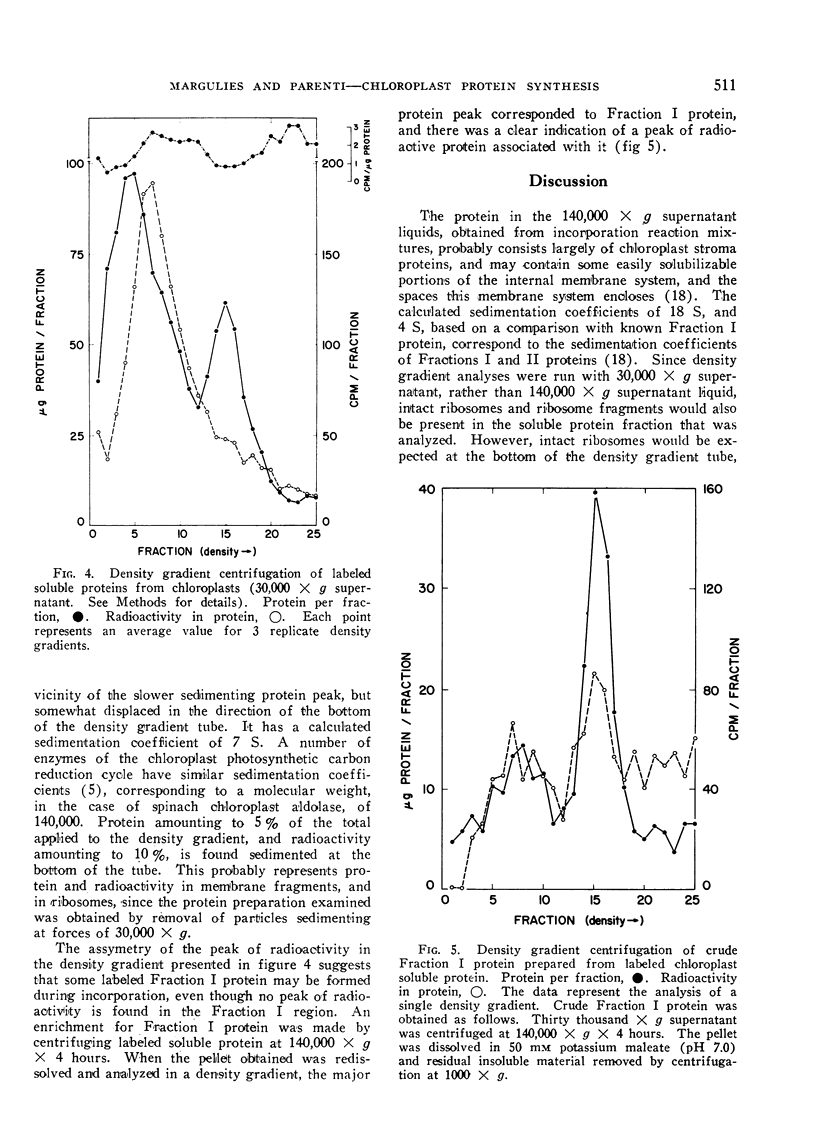
Abstract
Chloroplasts from leaves of plants which had been grown in the dark, and then illuminated for 12 hours were isolated, and allowed to incorporate 14C-leucine into protein, and the products of this incorporation were studied. Lamellar and soluble proteins are the principal products, and are formed in about equal amounts. Only some of the soluble proteins become heavily labeled. Those with highest specific activity have a molecular weight of the order of 140,000, while the higher molecular weight Fraction I protein has a much lower specific activity. The soluble protein as a whole does not serve as a precursor for the lamellar protein, and vice-versa, although a precursor-product relationship between a minor component of the soluble fraction and the lamellar fraction has not been ruled out. The relative protein synthesizing capabilities of chloroplasts and mitochondria are discussed with reference to the data presented.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamji M. S., Jagendorf A. T. Amino Acid incorporation by wheat chloroplasts. Plant Physiol. 1966 May;41(5):764–770. doi: 10.1104/pp.41.5.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman N. K., Francki R. I., Wildman S. G. Protein synthesis by cell-free extracts of tobacco leaves. 3. Comparison of the physical properties and protein synthesizing activities of 70 s chloroplast and 80 s cytoplasmic ribosomes. J Mol Biol. 1966 Jun;17(2):470–487. doi: 10.1016/s0022-2836(66)80157-2. [DOI] [PubMed] [Google Scholar]
- Boardman N. K. Ribosome composition and chloroplast development in Phaseolus vulgaris. Exp Cell Res. 1966 Sep;43(2):474–482. doi: 10.1016/0014-4827(66)90074-7. [DOI] [PubMed] [Google Scholar]
- Criddle R. S., Edwards D. L., Petersen T. G. Chemical studies on the homogeneity of the structural protein from mitochondria. Biochemistry. 1966 Feb;5(2):578–582. doi: 10.1021/bi00866a025. [DOI] [PubMed] [Google Scholar]
- EISENSTADT J., BRAWERMAN G. CHARACTERISTICS OF A CELL-FREE SYSTEM FROM EUGLENA GRACILIS FOR THE INCORPORATION OF AMINO ACIDS INTO PROTEIN. Biochim Biophys Acta. 1964 Mar 23;80:463–472. doi: 10.1016/0926-6550(64)90149-5. [DOI] [PubMed] [Google Scholar]
- GIBOR A., GRANICK S. PLASTIDS AND MITOCHONDRIA: INHERITABLE SYSTEMS. Science. 1964 Aug 14;145(3633):890–897. doi: 10.1126/science.145.3635.890. [DOI] [PubMed] [Google Scholar]
- Haldar D., Freeman K. B., Work T. S. The site of synthesis of mitochondrial proteins in Krebs II ascites-tumour cells. Biochem J. 1967 Mar;102(3):684–690. doi: 10.1042/bj1020684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LYTTLETON J. W., TS'O P. O. The localization of fraction I protein of green leaves in the chloroplasts. Arch Biochem Biophys. 1958 Jan;73(1):120–126. doi: 10.1016/0003-9861(58)90246-7. [DOI] [PubMed] [Google Scholar]
- Margulies M. M., Gantt E., Parenti F. In vitro Protein Synthesis by Plastids of Phaseolus vulgaris. II. The Probable Relation Between Ribonuclease Insensitive Amino Acid Incorporation and the Presence of Intact Chloroplasts. Plant Physiol. 1968 Apr;43(4):495–503. doi: 10.1104/pp.43.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti F., Margulies M. M. In Vitro Protein Synthesis by Plastids of Phaseolus vulgaris. I. Localization of Activity in the Chloroplasts of a Chloroplast Containing Fraction from Developing Leaves. Plant Physiol. 1967 Sep;42(9):1179–1186. doi: 10.1104/pp.42.9.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B. Protein synthesis in mitochondria. 3. The controlled disruption and subfractionation of mitochondria labelled in vitro with radioactive valine. Biochem J. 1962 Oct;85:177–189. doi: 10.1042/bj0850177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., REIS P. J., WORK T. S. Protein synthesis in mitochondria. Requirements for the incorporation of radioactive amino acids into mitochondrial protein. Biochem J. 1961 Jul;80:9–21. doi: 10.1042/bj0800009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., SUTTIE J. W., WORK T. S. Protein synthesis in mitochondria. 2. Rate of incorporation in vitro of radioactive amino acids into soluble proteins in the mitochondrial fraction, including catalase, malic dehydrogenase and cytochrome c. Biochem J. 1962 Apr;83:29–40. doi: 10.1042/bj0830029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER D., WILDMAN S. G. THE INCORPORATION OF AMINO ACIDS INTO PROTEIN BY CELL-FREE EXTRACTS FROM TOBACCO LEAVES. Biochemistry. 1964 Jul;3:954–959. doi: 10.1021/bi00895a019. [DOI] [PubMed] [Google Scholar]
- Spencer D. Protein synthesis by isolated spinach chloroplasts. Arch Biochem Biophys. 1965 Aug;111(2):381–390. doi: 10.1016/0003-9861(65)90200-6. [DOI] [PubMed] [Google Scholar]
- TROWN P. W. AN IMPROVED METHOD FOR THE ISOLATION OF CARBOXYDISMUTASE. PROBABLE IDENTITY WITH FRACTION I PROTEIN AND THE PROTEIN MOIETY OF PROTOCHLOROPHYLL HOLOCHROME. Biochemistry. 1965 May;4:908–918. doi: 10.1021/bi00881a018. [DOI] [PubMed] [Google Scholar]
- Truman D. E. The fractionation of proteins from ox-heart mitochondria labelled in vitro with radioactive amino acids. Biochem J. 1964 Apr;91(1):59–64. doi: 10.1042/bj0910059. [DOI] [PMC free article] [PubMed] [Google Scholar]



