Abstract
Background:
Quality reporting contributes to effective translation of health research in practice and policy. As an initial step in the development of a reporting guideline for scaling, the Standards for reporting stUdies of sCaling evidenCEd-informED interventions (SUCCEED), we performed a systematic review to identify relevant guidelines and compile a list of potential items.
Methods:
We conducted a systematic review according to Cochrane method guidelines. We searched the following databases: MEDLINE, Embase, PsycINFO, Cochrane Library, CINAHL, Web of Science, from their respective inceptions. We also searched websites of relevant organizations and Google. We included any document that provided instructions or recommendations, e.g., reporting guideline, checklist, guidance, framework, standard; could inform the design or reporting of scaling interventions; and related to the health sector. We extracted characteristics of the included guidelines and assessed their methodological quality using a 3-item internal validity assessment tool. We extracted all items from the guidelines and classified them according to the main sections of reporting guidelines (title, abstract, introduction, methods, results, discussion and other information). We performed a narrative synthesis based on descriptive statistics.
Results:
Of 7704 records screened (published between 1999 and 2019), we included 39 guidelines, from which data were extracted from 57 reports. Of the 39 guidelines, 17 were for designing scaling interventions and 22 for reporting implementation interventions. At least one female author was listed in 31 guidelines, and 21 first authors were female. None of the authors belonged to the patient stakeholder group. Only one guideline clearly identified a patient as having participated in the consensus process. More than half the guidelines (56%) had been developed using an evidence-based process. In total, 750 items were extracted from the 39 guidelines and distributed into the 7 main sections.
Conclusion:
Relevant items identified could inform the development of a reporting guideline for scaling studies of evidence-based health interventions. This and our assessment of guidelines could contribute to better reporting in the science and practice of scaling.
Keywords: quality reporting, reporting guideline, scale and spread, scaling, systematic review
1. Introduction
Health interventions that have been shown to be effective need to be scaled to maximize their potential impact on improving population health outcomes and promoting health equity. Hence there is growing interest in the science and practice of scaling.[1–8] In this project, we define the generic term “scaling” as a systematic process to broaden the reach and impact of evidence-based interventions so as to expand their benefits to individuals and society.[5,9,10]
The consistent and transparent reporting of health research can facilitate the use of study findings in making decisions about health policy and practice-based decision making. However, deficiencies in the quality of reporting of health research is well documented in the scientific and medical literature broadly. We also note a number of deficiencies in the reporting of scaling studies, e.g., in the areas of ethical and technical justification, scalability assessment, gender equity, and patient and public involvement.[1,5]
Reporting guidelines seek to address these deficiencies and facilitate adequate reporting of studies.[11] For example, a Cochrane systematic review showed that 25 out of 27 items of the Consolidated standards of reporting trials (CONSORT) statement were more completely reported in trials published in journals that endorsed the CONSORT than in trials published in journals that did not.[12] Reporting guidelines are used by authors during protocol development and manuscript preparation, by journal editors and peer-reviewers to assess quality reporting, and by readers when synthesizing the literature.
The Standards for reporting stUdies of sCaling evidenCEd-informED interventions (SUCCEED) project was initiated to help improve the reporting of scaling studies, as detailed in the published protocol.[5] An initial step in the development of the SUCCEED reporting guideline was to systematically compile a list of potential items to include.[13] The aim of this systematic review was to document and analyze guidelines relevant to scaling studies and extract relevant items for the development of the SUCCEED guideline.
2. Methods
We conducted the systematic review according to Cochrane method guidelines.[14] The protocol of the review was registered with the Open Science Framework (https://osf.io/vcwfx/) and is published elsewhere.[5] We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement to guide the report.[15] This study does not involve human participants and ethical approval was not required.
2.1. Eligibility criteria
We included any document that provided instructions or recommendations, e.g. a reporting guideline, checklist, guidance, framework, standard (referred thereafter as “guideline”); that could inform the design or reporting of scaling interventions; and was within the health sector. We included guidelines for reporting implementation interventions with the expectation that they could supply additional items relevant to scaling such as implementation strategies. We excluded documents such as formatting instructions produced by journal editors and publishers (Table 1).
Table 1.
Selection criteria for guidelines for designing and/or reporting scaling and implementation interventions.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Type of document | The document provides instructions or recommendations, e.g., reporting guideline, checklist, guidance, framework, standard. The document includes a list of the recommendations or a link to them. | Instructions to authors by journal editors. Does not include a list of recommendations. |
| Purpose of guidelines | The document informs the design (conduct, planning) or the reporting of scaling interventions. Synonyms of scaling include scale up, scale out, roll out, spread. The document informs the reporting of implementation of interventions. |
Is not about scaling or implementation. |
| Domain | Health | Other domains |
2.2. Information sources and search strategy
The search strategy was developed by our information specialist for MEDLINE, with iterative revisions by members of the research team and validation by a second information specialist using a Peer Review of Electronic Search Strategies tool[16] before being translated into the other electronic databases.[5] The full search strategy is provided in File S1, Supplemental Digital Content, http://links.lww.com/MD/L359. MEDLINE (Ovid), Embase (Elsevier), PsycINFO (Ovid), Cochrane Library (Methodology Register), CINAHL (EBSCOhost), and Web of Science were searched from their respective inceptions. The initial search was completed in May 2019.
For gray literature, we searched websites of relevant organizations (File S2, Supplemental Digital Content, http://links.lww.com/MD/L360) using the following terms: scaling up, scaling out, spread, scale up, scale out, upscaling, scalability, dissemination, diffusion, implementation. We also used the Google search engine, limiting results to the first 200 hits, after anonymizing the Google search link and applying the PDF filter to reduce the level of noise. For this search, we used each of the above-mentioned terms in combination with “reporting” and “health.” Finally, we searched the reference lists of identified reports.
2.3. Study selection
Results from the electronic databases were managed through Endnote X9 to identify duplicates. Records were then uploaded to Covidence, an Internet-based system, for independent screening. Four reviewers (A.G., A.B.C., G.E., and J.S.) independently screened a random sample of 5% of records to pilot test the eligibility criteria based on titles and abstracts. The eligibility form was validated with the members of the SUCCEED Executive Committee before pairs of the same 4 reviewers independently screened all the remaining records for titles and abstracts. Two reviewers (A.G. and G.E.) independently assessed the full texts. Discrepancies were resolved by consensus through discussion.
2.4. Data collection process
First, we developed a data dictionary informed by the Cochrane Checklist of items to consider in data collection[17] and guidelines to design scaling interventions.[6,9,18] The dictionary was enriched with elements supplied by members of the Executive Committee from their experience in the development of reporting guidelines and the science and practice of scaling. We then created an Excel extraction form from the data dictionary that was pilot tested by 3 reviewers (A.G., G.E., and O.A.). Pairs of the same reviewers independently extracted data from included guidelines and related reports. For each guideline, we retrieved all the necessary documents to assimilate the information required for extraction as follows:
We systematically searched for the guideline statement, the explanatory document, and any documents related to the development of the guideline (i.e., systematic reviews, Delphi studies).
We explored the website of the guideline or of the group that developed the guideline, if available.
We also retrieved any cited appendices or supplementary documents.
Extracted data included:
-
•
General characteristics (e.g., title, name of the guideline, number and sex of the authors and if first author, corresponding author and contact information including geographic location).
-
•
Type of the guideline (e.g., for designing scaling interventions).
-
•
Elements of the development process: theoretical framework; type of data collection (e.g., systematic review); consensus process, (e.g., Delphi) and panelists in consensus group (number, sex and type); validation or not.
-
•
Stakeholder involvement, i.e. number, sex and type (clinician, decision maker, journal editor/publisher, funder, patient, researcher).
-
•
Number and description of the items (for each included guideline, we extracted all items).
-
•
Presence of sex- and gender-related words and their correct use.[19]
-
•
Other information, e.g., funding source: public (governmental/intergovernmental), private (profit/nonprofit such as charities), public/private, and not provided (no funding as stated by the authors or no information); conflict of interest.
Disagreements were resolved by consensus or by discussion with a third reviewer (H.T.V.Z.).
2.5. Methodological quality assessment
We used a 3-item internal validity assessment tool to assess the methodological quality of development of the guidelines. The tool had been developed by members of the research team during a systematic review of sex and gender considerations in reporting guidelines.[20] Each of the following questions was coded “yes,” “no” or “unclear”: Did the developers of the guideline represent more than one stakeholder group? Did the developers report gathering any data for the creation of the guideline? and Did the developers report the use of a consensus process? Pairs of 3 reviewer authors of the tool (A.G., G.E., and H.T.V.Z.) independently conducted the assessment and any disagreements were resolved by consensus.
2.6. Data analysis and synthesis
We analyzed extracted data using descriptive statistics (numbers and percentages) and performed a narrative synthesis of the guidelines and items.
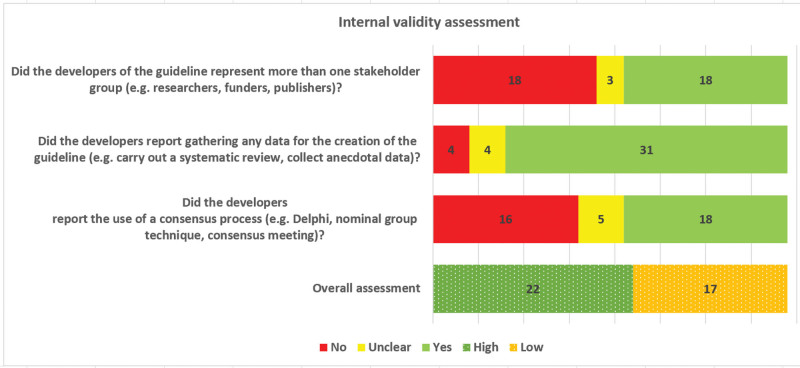
For the guidelines, we provided the numbers and percentages by type. We reported numbers and percentages of authors by stakeholder group (e.g., clinician, decision maker, journal editor, funder, patient, researcher) and by sex. We graphically synthesized the 3 criteria for methodological quality of the guidelines’ development, and gave it an overall rating of high internal validity (i.e., low risk of bias) if the number of “yes” ≥ 2 and low internal validity (i.e., high risk of bias) if the number of “yes” < 2.
The items generated were categorized into the main sections of any reporting guideline: title, abstract, introduction, methods, results, discussion and other information. For items extracted from reporting guidelines, we reported the categories as presented in their statements or checklists. For the classification of items extracted from the other type of guidelines (for designing scaling interventions), 4 review authors of several publications on scaling[1,5,21–24] (A.G., A.B.C., G.E., and H.T.V.Z.) conducted 2 pilot rounds. The classification was performed independently by pairs of 3 reviewers (A.G., G.E., and H.T.V.Z.) and disagreements were resolved through discussion. We calculated the percentage of items by type of guidelines included and by the main sections of a reporting guideline. SAS (SAS, version 9.4, Institute, Cary, NC) and Excel (Microsoft) were used to perform the analyses.
2.7. Patient and public involvement
We included 2 patient partners (E.A., K.P.) when establishing the Executive Committee, which is the first step recommended for developing health research reporting guidelines.[13] As members of the research team, they were coauthors on the published protocol[5] and provided feedback at each stage described in the methods: search strategy, study selection criteria, data extraction dictionary, and data synthesis. Given the methodological nature of the project and to optimize their participation, with their agreement we held working sessions to go through documents prior to Executive Committee meetings. They will continue to be involved in the subsequent steps of the development of SUCCEED reporting guideline, including the consensus process and the dissemination.
3. Results
3.1. Study selection
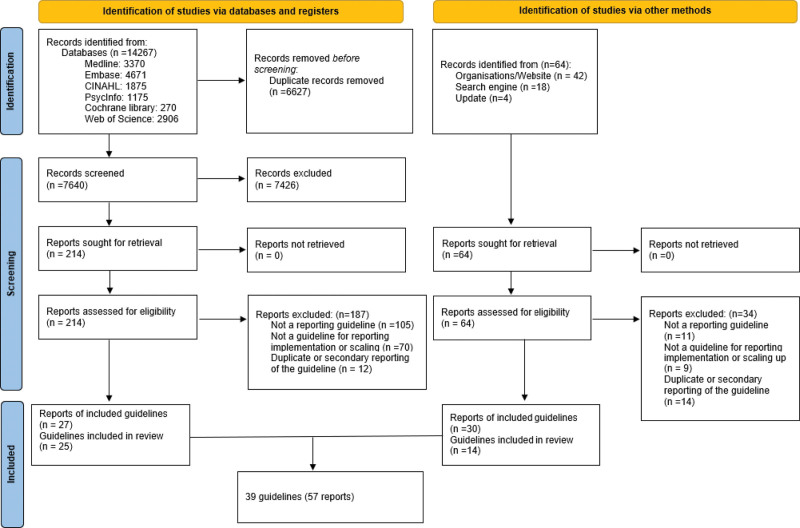
The PRISMA flow diagram (Fig. 1) was used to report the selection process. We included 39 guidelines (Table 2) whose data were extracted from 57 reports. The list of excluded reports is provided in File S3, Supplemental Digital Content, http://links.lww.com/MD/L361
Figure 1.
PRISMA 2020 flow diagram of new systematic reviews which included searches of databases, registers and other sources.
Table 2.
Characteristics of included guidelines and internal validity.
| Reference Name* (Year) Ref. Country |
Related reports [Ref.] | Focus i) Designing scaling intervention ii) Reporting implementation intervention | Number of items | Funding source† |
Conflict of interest information (Yes, No) | Internal validity‡ (High, Low) |
|---|---|---|---|---|---|---|
| Reach, efficacy, adoption, implementation, and maintenance framework (1999)[25] United States of America |
[26] | i | 31 | Public | No | Low |
| USAID and Management Sciences for Health (2002)[27] United States of America |
– | i | 10 | No info | No | Low |
| Reviewer Guidelines for Reports of Public Health Interventions (2003)[28] Canada |
– | ii | 19 | No info | No | Low |
| Transparent Reporting of Evaluations with nonrandomized Designs (2004)[29] United States of America |
– | ii | 58 | No info | No | High |
| ExpandNet/WHO framework for scaling up (2007)[8] Switzerland |
[18,30–32] | i | 27 | Public/private | No | High |
| Riley et al (2008)[33] Canada |
– | ii | 16 | Public/private | Yes | Low |
| Egan et al (2009)[34] United Kingdom |
– | ii | 10 | Public | Yes | Low |
| Framework for reporting health service delivery models for managing rheumatoid arthritis (2010)[35] Canada |
[36] | ii | 10 | Yes | High | |
| Bryce et al (2011)[37] United States of America |
– | i | 7 | Public/private | Yes | High |
| Conn & Groves (2011)[38] United States of America |
– | ii | 5 | Public | No | Low |
| Eaton et al (2011)[39] Nigeria |
– | i | 9 | No info | Yes | High |
| WHO/ExpandNet (2011)[40] Switzerland |
– | i | 12 | Public/private | No | High |
| Framework for explaining successful scale-up (2011)[41] United States of America |
– | i | 6 | No info | Yes | Low |
| AIDED model for scale-up (2012)[42] United States of America |
– | i | 5 | Private | Yes | Low |
| Reporting standards for studies of tailored interventions (2012)[43] United States of America |
– | ii | 7 | No info | Yes | Low |
| Guide for Monitoring Scale-up of Health Practices and Interventions (2013)[44] United States of America |
– | i | 10 | No info | No | Low |
| Workgroup for Intervention Development and Evaluation Research recommendations (2013)[45] Canada |
[46] | ii | 20 | Public | Yes | High |
| Duncan et al (2013)[47] United States of America |
– | ii | 21 | No info | No | Low |
| The Oxford Implementation Index (2013)[48] United Kingdom |
– | ii | 32 | Public | Yes | High |
| Proctor et al (2013)[49] United States of America |
– | ii | 10 | Public | Yes | Low |
| Dickson et al (2014)[50] United States of America |
– | i | 4 | Public/private | Yes | High |
| Template for intervention description and replication (2014)[51] United Kingdom |
– | ii | 12 | Public/private | Yes | High |
| Global framework implementation criteria for pilot test (2014)[52] United States of America |
– | ii | 17 | Public | Yes | High |
| Multiplicative scale-up framework (2014)[53] Switzerland |
– | i | 17 | No info | No | Low |
| mHealth Assessment and Planning for Scale (2015)[54] Switzerland |
– | i | 38 | Public/private | No | High |
| Neta et al (2015)[55] United States of America |
– | ii | 30 | Public | No | High |
| Guidelines for Reporting Evaluations based on Observational Methodology (2015)[56] Spain |
– | ii | 14 | Public | No | High |
| CCDR (2016)[57] Canada |
– | ii | 20 | No info | No | Low |
| Barker et al (2016)[58] United States of America |
– | i | 4 | Private | Yes | Low |
| Scaling Up Management Framework (2016)[59] United States of America |
– | i | 14 | Private | No | Low |
| Hales et al (2016)[60] Switzerland |
[61] | ii | 64 | Public | Yes | High |
| Milat et al (2016)[6] Australia |
[62–64] | i | 20 | Public | Yes | High |
| Standards for QUality Improvement Reporting Excellence (2016)[65] United States of America |
[66–69] | ii | 40 | Private | Yes | High |
| Indig et al (2017)[70] Australia |
– | i | 6 | Public | Yes | High |
| Programme Reporting Standards (2017)[71] Switzerland |
– | ii | 47 | Public | Yes | High |
| Standards for Reporting Implementation Studies (2017)[72] United Kingdom |
[73,74] | ii | 37 | Public | Yes | High |
| Consolidated advice for reporting ECD implementation research (2018)[75] United States of America |
– | ii | 21 | Public | Yes | High |
| McLean & Gargani (2019)[9] Canada |
[76] | i | 12 | Public | No | High |
| Reeves et al (2019)[77] Australia |
– | ii | 8 | No info | Yes | Low |
AIDED = model for scale up of family health innovations, CCDR = Canada Communicable Disease Report, USAID = United States Agency for International Development, WHO = World Health Organization.
Authors or organizations if no name was identified.
Public (governmental/intergovernmental), private (for profit/no-profit); no info (no info or no funding declared).
Based on a 3-item internal validity assessment tool (high if ≥2 “yes” and low if <2 “yes”).
3.2. Characteristics of the included guidelines
Included guidelines were published between 1999 and 2019. They originated in 7 countries, which were identified according to the location of the corresponding author’s institution or the location of the institution if no authors were listed (Table 2, File S4A, Supplemental Digital Content, http://links.lww.com/MD/L364). Regarding the type of guidelines, 43.6% (17/39) were guidelines to design scaling interventions and 56.4% (22/39) for reporting implementation interventions. None was found for reporting scaling interventions. Seventy-seven percent (30/39) mentioned the funding source and 59% (23/39) included a statement of conflict of interest. Most guidelines (41%, 16/39) were developed with public funding, while the private sector funded the fewest (13%, 5/39); combined public/private funding was used for 18% (7/39) and no information or source of funding were reported for 28% (11/39).
Regarding sex and patient representation in the study teams, at least one female author was listed on 80% (31/39) of included guidelines and as first author on 54 % (21/39), while at least one male author was listed on 72% (28/39). Seven guidelines were by female authors, and 4 guidelines by male authors only (Files S4 and S5, Supplemental Digital Content, http://links.lww.com/MD/L364 and http://links.lww.com/MD/L368). None of the authors belonged to the patient stakeholder group (File S6, Supplemental Digital Content, http://links.lww.com/MD/L369). Regarding sex and gender in guideline content, only one guideline (3%) mentioned at least one sex-related word and 3 (8%) mentioned at least one gender-related word. Of these 3 gender-related words, only one was used correctly (File S4A, Supplemental Digital Content, http://links.lww.com/MD/L364).
3.3. Development process and internal validity assessment of the guidelines
The development of the guidelines was predominantly based on data gathering through literature reviews (49%, 18/39) and systematic reviews (24%, 9/39). Only 13% (5/39) of the guidelines were developed using a theoretical framework. A consensus process was used in the development of 18 (46.2%) guidelines. The most common type of consensus was informal consensus (22%, 8/39) followed by Delphi (15.4%, 6/39) (Table 3, File S4A, Supplemental Digital Content, http://links.lww.com/MD/L364). Only one guideline (3%) clearly identified a patient as a panelist (File S7, Supplemental Digital Content, http://links.lww.com/MD/L371). Twenty-six percent of guidelines (10/39) used a validation process (pilot testing and/or expert feedback).
Table 3.
Elements of the development process of included guidelines.
| Designing scaling interventions N = 17 (%) |
Reporting implementation interventions N = 22 (%) |
Total N = 39 (%) |
|
|---|---|---|---|
| Theoretical framework | |||
| Yes | 3 (18) | 2 (9) | 5 (13) |
| No | 14 (82) | 20 (91) | 34 (87) |
| Type of data gathering* | |||
| Systematic review | 2 (13) | 7 (32) | 9 (24) |
| Other literature review | 8 (53) | 10 (45) | 18 (49) |
| Qualitative | 2 (13) | 0 (0) | 2 (5) |
| Other data source† | 5 (29) | 2 (9) | 7 (18) |
| Consensus process* | |||
| Delphi | 1 (6) | 5 (23) | 6 (15) |
| Informal consensus | 4 (27) | 4 (18) | 8 (22) |
| Consensus conference | 0 (0) | 4 (18) | 4 (11) |
| Validation | |||
| Yes | 3 (18) | 7 (32) | 10 (26) |
| No | 14 (82) | 15 (68) | 29 (74) |
Did not add up for only ‘yes’ value was reported.
e.g., expert opinion, field work.
A summary of our internal validity assessment, based on our 3-item tool, is presented in Figure 2. Development was evidence-based (high internal validity) in 56.4% (22/39) of the guidelines, and only in those developed since 2004 (Fig. 2, Table 2, File S8, Supplemental Digital Content, http://links.lww.com/MD/L372). The inclusion of more than one stakeholder group was the least fulfilled of the 3 validity criteria (46.2%, 18/39).
Figure 2.
Summary of internal validity assessment.
3.4. Identification and classification of potentially relevant items
In total, 750 items were extracted from the 39 guidelines and related documents with a mean number of 19.2 items per guideline (File S4B, Supplemental Digital Content, http://links.lww.com/MD/L367 for the full list of items). The numbers and means of items were 232 (13.6) for the guidelines for designing scaling interventions and 518 (23.5) for the guidelines for reporting implementation interventions. The distribution of the items per section of reporting guidelines is shown in Table 4. Sections that had the most items were “methods” (n = 519, 69%), “results” (n = 142, 19%) and “discussion” (n = 97, 13%). Examples of items identified for the methods section that have potential for inclusion in a scaling reporting guideline were:
Table 4.
Distribution of items by section of a reporting guideline.
| Section | Designing scaling interventions N (%) [232 items] |
Reporting implementation interventions N (%) [518 items] |
Total N (%) [750 items] |
|---|---|---|---|
| Title | 0 (0) | 20 (4) | 20 (3) |
| Abstract | 16 (7) | 51 (10) | 67 (9) |
| Introduction | 34 (15) | 58 (11) | 92 (12) |
| Methods | 207 (89) | 312 (60) | 519 (69) |
| Results | 33 (14) | 109 (21) | 142 (19) |
| Discussion | 15 (6) | 82 (16) | 97 (13) |
| Other information | 0 (0) | 9 (2) | 9 (1) |
-
-
Engage in a participatory process involving key stakeholders[78];
-
-
Adopt an approach to scaling up: specify the chosen delivery strategy (e.g., vertical, horizontal, cascade or phased approaches)[6,8,41];
-
-
Consider optimal scale (balances the magnitude, variety, sustainability, and equity of impacts in ways stakeholders endorse)[9];
-
-
Consider how much it will cost to scale up (e.g., estimating start-up costs, long-term running costs, economies of scale, the cost of alternatives).[79]
4. Discussion
An initial step in the development of a reporting guideline is the systematic review of the literature to identify potential items to include. To achieve this, we identified 39 guidelines: 22 for reporting implementation interventions and 17 for designing scaling interventions. We found no guidelines for reporting scaling studies. All but one guideline developer were located in high-income countries. No patient was listed as author, and of guideline developers who used a consensus process, only one included a patient as panelist. The 39 guidelines and their associated documents yielded 750 items distributed among 7 main sections of a reporting guideline.
We identified 2 types of guidelines. The first consisted of standards for reporting implementation interventions. Examples among the most recently published included Workgroup for Intervention Development and Evaluation Research, a Template for Intervention Description and Replication, Standards for Reporting Implementation Studies and Programme Reporting Standards,[45,51,71,72] with the expectation that they would improve aspects such as quality intervention reporting and the reporting of implementation strategies (scaling requires implementation strategies but not all implementation is scaling) and of any evidence-based interventions of interest.[80,81] However, they did not address some elements specific to scaling.[5] These elements were provided by the second type of guidelines, i.e. those that offered recommendations or steps for designing scaling interventions. Most of these guidelines were developed by nonacademic organizations that work mainly in low- and middle-income countries where most scaling programs occur.[1,10] Our assessment of evidence-based development of guidelines for designing scaling interventions would help actors in the field select the most appropriate ones while developing their scaling programs.
We found that none of the included guidelines involved patients in the development process as authors and only one included a patient as a panelist in the consensus process, despite the growing literature on the importance of patient engagement in health research. Interestingly, patients themselves expressed their willingness to be involved[82,83] and most journal editors support the nomination of patient partners as authors or coauthors on published biomedical research articles.[84] While recent years have seen an increase in the involvement of patients and members of the public in health research, they are rarely involved in methodological projects including in the development of reporting guidelines and in the science of implementation and scaling.[5,21] As such, the development of the SUCCEED project is innovative as it includes 2 patient partners on the Executive Committee. This corresponds to the highest level of patient engagement according to the continuum of engagement.[85] In addition, patients and members of the public will be recruited for the consensus process. One output from the involvement of patient partners in this research project was the development of a training presentation entitled “Initiation aux étapes d’une revue systématique: Implication des patientes et patients partenaires” [Introduction to the steps of a systematic review: Involvement of patient partners].
Items from 3 guidelines that did not meet the eligibility criteria for this systematic review will nevertheless be included in the consensus process because of their relevance to the development of the SUCCEED guideline. These 3 guidelines are Guidance for Reporting Involvement of Patients and the Public tools to improve reporting of patient and public involvement in research[86]; Sex and Gender Equity in Research guidelines [87]; and The CONSORT extension for the reporting of stepped wedge cluster randomized trials.[88] Items from the first two will be added because they address equity concerns, and the third because the stepped wedge design is considered one of the most appropriate for scaling studies.[22] Given the growth of open science in recent years and in accordance with recommended practices,[89,90] we will also formulate and add some items related to preregistration and data sharing practices. Indeed, an increasing number of funding agencies are requiring data management plans including data sharing statements[90,91] and are consequently relevant for reporting guidelines. Some existing reporting guidelines (e.g., PRISMA) were updated or are being updated (e.g., CONSORT) accordingly.[15]
There are a few limitations to note. Scaling is a growing field, including the concept of “the science of scaling”[5] and there is no consensus on terminology and frameworks. As such, we may not have identified all relevant guidelines on scaling. As our search for this study was completed in 2019 we may have missed recent scaling guidelines or work that is still in progress or not yet published. However, our identified guidelines included the framework (WHO/ExpandNet framework) that as of 2022 is still the one most frequently used by actors developing and reporting scaling programs.[10] We will also enrich the list of items by reviewing studies included in our umbrella review on evidence on scaling in health and social care[10] and with the input of experts in the field during our forthcoming international consensus study. Finally, we will conduct living reviews[4] to incorporate new items as the science evolves.
5. Conclusion
This review identified relevant guidelines for the development of the SUCCEED reporting guideline. We assessed the evidence-based development of included guidelines, allowing the actors in the science of scaling to choose the best guidelines to inform their program design. The generated list of items will be enriched through a consensus process. Findings from this study and those of subsequent steps in the development of the SUCCEED guideline will help increase awareness of the importance of quality reporting and contribute to a better understanding of the science and practice of scaling health innovations.
Acknowledgments
We thank Becky Skidmore, MLS for peer review of the MEDLINE search strategy. We thank Jasmine Sawadogo (JS) and Odilon Assan (OA) for their contributions in the screening of records or data extraction. We also thank Louisa Blair for editing this manuscript.
Author contributions
Conceptualization: Amédé Gogovor, Hervé Tchala Vignon Zomahoun, Ali Ben Charif, David Moher, Robert K. D. McLean, Andrew Milat, Luke Wolfenden, Karina Prévost, Emmanuelle Aubin, Paula Rochon, France Légaré.
Data curation: Giraud Ekanmian, Nathalie Rheault.
Formal analysis: Giraud Ekanmian.
Funding acquisition: France Légaré.
Investigation: Amédé Gogovor, Hervé Tchala Vignon Zomahoun, Ali Ben Charif, David Moher, Robert K. D. McLean, Andrew Milat, Luke Wolfenden, Karina Prévost, Emmanuelle Aubin, Paula Rochon, Nathalie Rheault, France Légaré.
Methodology: Amédé Gogovor, Hervé Tchala Vignon Zomahoun, Ali Ben Charif, David Moher, Robert K. D. McLean, Andrew Milat, Luke Wolfenden, Paula Rochon, France Légaré.
Supervision: Amédé Gogovor, Hervé Tchala Vignon Zomahoun, David Moher, France Légaré.
Writing – original draft: Amédé Gogovor.
Writing – review & editing: Amédé Gogovor, Hervé Tchala Vignon Zomahoun, Ali Ben Charif, Giraud Ekanmian, David Moher, Robert K. D. McLean, Andrew Milat, Luke Wolfenden, Karina Prévost, Emmanuelle Aubin, Paula Rochon, Nathalie Rheault, France Légaré.
Supplementary Material
Abbreviations:
- CONSORT
- consolidated standards of reporting trials
- Portable Document Format
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SAS
- Statistical Analysis System
- SUCCEED
- Standards for reporting studies of scaling evidenced-informed interventions
- WHO
- World Health Organization
The study was supported by the Canadian Institutes of Health Research (CIHR) grant awarded to the Quebec SPOR Support Unit (QSSU) (#SU1139759) and the CIHR Foundation Grant (#FDN-159931). AG was supported by a CIHR Patient-Oriented Research fellowship. DM is on the executive of the EQUATOR Network.
This study does not involve human participants and ethical approval was not required.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental Digital Content is available for this article.
How to cite this article: Gogovor A, Zomahoun HTV, Ben Charif A, Ekanmian G, Moher D, McLean RKD, Milat A, Wolfenden L, Prévost K, Aubin E, Rochon P, Rheault N, Légaré F. Informing the development of the SUCCEED reporting guideline for studies on the scaling of health interventions: A systematic review. Medicine 2024;103:7(e37079).
Protocol registration: https://osf.io/vcwfx.
Contributor Information
Hervé Tchala Vignon Zomahoun, Email: herve-tchala.zomahoun.1@ulaval.ca.
Ali Ben Charif, Email: ali.bencharif@cubecxpert.ca.
Giraud Ekanmian, Email: codjo-giraud-ulrich.ekanmian.1@ulaval.ca.
David Moher, Email: dmoher@ohri.ca.
Robert K. D. McLean, Email: rmclean@idrc.ca.
Andrew Milat, Email: andrew.milat@sydney.edu.au.
Luke Wolfenden, Email: luke.wolfenden@hnehealth.nsw.gov.au.
Karina Prévost, Email: karinaprevost@hotmail.com.
Emmanuelle Aubin, Email: emma@ecoetat.com.
Paula Rochon, Email: paula.rochon@utoronto.ca.
Nathalie Rheault, Email: nathalrhe@hotmail.com.
France Légaré, Email: france.legare@fmed.ulaval.ca.
References
- [1].Ben Charif A, Zomahoun HTV, LeBlanc A, et al. Effective strategies for scaling up evidence-based practices in primary care: a systematic review. Implement Sci. 2017;12:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gargani J, McLean R. Scaling science. Stanford Soc Innov Rev. 2017;15:34–9. [Google Scholar]
- [3].Leeman J, Boisson A, Go V. Scaling up public health interventions: engaging partners across multiple levels. Annu Rev Public Health. 2022;43:null. [DOI] [PubMed] [Google Scholar]
- [4].Legare F, Plourde KV, Charif AB, et al. Evidence on scaling in health and social care: protocol for a living umbrella review. Syst Rev. 2021;10:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gogovor A, Zomahoun HTV, Ben Charif A, et al. Essential items for reporting of scaling studies of health interventions (SUCCEED): protocol for a systematic review and Delphi process. Syst Rev. 2020;9:11–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Milat AJ, Newson R, King L, et al. A guide to scaling up population health interventions. Public Health Res Pract. 2016;26:e2611604. [DOI] [PubMed] [Google Scholar]
- [7].scalingXchange. Scaling the impact of innovation and research: a call to action from the global South. Available at: https://www.scalingxchange.org/ [access date March 23, 2022].
- [8].Simmons R, Fajans P, Ghiron L. (eds). Scaling Up Health Service Delivery: From Pilot Innovations to Policies and Programmes. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- [9].McLean R, Gargani J. Scaling Impact: Innovation for the Public Good. New York City: Routledge; 2019. [Google Scholar]
- [10].De Carvalho Corôa R, Gogovor A, Ben Charif A, et al. Evidence on scaling in health and social care: an umbrella review. Milbank Q. 2023;101:881–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Simera I, Moher D, Hirst A, et al. Transparent and accurate reporting increases reliability, utility, and impact of your research: reporting guidelines and the EQUATOR network. BMC Med. 2010;8:24–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bartlett C, Simpson K, Turner AN. Patient access to complex chronic disease records on the internet. BMC Med Inform Decis Mak. 2012;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Moher D, Schulz KF, Simera I, et al. Guidance for developers of health research reporting guidelines. PLoS Med. 2010;7:e1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. version 6.2 ed. Cochrane, 2021. Available at: https://training.cochrane.org/handbook/archive/v6.2 [access date January 31, 2022]. [Google Scholar]
- [15].Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McGowan J, Sampson M, Salzwedel DM, et al. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- [17].Cochrane Handbook “Checklist of items to consider in data collection” The Cochrane collaboration. Cochrane handbook for systematic reviews of interventions version 5.1.0. Web site. Available at: https://handbook-5-1.cochrane.org/. Published 2011. [access date October 17, 2019].
- [18].World Health Organization-ExpandNet. Nine Steps for Developing a Scaling-up Strategy. Geneva, Switzerland: WHO; 2010. [Google Scholar]
- [19].Adisso EL, Zomahoun HTV, Gogovor A, et al. Sex and gender considerations in implementation interventions to promote shared decision making: a secondary analysis of a cochrane systematic review. PLoS One. 2020;15:e0240371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gogovor A, Zomahoun HTV, Ekanmian G, et al. Sex and gender considerations in reporting guidelines for health research: a systematic review. Biol Sex Differ. 2021;12:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ben Charif A, Plourde KV, Guay-Belanger S, et al.; RePOS Network. Strategies for involving patients and the public in scaling-up initiatives in health and social services: protocol for a scoping review and Delphi survey. Syst Rev. 2021;10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ben Charif A, Zomahoun HTV, Gogovor A, et al. Tools for assessing the scalability of innovations in health: a systematic review. Health Res Policy Syst. 2022;20:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ben Charif A, Zomahoun HTV, Massougbodji J, et al. Assessing the scalability of innovations in primary care: a cross-sectional study. CMAJ Open. 2020;8:E613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zomahoun HTV, Ben Charif A, Freitas A, et al. The pitfalls of scaling up evidence-based interventions in health. Glob Health Action. 2019;12:1670449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].RE-AIM – Measures and Checklists. Available at: https://re-aim.org/resources-and-tools/measures-and-checklists/ [access date December 16, 2022].
- [27].Ten Dimensions of Scaling Up Reproductive Health Programs: An Introduction (Report). Washington, DC: US Agency for International Development and Management Sciences for Health; 2002. [Google Scholar]
- [28].Huston P. Reporting on innovative public health interventions. Can J Public Health. 2003;94:326–8. [Google Scholar]
- [29].Des Jarlais DC, Lyles C, Crepaz N, et al. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94:361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].World Health Organization. Scaling up Projects and Initiatives for Better Health: From Concepts to Practice. Geneva, Switzerland: WHO; 2016. [Google Scholar]
- [31].World Health Organization. WHO Concise Guide to Implementing and Scaling Up Family Planning Service Improvements. Geneva, Switzerland: WHO; 2018. [Google Scholar]
- [32].World Health Organization-ExpandNet. Practical Guidance for Scaling Up Health Service Innovations. Geneva, Switzerland: WHO; 2009. [Google Scholar]
- [33].Riley BL, MacDonald J, Mansi O, et al. Is reporting on interventions a weak link in understanding how and why they work? A preliminary exploration using community heart health exemplars. Implement Sci. 2008;3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Egan M, Bambra C, Petticrew M, et al. Reviewing evidence on complex social interventions: appraising implementation in systematic reviews of the health effects of organisational-level workplace interventions. J Epidemiol Community Health. 2009;63:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].O’Donnell S, Li LC, King J, et al. Development of a framework for reporting health service models for managing rheumatoid arthritis. Clin Rheumatol. 2010;29:151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li LC, Bombardier C. Setting priorities in arthritis care: care III conference. J Rheumatol. 2006;33:1891–4. [PubMed] [Google Scholar]
- [37].Bryce J, Victora CG, Boerma T, et al. Evaluating the scale-up for maternal and child survival: a common framework. Int Health. 2011;3:139–46. [DOI] [PubMed] [Google Scholar]
- [38].Conn VS, Groves PS. Protecting the power of interventions through proper reporting. Nurs Outlook. 2011;59:318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Eaton J, McCay L, Semrau M, et al. Scale up of services for mental health in low-income and middle-income countries. Lancet. 2011;378:1592–603. [DOI] [PubMed] [Google Scholar]
- [40].World Health Organization/ExpandNet. Beginning with the End in Mind: Planning Pilot Projects and Other Programmatic Research for Successful Scaling Up. Geneva: World Health Organization; 2011. [Google Scholar]
- [41].Yamey G. Scaling up global health interventions: a proposed framework for success. PLoS Med. 2011;8:e1001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bradley EH, Curry LA, Taylor LA, et al. A model for scale up of family health innovations in low-income and middle-income settings: a mixed methods study. BMJ Open. 2012;2:e000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Harrington NG, Noar SM. Reporting standards for studies of tailored interventions. Health Educ Res. 2012;27:331–42. [DOI] [PubMed] [Google Scholar]
- [44].Adamou B, Curran J, Wilson L, et al. Guide for Monitoring Scale-up of Health Practices and Interventions. Chapel Hill: MEASURE Evaluation; 2013. [Google Scholar]
- [45].Albrecht L, Archibald M, Arseneau D, et al. Development of a checklist to assess the quality of reporting of knowledge translation interventions using the Workgroup for Intervention Development and Evaluation Research (WIDER) recommendations. Implement Sci. 2013;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].WIDER recommendations to improve reporting of the content of behaviour change interventions. Available at: https://mspace.lib.umanitoba.ca/xmlui/bitstream/handle/1993/12546/1748-5908-7-70-S4.PDF [access date December 16, 2022].
- [47].Duncan JR, Larson DB, Kruskal JB. Standardization of quality initiative reporting. Radiographics. 2013;33:373–4. [DOI] [PubMed] [Google Scholar]
- [48].Montgomery P, Underhill K, Gardner F, et al. The Oxford implementation index: a new tool for incorporating implementation data into systematic reviews and meta-analyses. J Clin Epidemiol. 2013;66:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013;8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dickson KE, Simen-Kapeu A, Kinney MV, et al.; Lancet Every Newborn Study Group. Every newborn: health-systems bottlenecks and strategies to accelerate scale-up in countries. Lancet. 2014;384:438–54. [DOI] [PubMed] [Google Scholar]
- [51].Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- [52].Luoto J, Shekelle PG, Maglione MA, et al. Reporting of context and implementation in studies of global health interventions: a pilot study. Implement Sci. 2014;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].World Health Organization. An Approach to Rapid Scale Up Using HIV/AIDS Treatment and Care as an Example. Geneva, Switzerland: WHO; 2014. [Google Scholar]
- [54].World Health Organization. The MAPS Toolkit: mHealth Assessment and Planning for Scale. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- [55].Neta G, Glasgow RE, Carpenter CR, et al. A framework for enhancing the value of research for dissemination and implementation. Am J Public Health. 2015;105:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Portell M, Anguera MT, Chacon-Moscoso S, et al. Guidelines for reporting evaluations based on observational methodology. Psicothema. 2015;27:283–9. [DOI] [PubMed] [Google Scholar]
- [57].CCDR. A reporting guide for implementation science articles. Can Commun Dis Rep. 2016;42:175–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Barker PM, Reid A, Schall MW. A framework for scaling up health interventions: lessons from large-scale improvement initiatives in Africa. Implement Sci. 2016;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cooley L, Kohl R, Ved RR. Scaling Up – From Vision to Large-scale Change: A Management Framework for Practitioners. Arlington: Management Systems International; 2016. [Google Scholar]
- [60].Hales S, Lesher-Trevino A, Ford N, et al. Reporting guidelines for implementation and operational research. Bull World Health Organ. 2016;94:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Maher D. Reporting guidelines for implementation and operational research. Public Health Action. 2016;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Milat AJ, Newson R, King L; Centre for Epidemiology and Evidence. Increasing the Scale of Population Health Interventions: A Guide. Sydney, NSW: NSW Ministry of Health; 2014. [Google Scholar]
- [63].Milat AJ, King L, Bauman AE, et al. The concept of scalability: increasing the scale and potential adoption of health promotion interventions into policy and practice. Health Promot Int. 2013;28:285–98. [DOI] [PubMed] [Google Scholar]
- [64].Milat AJ, King L, Newson R, et al. Increasing the scale and adoption of population health interventions: experiences and perspectives of policy makers, practitioners, and researchers. Health Res Policy Syst. 2014;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25:986–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].SQUIRE. Available at: http://squire-statement.org/ [access date December 16, 2022].
- [67].Davidoff F, Batalden P, Stevens D, et al.; SQUIRE Development Group. Publication guidelines for quality improvement in health care: evolution of the SQUIRE project. Qual Saf Health Care. 2008;17(Suppl_1):i3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Goodman D, Ogrinc G, Davies L, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016;25:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17(Suppl_1):i13–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Indig D, Lee K, Grunseit A, et al. Pathways for scaling up public health interventions. BMC Public Health. 2017;18:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kagesten AE, Tuncalp O, Portela A, et al. Programme Reporting Standards (PRS) for improving the reporting of sexual, reproductive, maternal, newborn, child and adolescent health programmes. BMC Med Res Methodol. 2017;17:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Pinnock H, Barwick M, Carpenter CR, et al.; StaRI Group. Standards for Reporting Implementation Studies (StaRI) Statement. BMJ. 2017;356:i6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Pinnock H, Epiphaniou E, Sheikh A, et al. Developing standards for reporting implementation studies of complex interventions (StaRI): a systematic review and e-Delphi. Implement Sci. 2015;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Pinnock H, Barwick M, Carpenter CR, et al.; StaRI Group. Standards for Reporting Implementation Studies (StaRI): explanation and elaboration document. BMJ Open. 2017;7:e013318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yousafzai AK, Aboud FE, Nores M, et al. Reporting guidelines for implementation research on nurturing care interventions designed to promote early childhood development. Ann N Y Acad Sci. 2018;1419:26–37. [DOI] [PubMed] [Google Scholar]
- [76].Price-Kelly H, van Haeren L, McLean R. The Scaling Playbook: A Practical Guide for researchers. Ottawa: International Development Research Centre; 2020. [Google Scholar]
- [77].Reeves P, Edmunds K, Searles A, et al. Economic evaluations of public health implementation-interventions: a systematic review and guideline for practice. Public Health. 2019;169:101–13. [DOI] [PubMed] [Google Scholar]
- [78].Bauer GR, Braimoh J, Scheim AI, et al. Transgender-inclusive measures of sex/gender for population surveys: mixed-methods evaluation and recommendations. PLoS One. 2017;12:e0178043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Aaronson N, Alonso J, Burnam A, et al. Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res. 2002;11:193–205. [DOI] [PubMed] [Google Scholar]
- [80].Palmer W, Okonya O, Jellison S, et al. Intervention reporting of clinical trials published in high-impact cardiology journals: effect of the TIDieR checklist and guide. BMJ Evid Based Med. 2021;26:91–7. [DOI] [PubMed] [Google Scholar]
- [81].Wolfenden L, Foy R, Presseau J, et al. Designing and undertaking randomised implementation trials: guide for researchers. BMJ. 2021;372:m3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bornstein MH, Putnick DL, Bradley RH, et al. Gender in low- and middle-income countries: introduction. Monogr Soc Res Child Dev. 2016;81:7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].McCarron TL, Clement F, Rasiah J, et al. Patients as partners in health research: a scoping review. Health Expect. 2021;24:1378–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cobey KD, Monfaredi Z, Poole E, et al. Editors-in-chief perceptions of patients as (co) authors on publications and the acceptability of ICMJE authorship criteria: a cross-sectional survey. Res Involv Engagem. 2021;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Karazivan P, Dumez V, Flora L, et al. The patient-as-partner approach in health care: a conceptual framework for a necessary transition. Acad Med. 2015;90:437–41. [DOI] [PubMed] [Google Scholar]
- [86].Staniszewska S, Brett J, Simera I, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358:j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Heidari S, Babor TF, De Castro P, et al. Sex and gender equity in research: rationale for the SAGER guidelines and recommended use. Res Integrity Peer Rev. 2016;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hemming K, Taljaard M, McKenzie JE, et al. Reporting of stepped wedge cluster randomised trials: extension of the CONSORT 2010 statement with explanation and elaboration. BMJ. 2018;363:k1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Foster ED, Deardorff A. Open Science Framework (OSF). J Med Libr Assoc. 2017;105:203– 6. [Google Scholar]
- [90].McKiernan EC, Bourne PE, Brown CT, et al. How open science helps researchers succeed. Elife. 2016;5:e16800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Gogovor A. An infographic of data sharing in North America. OSF Preprints. 2022. Available at: osf.io/jm3hf [access date December 16, 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.