Abstract
Beet discs aged in 0.5 mM CaSO4 develop a capacity to absorb K+ and Cl− from solutions of low concentration. The initial influx of these ions is described by a hyperbolic relationship with concentration in the range 0.01 to 0.5 mM KCl, which is identical with the system 1 absorption isotherm found in other tissues. A second hyperbolic isotherm, attributable to system 2, is found at higher concentrations (1-50 mM KCl).
When the transport of labeled ion to the vacuole is studied by wash-exchanging the bulk of the cytoplasmic label following the absorption period, it is noted that in the range of system 1, isotope influx to the vacuole increases with time as the concentration of labeled ions in the cytoplasm increases, while in the range of system 2, influx to the vacuole is constant from the beginning. Diminution of the cytoplasmic specific activity during radio-isotope absorption by prefilling the cytoplasm with the analogous unlabeled salt, markedly reduces subsequent radioisotope uptake to the vacuole only in the range of system 1. These experiments suggest that the cytoplasm serves as a mixing chamber, and that the plasma membrane controls ion uptake to the tissue at low concentrations, indicating that the system 1 isotherm reflects ion movement into the cytoplasm through the plasma membrane. Flux experiments support this conclusion, showing that development with age of the system 1 isotherm corresponds to a quantitatively similar increase in plasma membrane influx in 0.2 mM KCl.
At higher concentrations the outer membrane no longer rate-limits entry of ions to the vacuole. Isotope influx under these conditions, described by the system 2 isotherm, presumably reflects movement across the tonoplast.
Full text
PDF

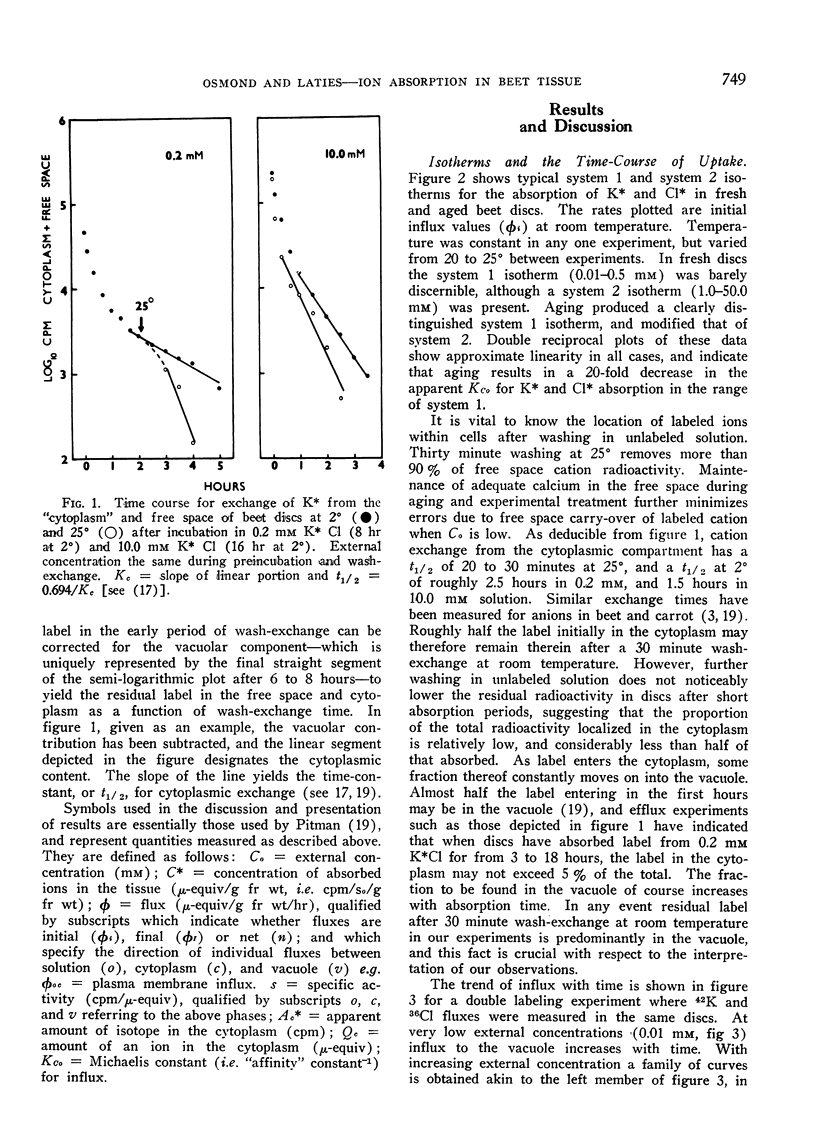
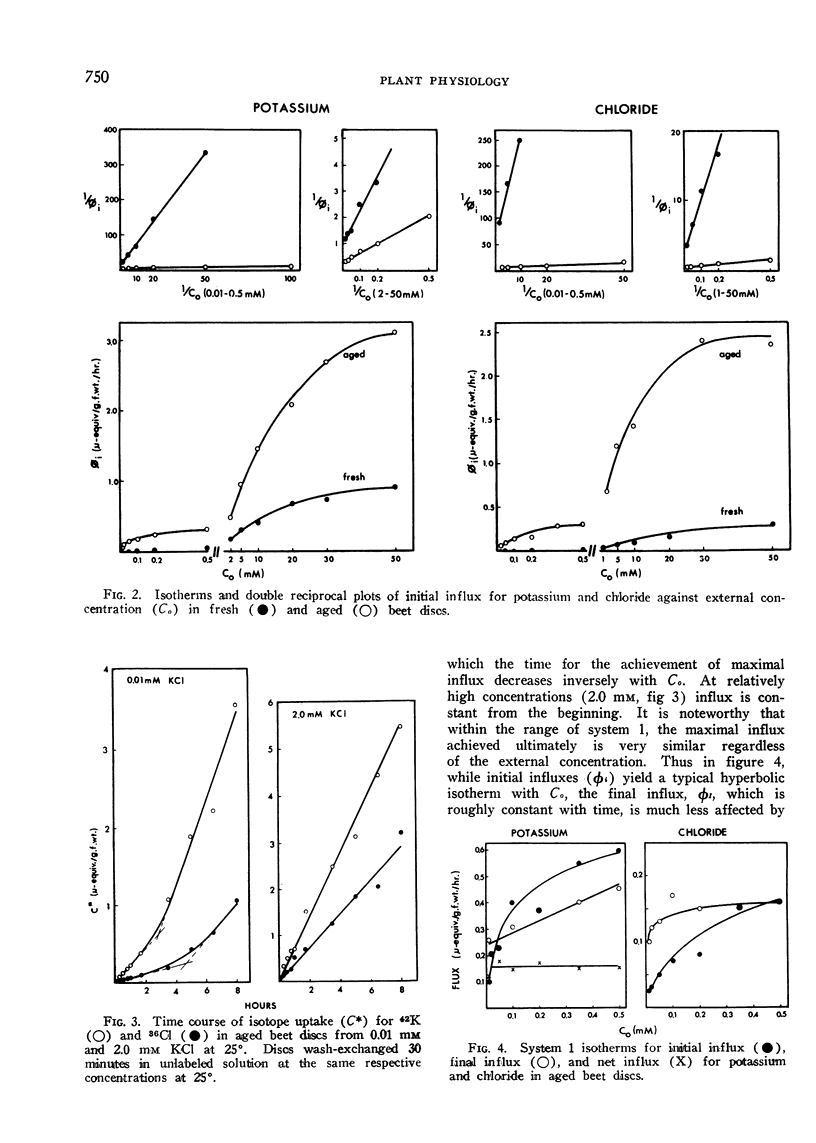
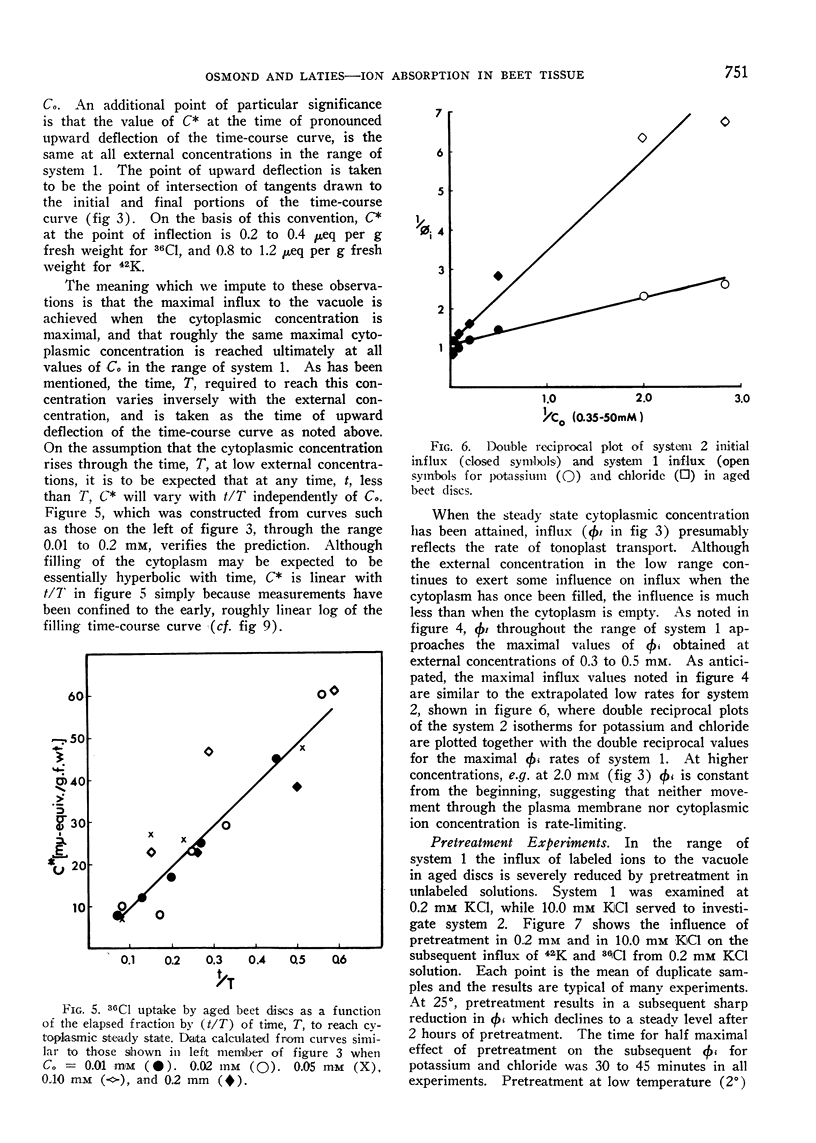
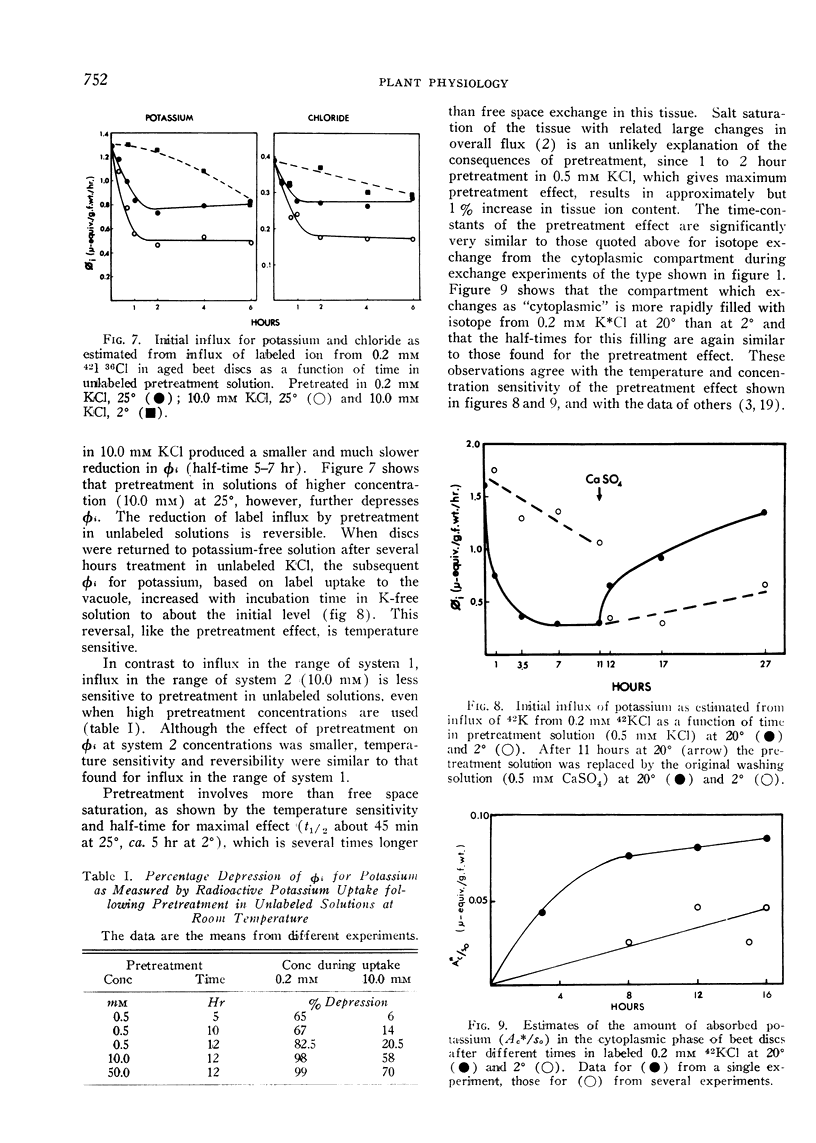


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Etherton B. Relationship of Cell Transmembrane Electropotential to Potassium and Sodium Accumulation Ratios in Oat and Pea Seedlings. Plant Physiol. 1963 Sep;38(5):581–585. doi: 10.1104/pp.38.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland D. R., Broyer T. C. GENERAL NATURE OF THE PROCESS OF SALT ACCUMULATION BY ROOTS WITH DESCRIPTION OF EXPERIMENTAL METHODS. Plant Physiol. 1936 Jul;11(3):471–507. doi: 10.1104/pp.11.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties G. G., Macdonald I. R., Dainty J. Influence of the Counter-ion on the Absorption Isotherm for Chloride at Low Temperature. Plant Physiol. 1964 Mar;39(2):254–262. doi: 10.1104/pp.39.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald I. R., Laties G. G. Kinetic Studies of Anion Absorption by Potato Slices at 0 C. Plant Physiol. 1963 Jan;38(1):38–44. doi: 10.1104/pp.38.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G., Saddler H. D. Active sodium and potassium transport in cells of barley roots. Proc Natl Acad Sci U S A. 1967 Jan;57(1):44–49. doi: 10.1073/pnas.57.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii K., Laties G. G. Dual mechanisms of ion uptake in relation to vacuolation in corn roots. Plant Physiol. 1966 May;41(5):863–870. doi: 10.1104/pp.41.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]


