Abstract
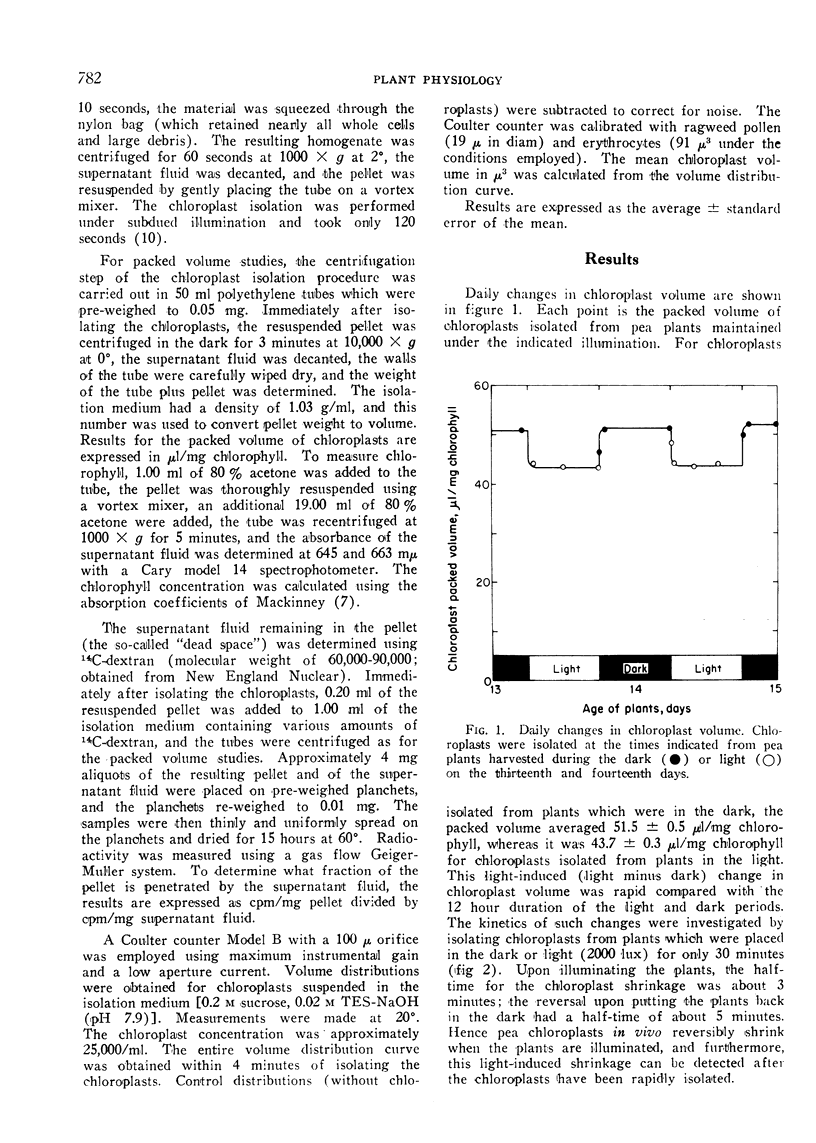
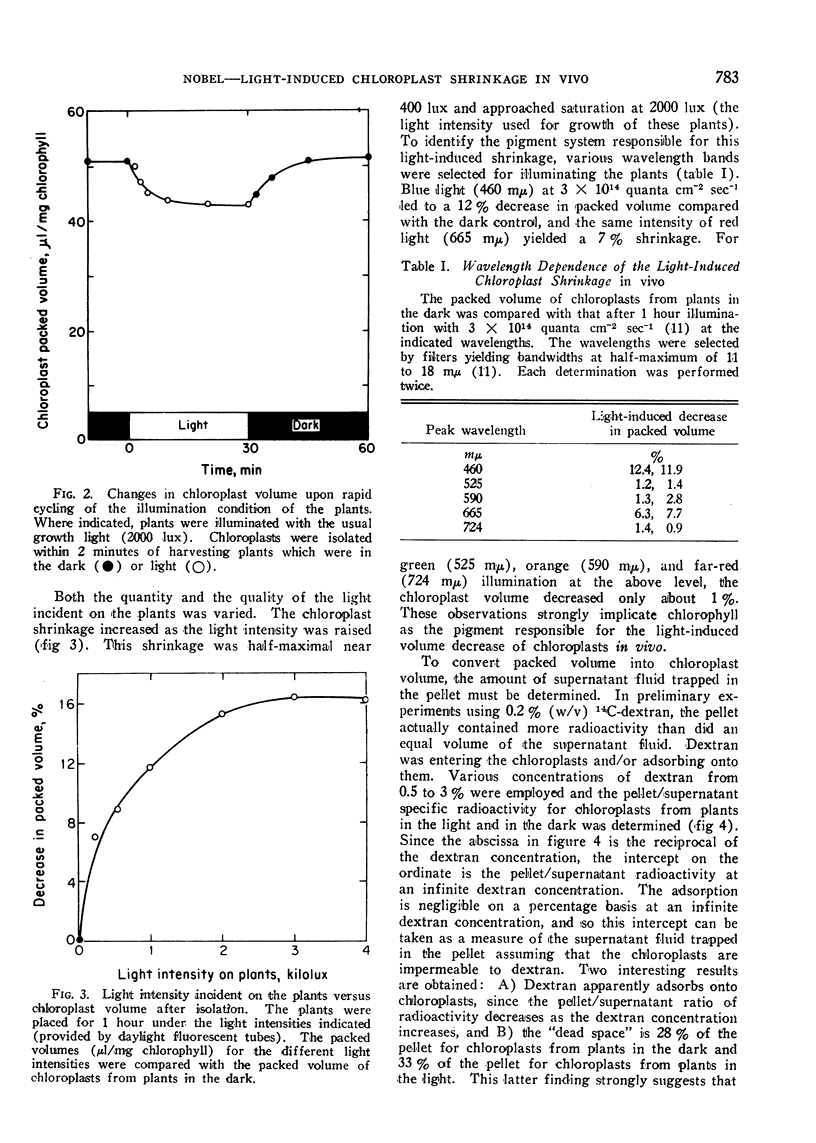
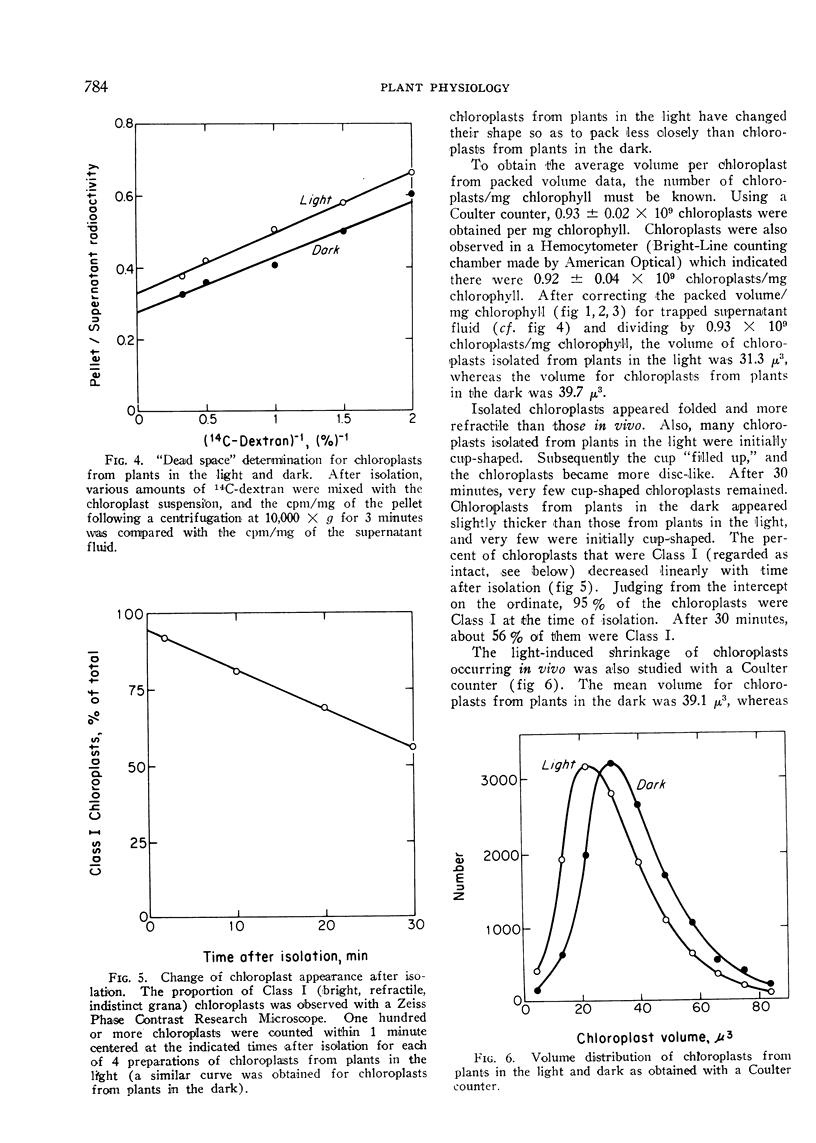
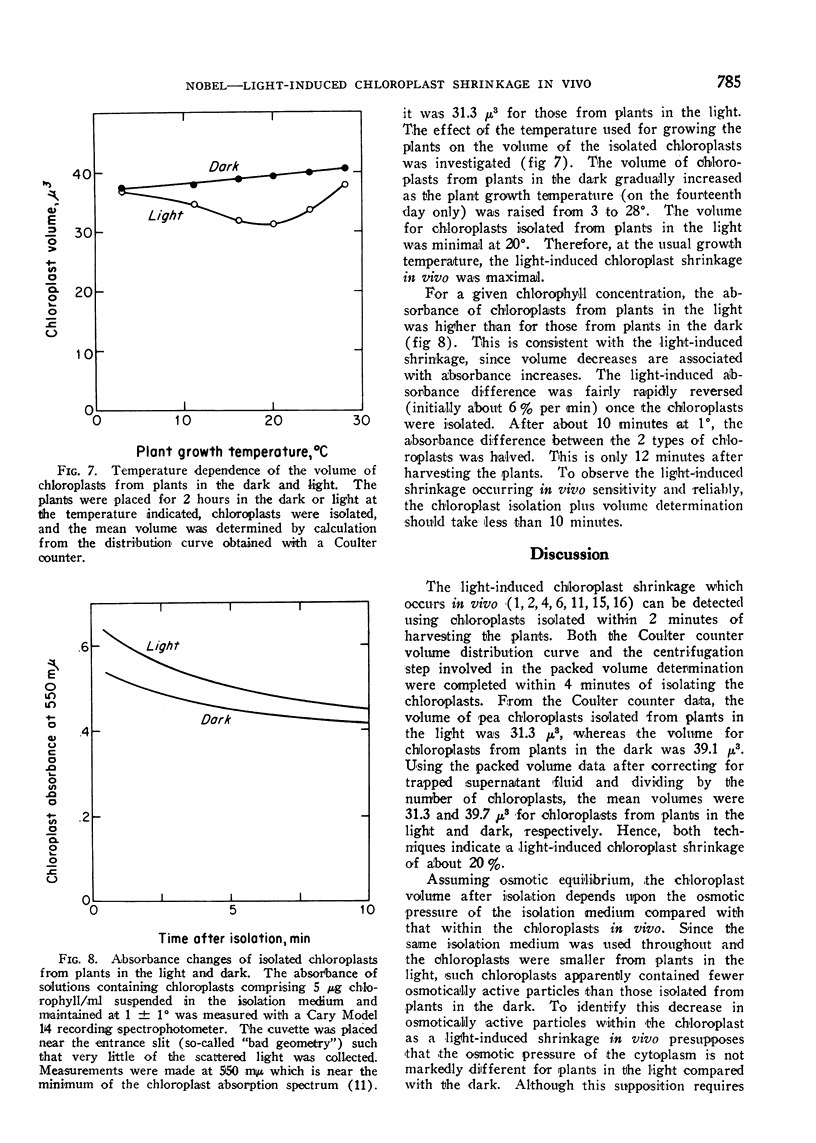
A light-induced shrinkage of chloroplasts in vivo could be detected with chloroplasts isolated within 2 minutes of harvesting pea plants. As determined both by packed volume and Coulter counter, the mean volume of chloroplasts from plants in the dark was 39 μ3, whereas it was 31 μ3 for chloroplasts from plants in the light. Upon illumination of the plants, the half-time for the chloroplast shrinkage in vivo was about 3 minutes, and the half-time for the reversal in the dark was about 5 minutes. A plant growth temperature of 20° was optimal for the volume change. The chloroplast shrinkage was half-maximal for a light intensity of 400 lux incident on the plants and was light-saturated near 2000 lux. The light-absorbing pigment responsible for the volume change was chlorophyll. This light-induced shrinkage resulted in a flattening and slight indenting of the chloroplasts. This chloroplast flattening upon illumination of the plants may accompany an increase in the photosynthetic efficiency of chloroplasts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dilley R. A., Rothstein A. Chloroplast membrane characteristics. Biochim Biophys Acta. 1967 Jul 3;135(3):427–443. doi: 10.1016/0005-2736(67)90032-6. [DOI] [PubMed] [Google Scholar]
- KUSHIDA H., ITOH M., IZAWA S., SHIBATA K. DEFORMATIONS OF CHLOROPLASTS ON ILLUMINATION IN INTACT SPINACH LEAVES. Biochim Biophys Acta. 1964 Jan 27;79:201–203. doi: 10.1016/0926-6577(64)90051-8. [DOI] [PubMed] [Google Scholar]
- Nobel P. S. A rapid technique for isolating chloroplasts with high rates of endogenous photophosphorylation. Plant Physiol. 1967 Oct;42(10):1389–1394. doi: 10.1104/pp.42.10.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel P. S. Chloroplast shrinkage and increased photophosphorylation in vitro upon illuminating intact plants of Pisum sativum. Biochim Biophys Acta. 1968 Jan 15;153(1):170–182. doi: 10.1016/0005-2728(68)90157-6. [DOI] [PubMed] [Google Scholar]
- Nobel P. S., Packer L. Light-Dependent Ion Translocation in Spinach Chloroplasts. Plant Physiol. 1965 Jul;40(4):633–640. doi: 10.1104/pp.40.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer L., Barnard A. C., Deamer D. W. Ultrastructural and Photometric Evidence for Light-Induced Changes in Chloroplast Structure in vivo. Plant Physiol. 1967 Feb;42(2):283–293. doi: 10.1104/pp.42.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolberg A. B., Macey R. I. Osmotic behavior of spinach chloroplasts. Biochim Biophys Acta. 1965 Nov 29;109(2):424–430. doi: 10.1016/0926-6585(65)90168-8. [DOI] [PubMed] [Google Scholar]
- Vanden Driessche T. Circadian rhythms in Acetabularia: photosynthetic capacity and chloroplast shape. Exp Cell Res. 1966 Apr;42(1):18–30. doi: 10.1016/0014-4827(66)90315-6. [DOI] [PubMed] [Google Scholar]


