Abstract
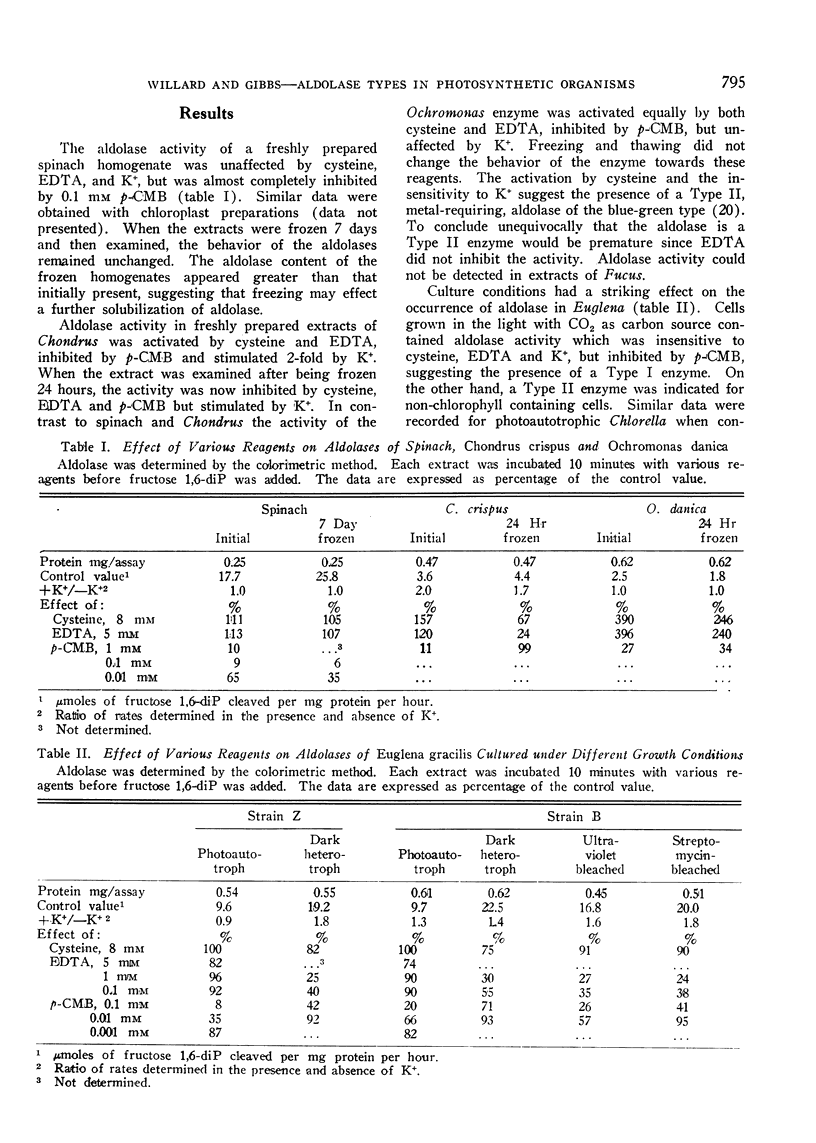
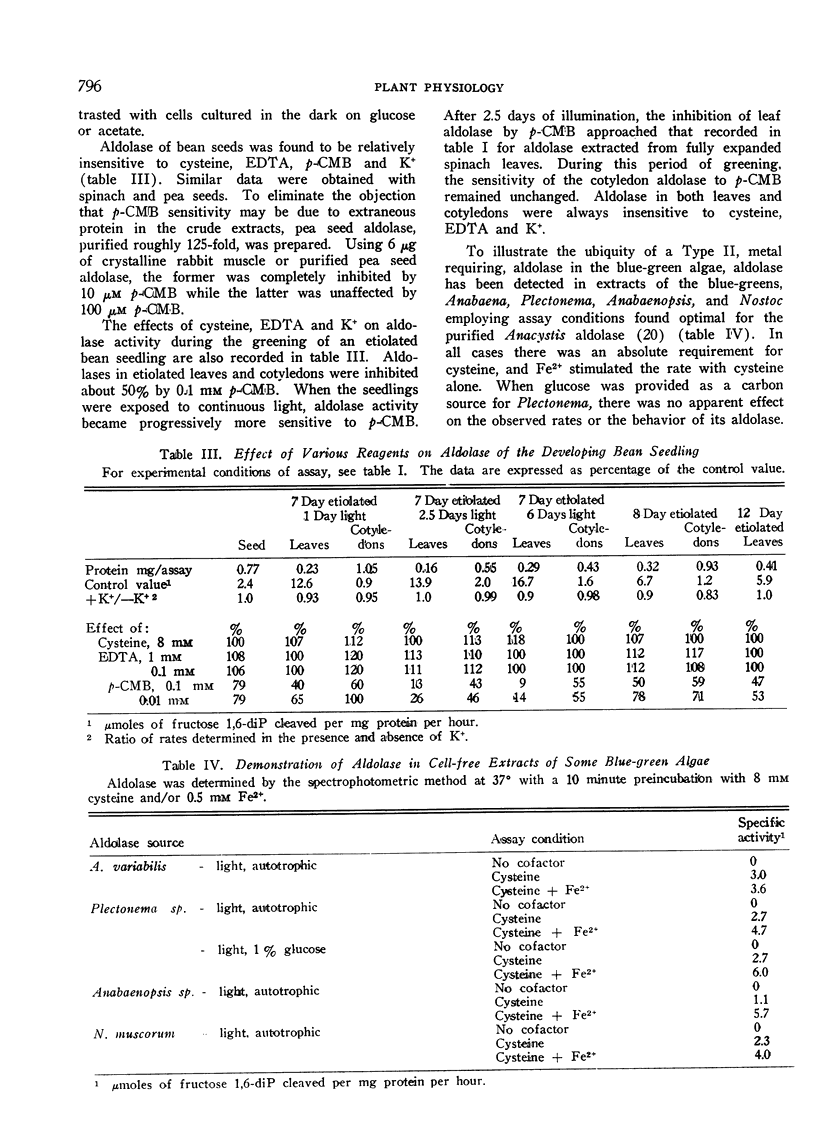
Spinach leaves and photoautotrophically grown Euglena and Chlorella possess fructose 1,6-diphosphate aldolases inhibited by p-chloromercuribenzoate but insensitive to K+ or ethylenediamine tetraacetate (Type I). Dark grown Euglena and Chlorella have aldolases inhibited by p-chloromercuribenzoate and ethylenediamine tetraacetate but stimulated by K+ (Type II). The red alga, Chondrus, and the golden-brown alga, Ochromonas, appear to possess both types. Bean, pea, and spinach seeds and the leaves and cotyledons of etiolated bean seedlings contain a p-chloromercuribenzoate insensitive, apparently non-sulfhydryl variant of Type I. Sensitivity of leaf aldolase to p-chloromercuribenzoate occurs in etiolated bean seedlings only after an extended period of illumination. Type II aldolase activity in cell-free extracts of 4 blue-green algae has been demonstrated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fewson C. A., Al-Hafidh M., Gibbs M. Role of Aldolase in Photosynthesis. I. Enzyme Studies With Photosynthetic Organisms With Special Reference to Blue-Green Algae. Plant Physiol. 1962 May;37(3):402–406. doi: 10.1104/pp.37.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. C., Gibbs M. Intracellular and Phylogenetic Distribution of Ribulose 1,5-Diphosphate Carboxylase and D-Glyceraldehyde-3-Phosphate Dehydrogenases. Plant Physiol. 1959 May;34(3):324–329. doi: 10.1104/pp.34.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBS M. Triosephosphate dehydrogenase and glucose-6-phosphate dehydrogenase in the pea plant. Nature. 1952 Jul 26;170(4317):164–165. doi: 10.1038/170164a0. [DOI] [PubMed] [Google Scholar]
- HAGEMAN R. H., ARNON D. I. Changes in glyceraldehyde phosphate dehydrogenase during the life cycle of a green plant. Arch Biochem Biophys. 1955 Aug;57(2):421–436. doi: 10.1016/0003-9861(55)90304-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LYMAN H., EPSTEIN H. T., SCHIFF J. A. Studies of chloroplast development in Euglena. I. Inactivation of green colony formation by u.v. light. Biochim Biophys Acta. 1961 Jun 24;50:301–309. doi: 10.1016/0006-3002(61)90328-6. [DOI] [PubMed] [Google Scholar]
- Russell G. K., Gibbs M. Partial purification and characterization of two fructose diphosphate aldolases from Chlamydomonas mundana. Biochim Biophys Acta. 1967 Jan 11;132(1):145–154. doi: 10.1016/0005-2744(67)90200-8. [DOI] [PubMed] [Google Scholar]
- TURNER J. F., BLACK C. C., GIBBS M. Studies on photosynthetic processes. I. The effect of light intensity on triphosphopyridine nucleotide reduction, adenosine triphosphate formation, and carbon dioxide assimilation in spinach chloroplasts. J Biol Chem. 1962 Feb;237:577–579. [PubMed] [Google Scholar]
- Van Baalen C. Aldolase in blue-green algae. Nature. 1965 Apr 10;206(980):193–195. doi: 10.1038/206193a0. [DOI] [PubMed] [Google Scholar]
- Willard J. M., Schulman M., Gibbs M. Aldolase in Anacystis nidulans and Rhodopseudomonas spheroides. Nature. 1965 Apr 10;206(980):195–195. doi: 10.1038/206195a0. [DOI] [PubMed] [Google Scholar]


