Abstract
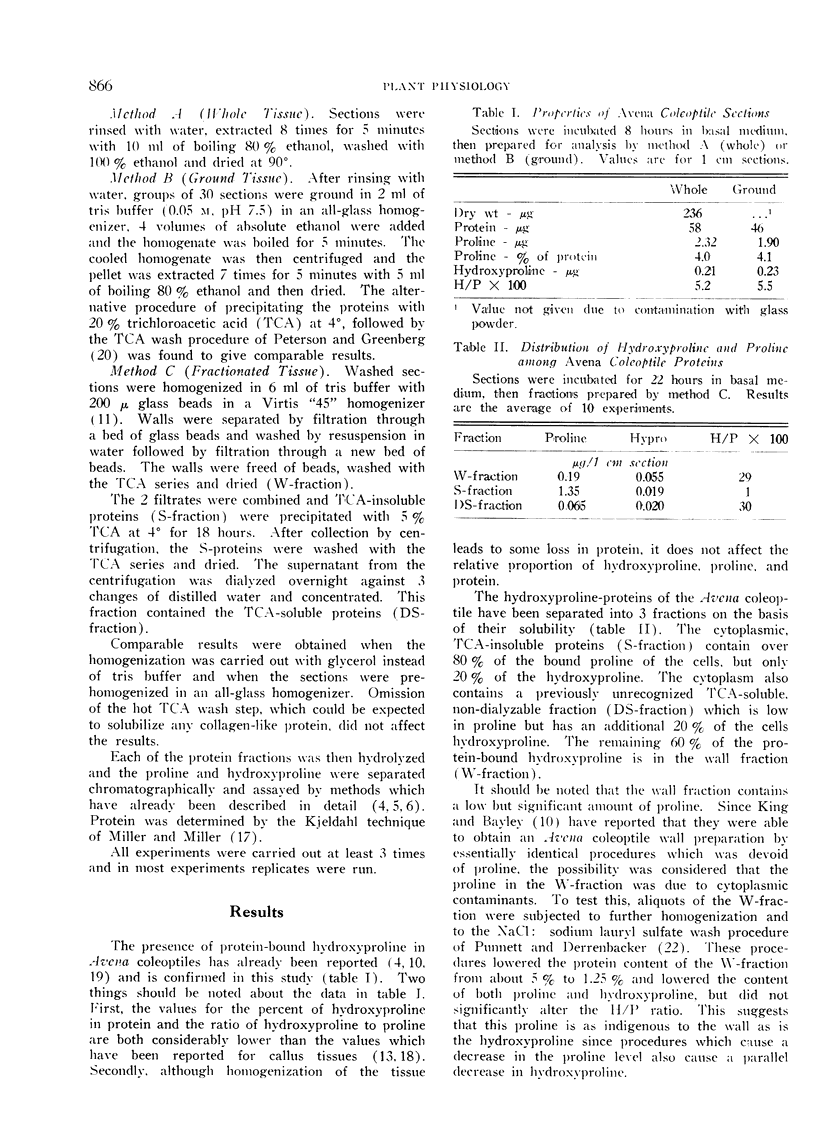
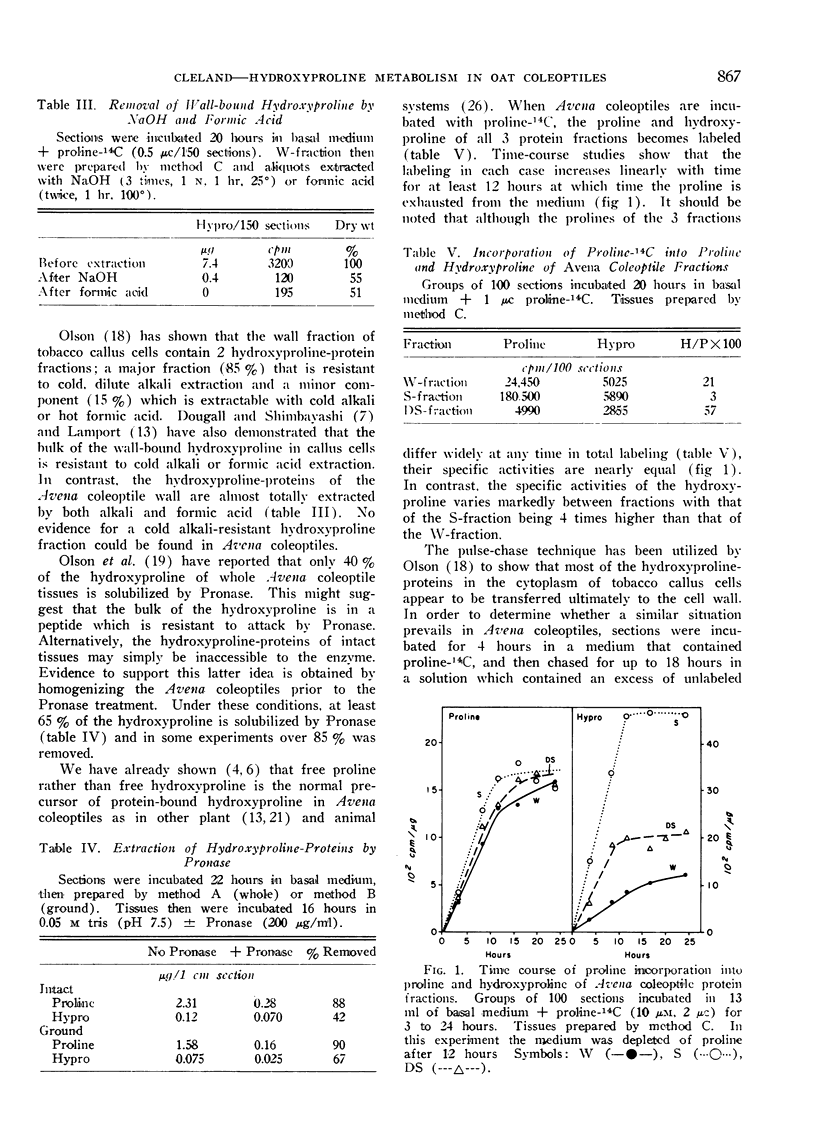
A study has been made of the distribution and metabolism of protein-bound hydroxyproline in an elongating tissue, the excised Avena coleoptile. The hydroxyproline-containing proteins of this tissue have been separated into 3 fractions on the basis of their solubilities. The cytoplasmic, trichloroacetic acid-insoluble proteins (S-fraction) contain the bulk of the proline of the cells but only 20% of the hydroxyproline. The cytoplasm also contains a previously unrecognized trichloroacetic acid-soluble, non-dialyzable fraction (DS-fraction) which is low in proline but contains 20% of the hydroxyproline. The remaining 60% of the hydroxyproline is in the wall-bound, cold alkali-soluble fraction (extensin).
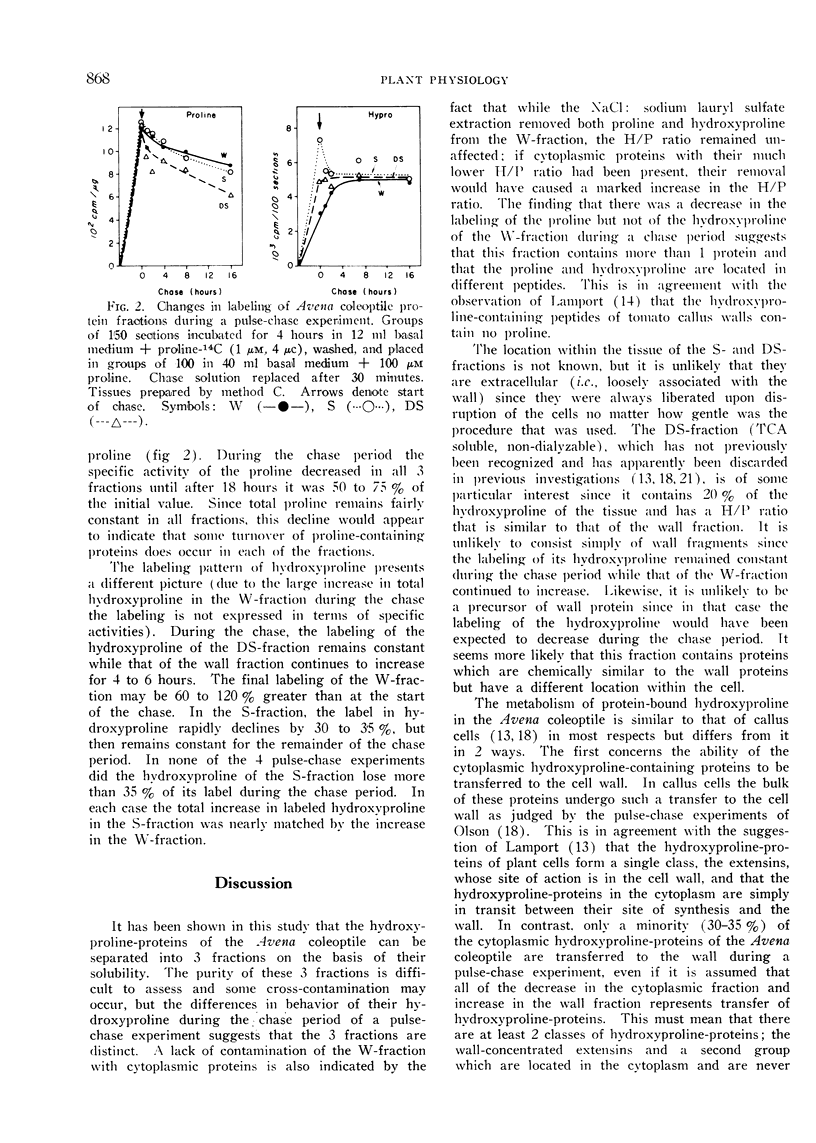
Incorporation of free proline into the proline and hydroxyproline of all fractions is linear with time for at least 12 hours. The specific activity of the proline at any time is the same in all 3 fractions while the specific activity of the hydroxyproline is 4-times greater in the S-fraction than in the W-fraction. During a pulse-chase experiment the specific activity of the proline decreases 25 to 40% in all fractions during the chase. The labeling of hydroxyproline in the wall increases during the chase while that of the DS-fraction remains constant. In the S-fraction, the labeling in hydroxyproline rapidly drops 30 to 35% during the chase but then remains constant. It is concluded that the majority of the hydroxyproline-proteins in the cytoplasm are not transported to the wall. It is suggested that a sizeable portion of the cytoplasmic hydroxyproline may be located in enzymatic proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boundy J. A., Wall J. S., Turner J. E., Woychik J. H., Dimler R. J. A mucopolysaccharide containing hydroxyproline from corn pericarp. Isolation and composition. J Biol Chem. 1967 May 25;242(10):2410–2415. [PubMed] [Google Scholar]
- Cleland R. Inhibition of cell elongation in Avena coleoptile by hydroxyproline. Plant Physiol. 1967 Feb;42(2):271–274. doi: 10.1104/pp.42.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. Inhibition of formation of protein-bound hydroxyproline by free hydroxyproline in Avena coleoptiles. Plant Physiol. 1967 Sep;42(9):1165–1170. doi: 10.1104/pp.42.9.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R., Olson A. C. Metabolism of free hydroxyproline in Avena coleoptiles. Biochemistry. 1967 Jan;6(1):32–36. doi: 10.1021/bi00853a007. [DOI] [PubMed] [Google Scholar]
- Dougall D. K., Shimbayashi K. Factors Affecting Growth of Tobacco Callus Tissue and Its Incorporation of Tyrosine. Plant Physiol. 1960 May;35(3):396–404. doi: 10.1104/pp.35.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A. C. Proteins and Plant Cell Walls. Proline to Hydroxyproline in Tobacco Suspension Cultures. Plant Physiol. 1964 Jul;39(4):543–550. doi: 10.1104/pp.39.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON E. A., GREENBERG D. M. Characteristics of the amino acid-incorporating system of liver homogenates. J Biol Chem. 1952 Jan;194(1):359–375. [PubMed] [Google Scholar]
- Roberts R. M., Loewus F. Inositol Metabolism in Plants. III. Conversion of Myo-inositol-2-H to Cell Wall Polysaccharides in Sycamore (Acer pseudoplatanus L.) Cell Culture. Plant Physiol. 1966 Nov;41(9):1489–1498. doi: 10.1104/pp.41.9.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]


