Abstract
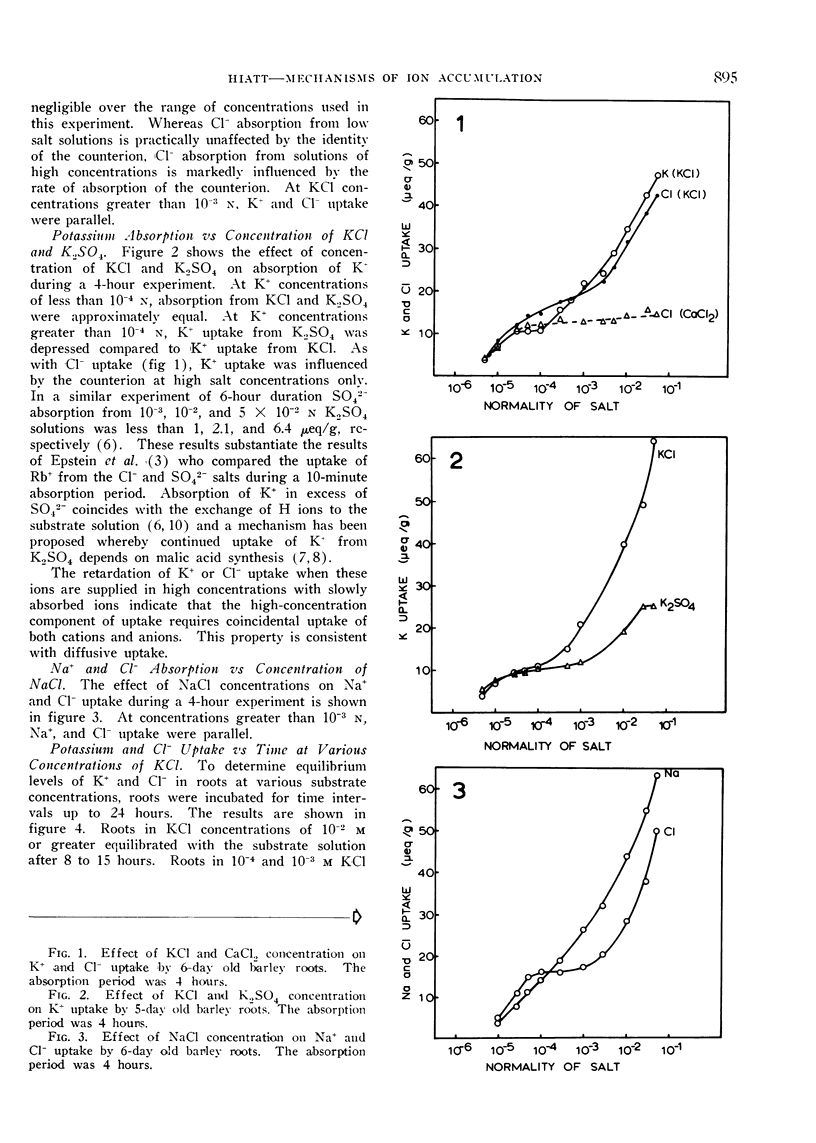
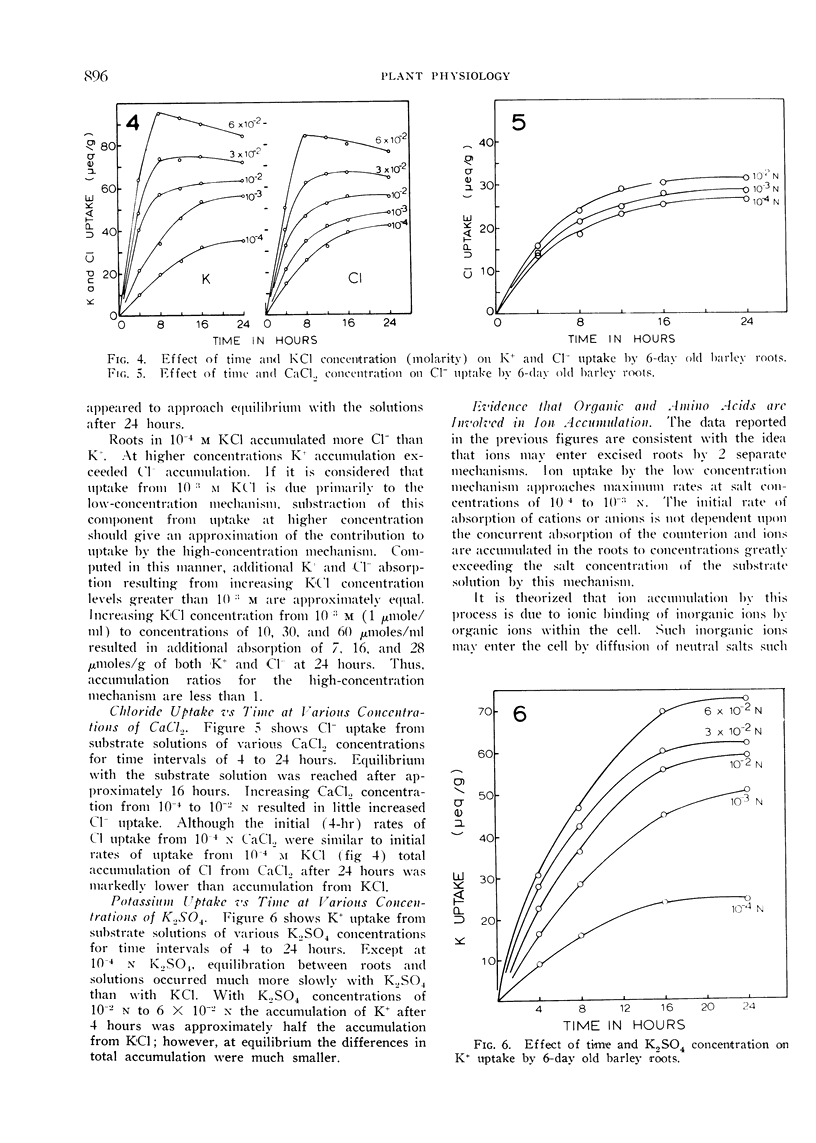
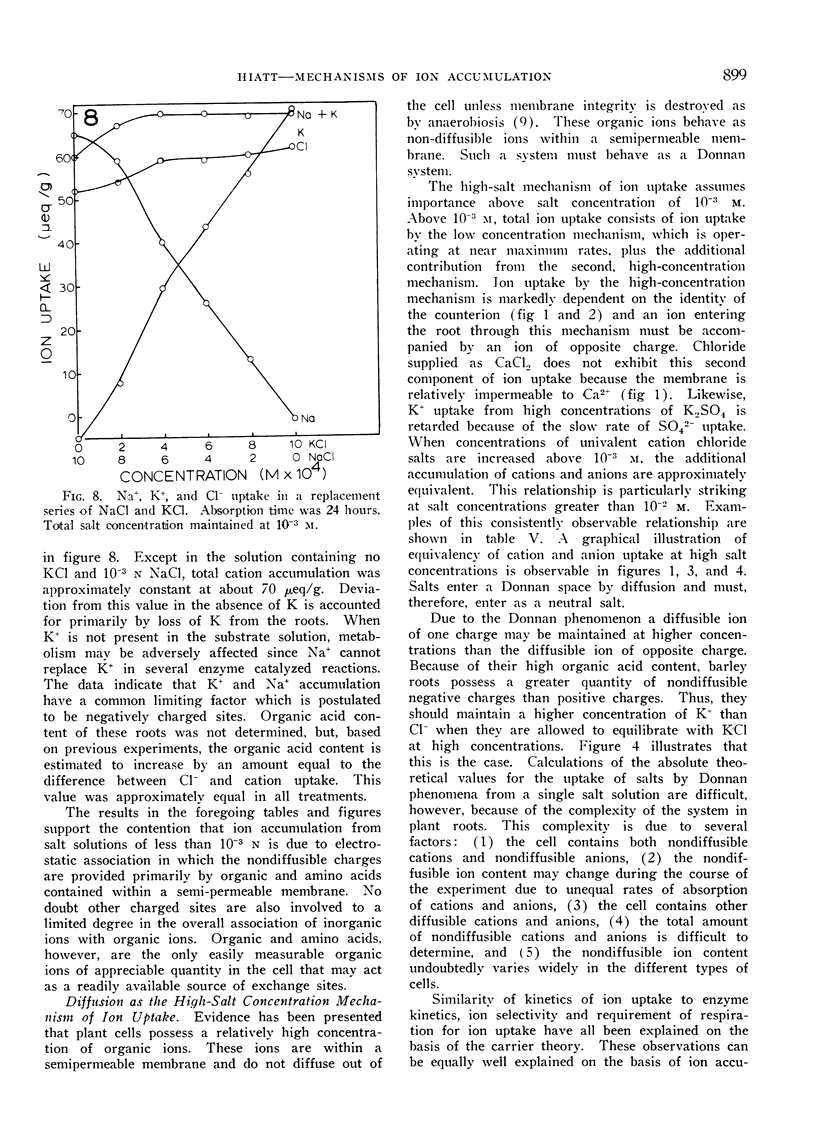
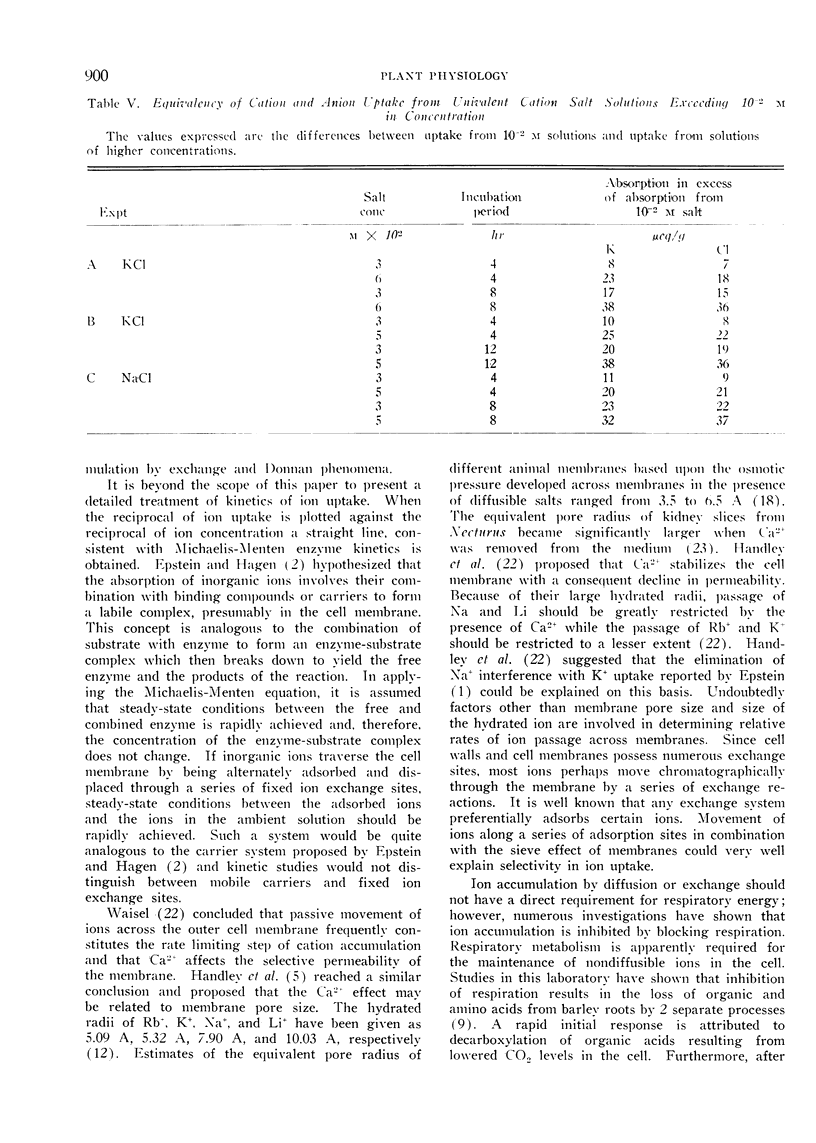
Excised roots of barley (Hordeum vulgare, var. Campana) were incubated for periods up to 24 hours in salt solutions of various concentrations and ion accumulation was determined at various time intervals. The data were consistent with the existence of 2 components of ion uptake, one accounting for ion uptake from solutions below 1 mm and both components contributing to uptake from solutions of concentrations higher than 1 mm.
It is proposed that organic and amino acids play an important role in ion accumulation by providing nondiffusible charges which may bind or retain inorganic ions within the cell. Ions would enter the cell by diffusion or exchange from salt solutions of low concentration and become associated with nondiffusible organic ions, principally organic and amino acids. The electrostatic association between inorganic and organic ions would maintain a gradient and diffusion-exchange would occur until equilibrium between the cell and the external solution was reached. It is proposed that the additional component of ion uptake which becomes important at salt concentrations higher than 1 mm is a result of diffusion of neutral salts according to Donnan phenomena. Ion uptake by this proposed mechanism would not necessarily involve the action of carriers.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Epstein E., Hagen C. E. A KINETIC STUDY OF THE ABSORPTION OF ALKALI CATIONS BY BARLEY ROOTS. Plant Physiol. 1952 Jul;27(3):457–474. doi: 10.1104/pp.27.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Noggle J. C. Multiple Site Uptake of Individual Cations by Roots as Affected by Hydrogen Ion. Plant Physiol. 1958 Mar;33(2):139–144. doi: 10.1104/pp.33.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley R., Metwally A., Overstreet R. Effects of Ca Upon Metabolic and Nonmetabolic Uptake of Na and Rb by Root Segments of Zea mays. Plant Physiol. 1965 May;40(3):513–520. doi: 10.1104/pp.40.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt A. J., Lowe R. H. Loss of organic acids, amino acids, k, and cl from barley roots treated anaerobically and with metabolic inhibitors. Plant Physiol. 1967 Dec;42(12):1731–1736. doi: 10.1104/pp.42.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt A. J. Relationship of Cell Sap pH to Organic Acid Change During Ion Uptake. Plant Physiol. 1967 Feb;42(2):294–298. doi: 10.1104/pp.42.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON P. C., ADAMS H. R. Cation-anion balance during potassium and sodium absorption by barley roots. J Gen Physiol. 1963 Jan;46:369–386. doi: 10.1085/jgp.46.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G., Dolan T., Gee R., Saltman P. Sodium Chloride Effect on Dark Fixation of CO(2) by Marine & Terrestrial Plants. Plant Physiol. 1962 May;37(3):446–449. doi: 10.1104/pp.37.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett J. E., Heald W. R., Hendricks S. B. Cation binding by baker's yeast and resins. Plant Physiol. 1965 Jul;40(4):665–671. doi: 10.1104/pp.40.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H., Handley R., Overstreet R. Potassium Loss and Changes in the Fine Structure of Corn Root Tips Induced by H-ion. Plant Physiol. 1966 Dec;41(10):1725–1735. doi: 10.1104/pp.41.10.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITTEMBURY G., SUGINO N., SOLOMON A. K. Effect of antidiuretic hormone and calcium on the equivalent pore radius of kidney slices from Necturus. Nature. 1960 Aug 20;187:699–701. doi: 10.1038/187699a0. [DOI] [PubMed] [Google Scholar]


