Abstract
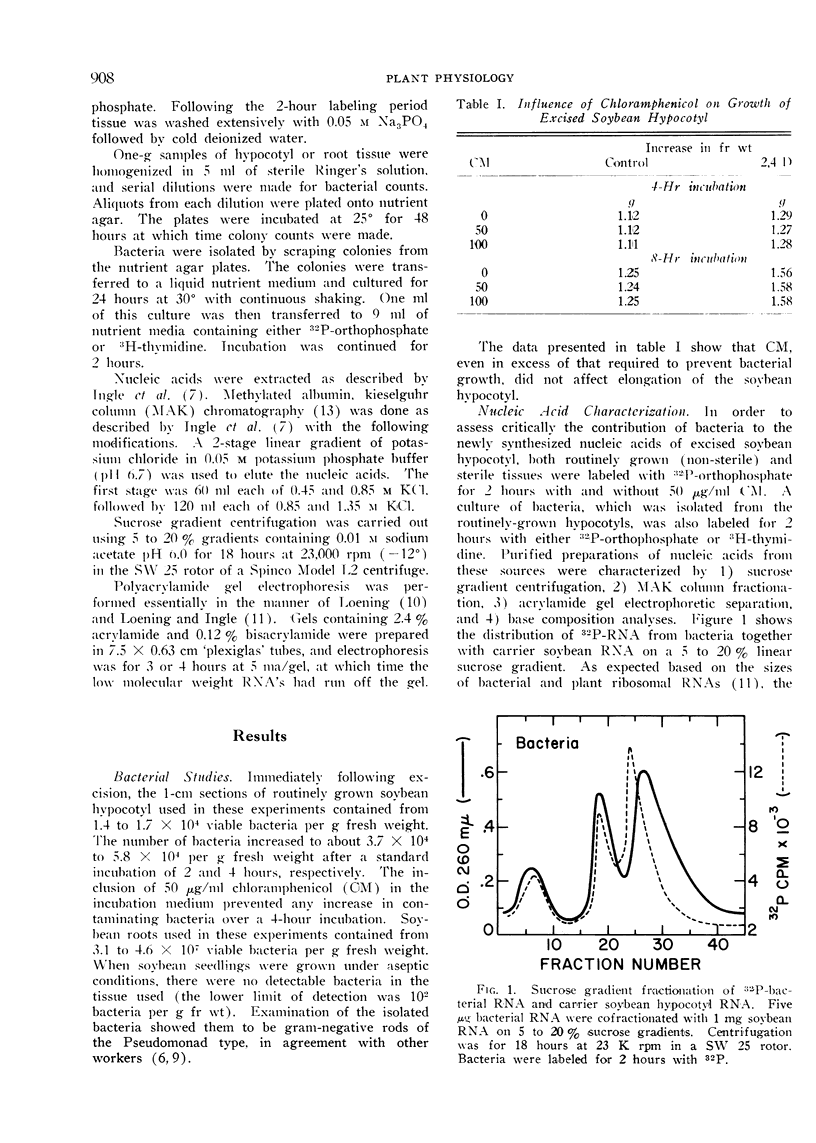
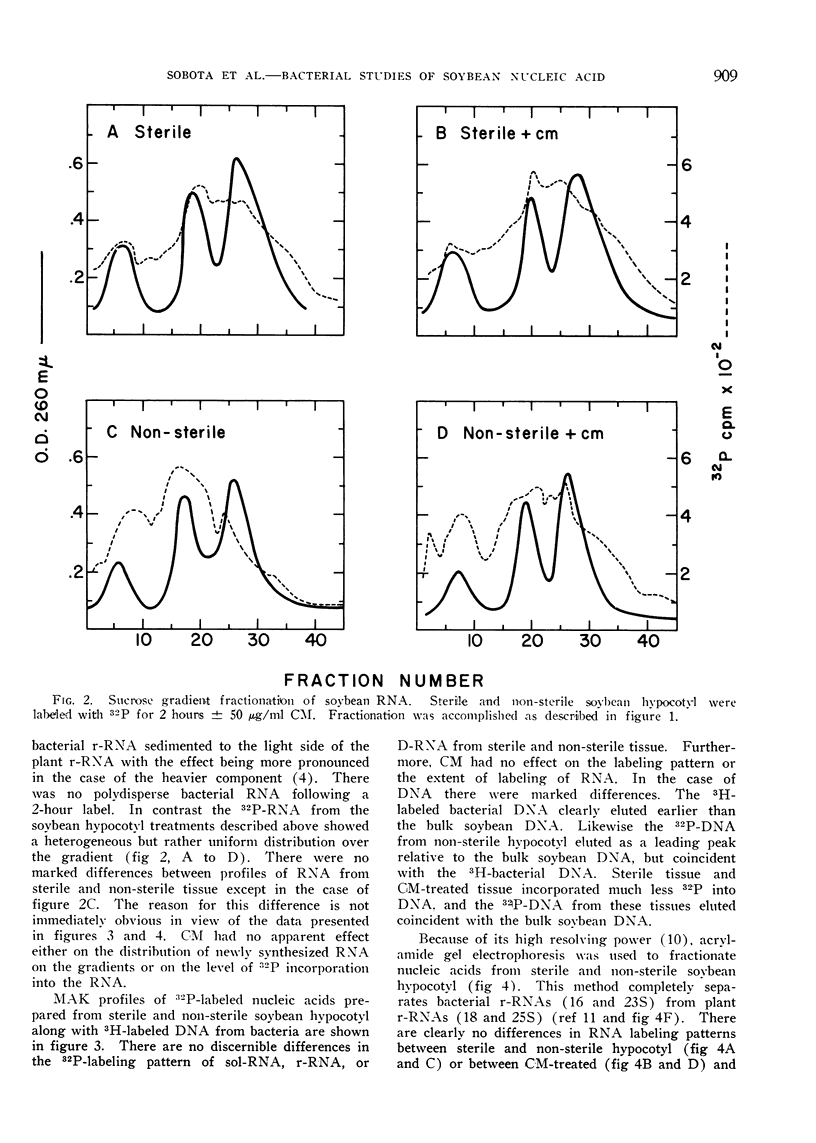
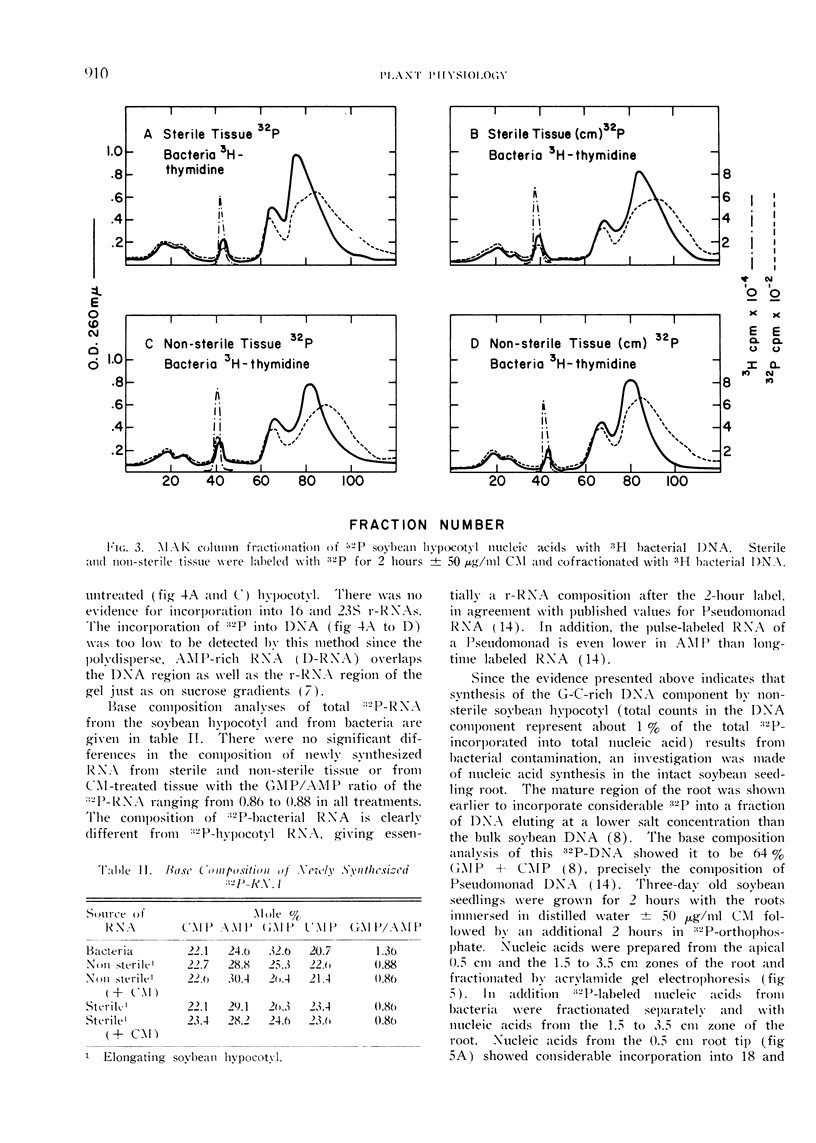
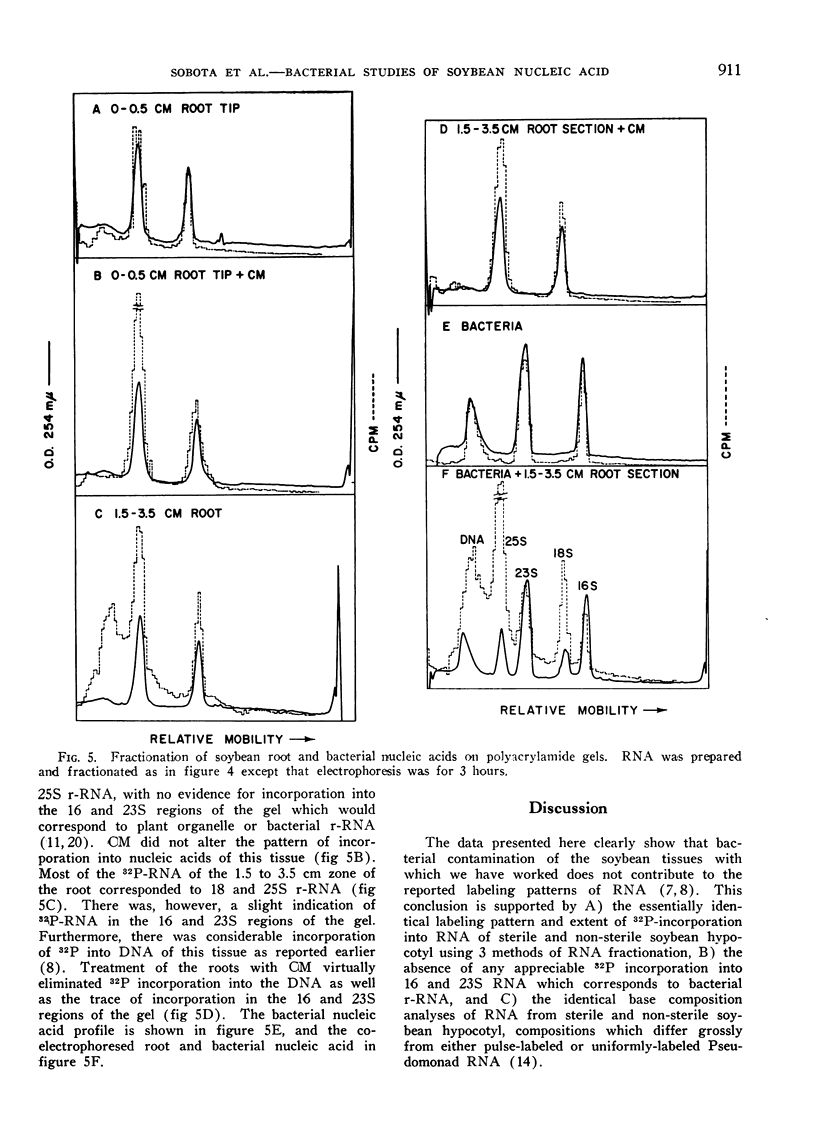
An investigation of the possible contribution of bacteria to the labeling patterns of soybean seedling nucleic acid was made. The results using sucrose gradient, MAK column, and acrylamide gel electrophoretic fractionation together with base composition analyses of nucleic acid preparations show that contaminating bacteria do not contribute to the incorporation of 32P-orthophosphate into the RNA of excised hypocotyl or soybean root tip. Sterile, non-sterile, and CM-treated soybean hypocotyl synthesize D-RNA to the same extent. The contaminating bacteria do not synthesize an AMP-rich RNA. The G-C rich 32P-DNA component of the soybean tissues used in these studied results, at least primarily, from the incorporation by contaminating bacteria. CM can be used successfully to eliminate the contribution of bacteria to the labeling of nucleic acids by etiolated plant tissues. Bacterial counts, although valuable, are not sufficient to determine if contaminating bacteria will significantly contribute to nucleic acid labeling in plants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHERRY J. H. ASSOCIATION OF RAPIDLY METABOLIZED DNA AND RNA. Science. 1964 Nov 20;146(3647):1066–1069. doi: 10.1126/science.146.3647.1066. [DOI] [PubMed] [Google Scholar]
- CHUN E. H., VAUGHAN M. H., Jr, RICH A. THE ISOLATION AND CHARACTERIZATION OF DNA ASSOCIATED WITH CHLOROPLAST PREPARATIONS. J Mol Biol. 1963 Aug;7:130–141. doi: 10.1016/s0022-2836(63)80042-x. [DOI] [PubMed] [Google Scholar]
- Chroboczek H., Cherry J. H. Production of messenger RNA during seed germination. Biochem Biophys Res Commun. 1965 Sep 22;20(6):774–779. doi: 10.1016/0006-291x(65)90085-9. [DOI] [PubMed] [Google Scholar]
- Click R. E., Tint B. L. Comparative sedimentation rates of plant, bacterial and animal ribosomal RNA. J Mol Biol. 1967 Apr 14;25(1):111–122. doi: 10.1016/0022-2836(67)90282-3. [DOI] [PubMed] [Google Scholar]
- GIERER A., SCHRAMM G. Infectivity of ribonucleic acid from tobacco mosaic virus. Nature. 1956 Apr 14;177(4511):702–703. doi: 10.1038/177702a0. [DOI] [PubMed] [Google Scholar]
- Hock B. Nature of a rapidly labeled RNA fraction described in higher plant systems. Plant Physiol. 1967 Aug;42(8):1149–1152. doi: 10.1104/pp.42.8.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGLE J., KEY J. L., HOLM R. E. DEMONSTRATION AND CHARACTERIZATION OF A DNA-LIKE RNA IN EXCISED PLANT TISSUE. J Mol Biol. 1965 Apr;11:730–746. doi: 10.1016/s0022-2836(65)80031-6. [DOI] [PubMed] [Google Scholar]
- Ingle J., Key J. L. A comparative evaluation of the synthesis of DNA-like RNA in excised and intact plant tissues. Plant Physiol. 1965 Nov;40(6):1212–1219. doi: 10.1104/pp.40.6.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E., Ingle J. Diversity of RNA components in green plant tissues. Nature. 1967 Jul 22;215(5099):363–367. doi: 10.1038/215363a0. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Studies on the role of ribonucleic acid in the growth of bacteria. Biochim Biophys Acta. 1960 Jul 29;42:99–116. doi: 10.1016/0006-3002(60)90757-5. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMPSON M., KATOH A., HOTTA Y., STERN H. METABOLICALLY LABILE DEOXYRIBONUCLEIC ACID. Proc Natl Acad Sci U S A. 1963 Sep;50:459–463. doi: 10.1073/pnas.50.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Stutz E., Noll H. Characterization of cytoplasmic and chloroplast polysomes in plants: evidence for three classes of ribosomal RNA in nature. Proc Natl Acad Sci U S A. 1967 Mar;57(3):774–781. doi: 10.1073/pnas.57.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester C. F., Dure L. S., 3rd Nucleic acid synthesis during the hormone-stimulated growth of excised oat coleoptiles. Biochemistry. 1967 Aug;6(8):2532–2538. doi: 10.1021/bi00860a034. [DOI] [PubMed] [Google Scholar]
- Waters L. C., Dure L. S., 3rd Ribonucleic acid synthesis in germinating cotton seeds. J Mol Biol. 1966 Aug;19(1):1–27. doi: 10.1016/s0022-2836(66)80046-3. [DOI] [PubMed] [Google Scholar]
- Wilson C. M. Bacteria, antibiotics and amino Acid incorporation into maize endosperm protein bodies. Plant Physiol. 1966 Feb;41(2):325–327. doi: 10.1104/pp.41.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]


