Abstract
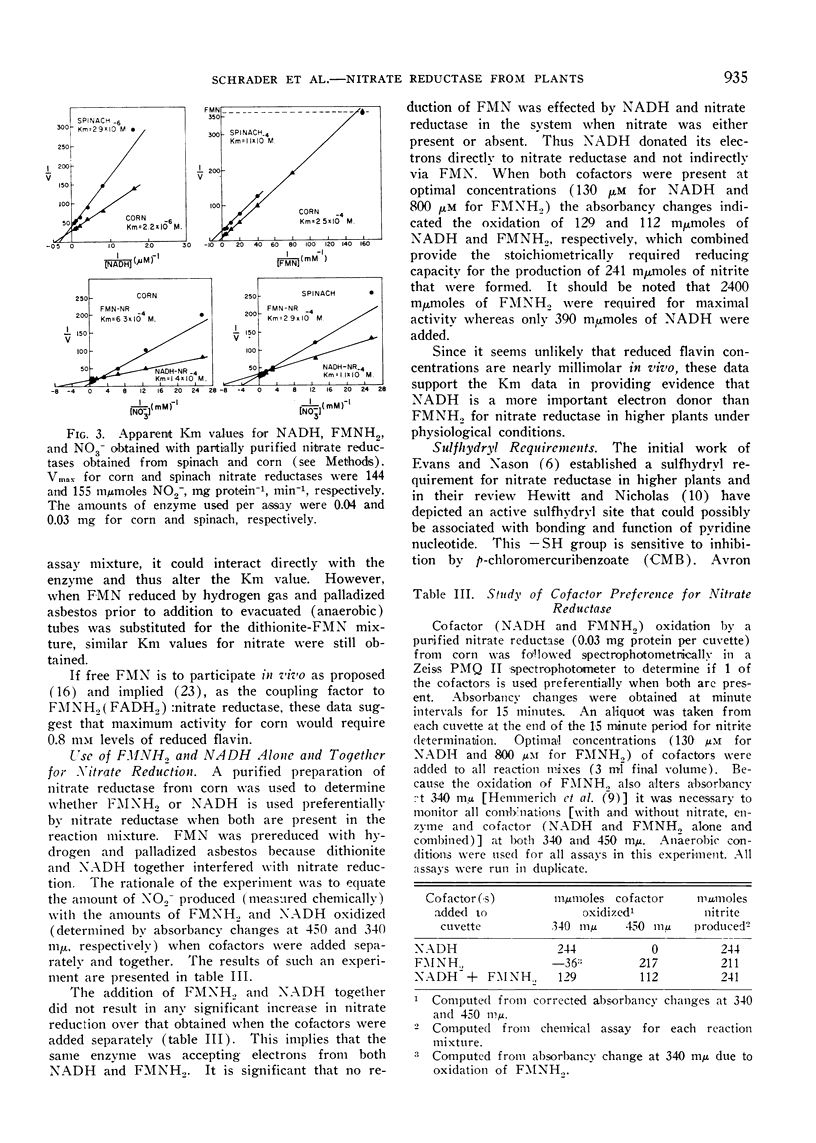
With respect to cofactor requirements, NADH, and FMNH2 were equally effective as electron donors for nitrate reductase obtained from leaves of maize, marrow, and spinach, when the cofactors were supplied in optimal concentrations. The concentration of FMNH2 required to obtain half-maximal activity was from 40- to 100-fold higher than for NADH. For maximal activity with the corn enzyme, 0.8 millimolar FMNH2 was required. In contrast, NADPH was functional only when supplied with NADP:reductase and exogenous FMN (enzymatic generation of FMNH2).
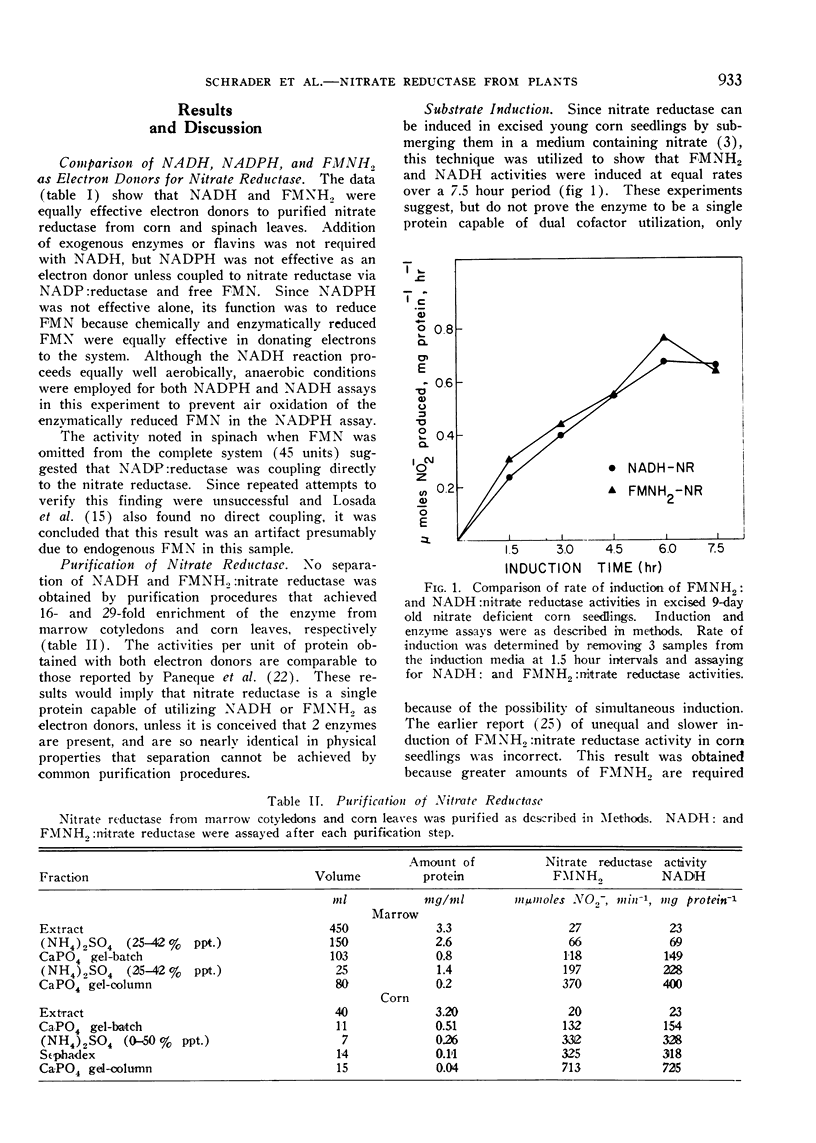
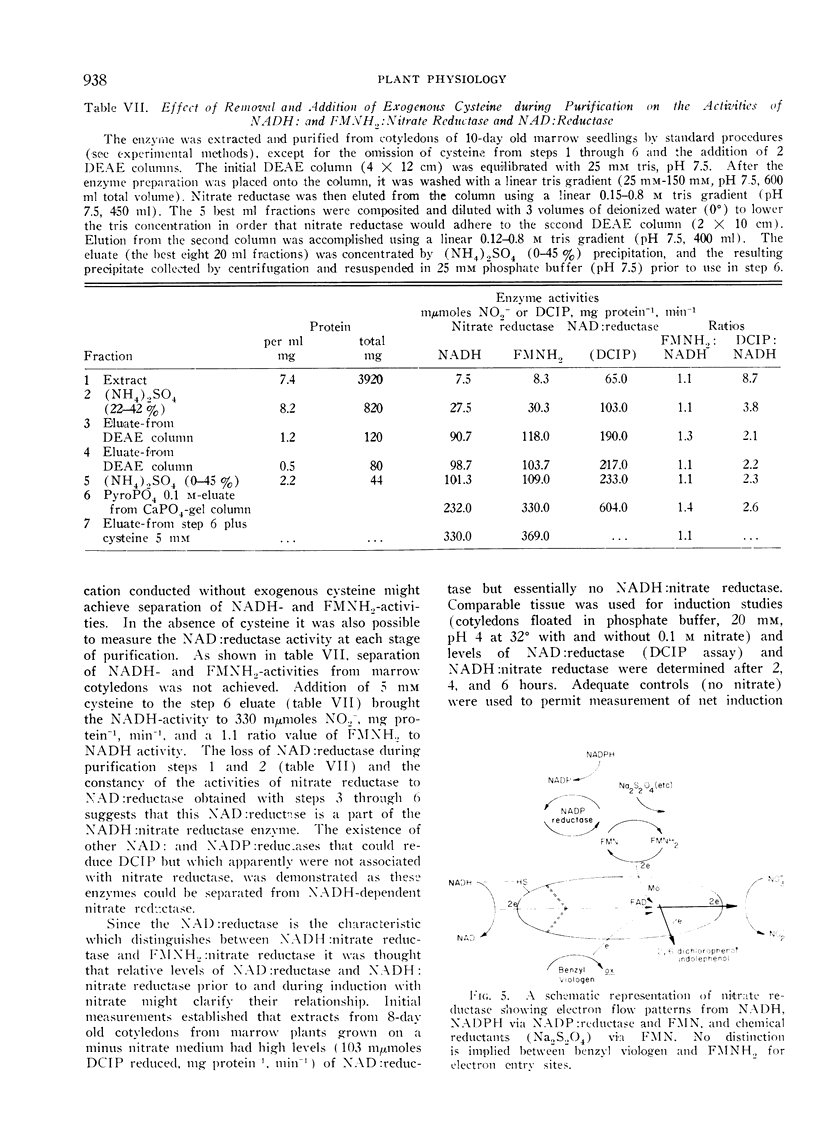
All attempts to separate the NADH2- and FMNH2-dependent nitrate reductase activities were unsuccessful and regardless of cofactor used equal activities were obtained, if cofactor concentration was optimal. Unity of NADH to FMNH2 activities were obtained during: A) purification procedures (4 step, 30-fold); B) induction of nitrate reductase in corn seedlings with nitrate; and C) inactivation of nitrate reductase in intact or excised corn seedlings. The NADH- and FMNH2-dependent activities were not additive.
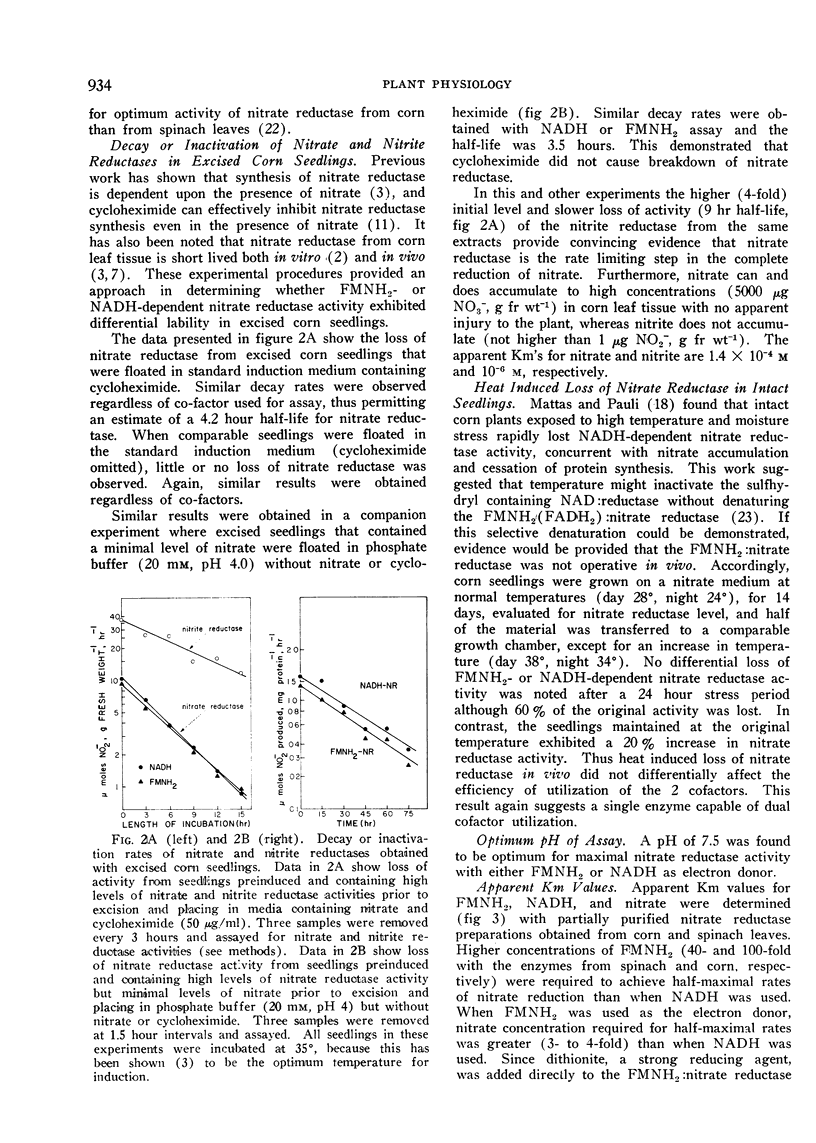
A half-life for nitrate reductase of approximately 4 hours was estimated from the inactivation studies with excised corn seedlings. Similar half-life values were obtained when seedlings were incubated at 35° in a medium containing nitrate and cycloheximide (to inhibit protein synthesis), or when both nitrate and cycloheximide were omitted.
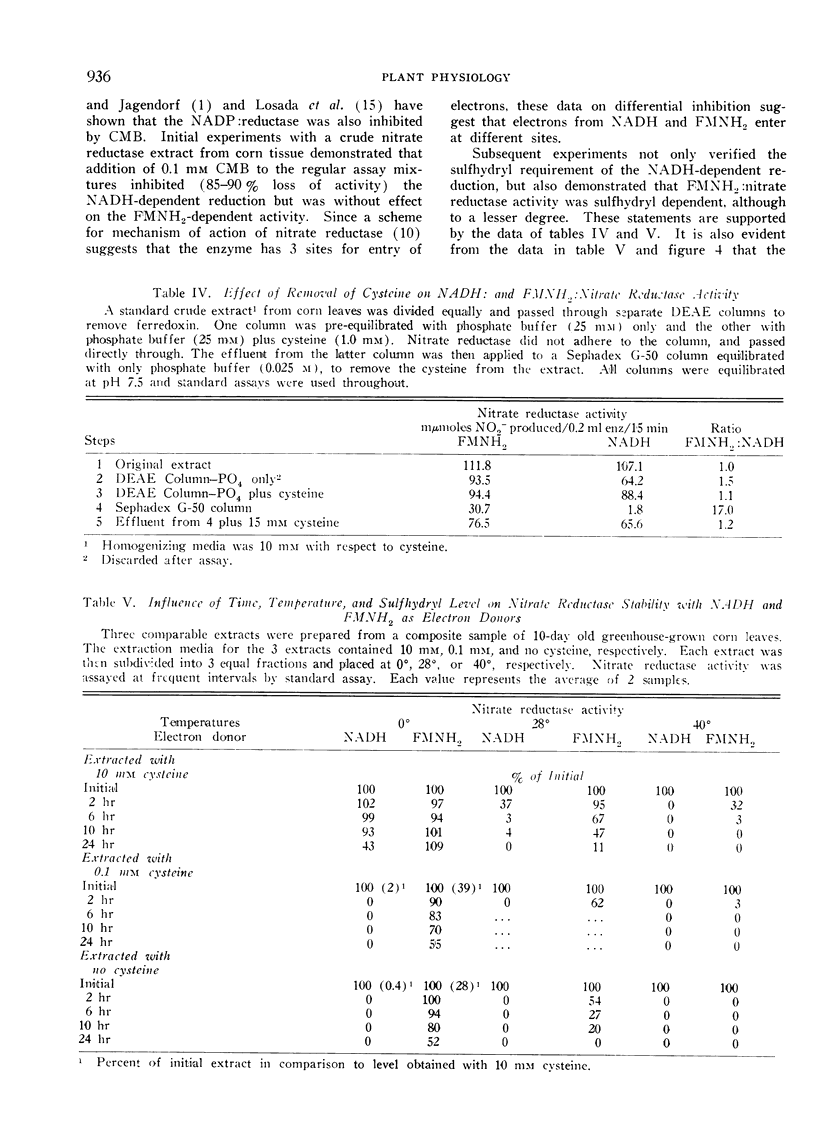
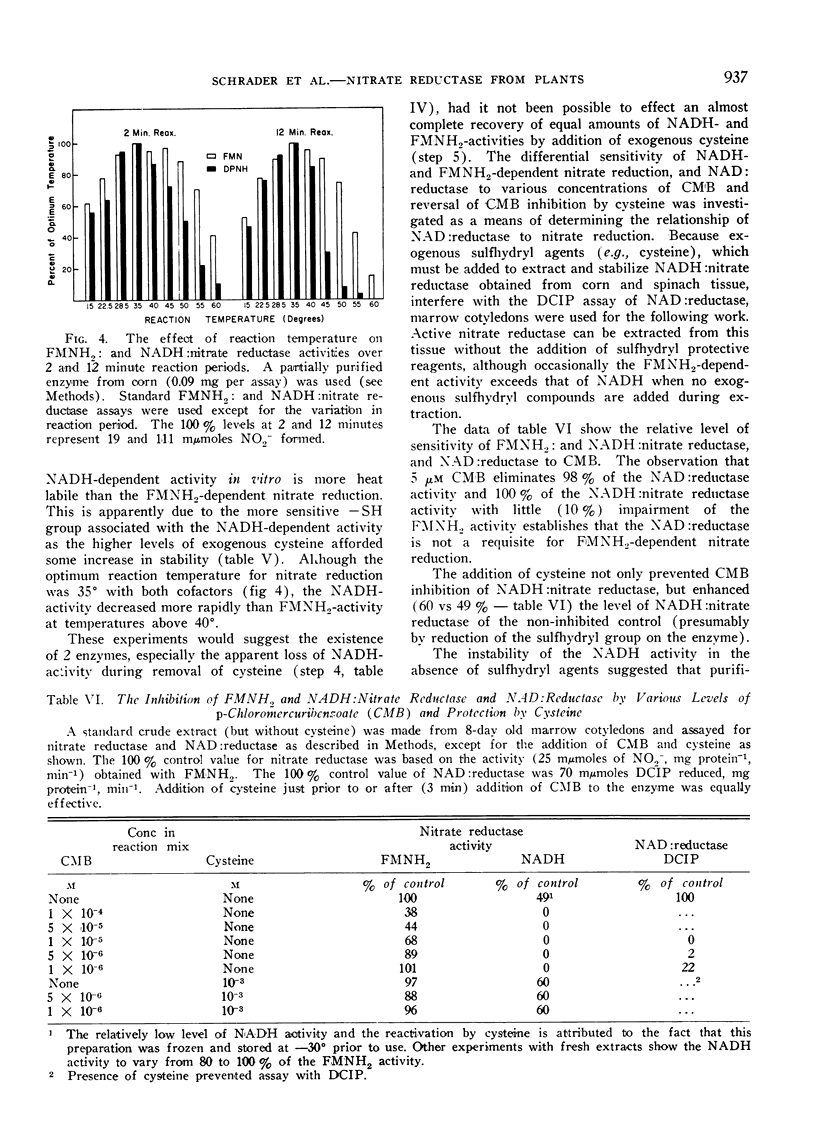
In those instances where NADH activity but not FMNH2 activity was lost due to treatment (temperature, removal of sulfhydryl agents, addition of p-chloromercuribenzoate), the loss could be explained by inactivation of the sulfhydryl group (s) required for NADH activity. This was verified by reactivation with exogenous cysteine.
Based on these current findings, and previous work, it is concluded that nitrate reductase is a single moiety with the ability to utilize either NADH or FMNH2 as cofactor. However the high concentration of FMNH2 required for optimal activity suggests that in vivo NADH is the electron donor and that nitrate reductase in higher plants should be designated NADH:nitrate reductase (E.C. 1.6.6.1).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., JAGENDORF A. T. A TPNH diaphorase from chloroplasts. Arch Biochem Biophys. 1956 Dec;65(2):475–490. doi: 10.1016/0003-9861(56)90207-7. [DOI] [PubMed] [Google Scholar]
- BEEVERS L., FLESHER D., HAGEMAN R. H. STUDIES ON THE PYRIDINE NUCLEOTIDE SPECIFICITY OF NITRATE REDUCTASE IN HIGHER PLANTS AND ITS RELATIONSHIP TO SULFHYDRYL LEVEL. Biochim Biophys Acta. 1964 Sep 18;89:453–464. doi: 10.1016/0926-6569(64)90071-9. [DOI] [PubMed] [Google Scholar]
- Beevers L., Schrader L. E., Flesher D., Hageman R. H. The Role of Light and Nitrate in the Induction of Nitrate Reductase in Radish Cotyledons and Maize Seedlings. Plant Physiol. 1965 Jul;40(4):691–698. doi: 10.1104/pp.40.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Nason A. Pyridine Nucleotide-Nitrate Reductase from Extracts of Higher Plants. Plant Physiol. 1953 Apr;28(2):233–254. doi: 10.1104/pp.28.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman R. H., Flesher D. Nitrate Reductase Activity in Corn Seedlings as Affected by Light and Nitrate Content of Nutrient Media. Plant Physiol. 1960 Sep;35(5):700–708. doi: 10.1104/pp.35.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman R. H., Waygood E. R. Methods for the Extraction of Enzymes from Cereal Leaves with Especial Reference to the Triosephosphate Dehydrogenases. Plant Physiol. 1959 Jul;34(4):396–400. doi: 10.1104/pp.34.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerich P., Veeger C., Wood H. C. Progress in the chemistry and molecular biology of flavins and flavocoenzymes. Angew Chem Int Ed Engl. 1965 Aug;4(8):671–687. doi: 10.1002/anie.196506711. [DOI] [PubMed] [Google Scholar]
- Ingle J. The regulation of activity of the enzymes involved in the assimilation of nitrate by higher plants. Biochem J. 1966 Sep;100(3):577–588. doi: 10.1042/bj1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy K. W., Hageman R. H. The purification and properties of nitrite reductase from higher plants, and its dependence on ferredoxin. Biochem J. 1966 Jul;100(1):263–273. doi: 10.1042/bj1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada M., Ramírez J. M., Paneque A., Del Campo F. F. Light and dark reduction of nitrate in a reconstituted chloroplast system. Biochim Biophys Acta. 1965 Sep 27;109(1):86–96. doi: 10.1016/0926-6585(65)90093-2. [DOI] [PubMed] [Google Scholar]
- MEDINA A., NICHOLAS D. J. Interference by reduced pyridine nucleotides in the diazotization of nitrite. Biochim Biophys Acta. 1957 Feb;23(2):440–442. doi: 10.1016/0006-3002(57)90355-4. [DOI] [PubMed] [Google Scholar]
- NICHOLAS D. J., NASON A. Mechanism of action of nitrate reductase from Neurospora. J Biol Chem. 1954 Nov;211(1):183–197. [PubMed] [Google Scholar]
- Paneque A., Del Campo F. F., Ramírez J. M., Losada M. Flavin nucleotide nitrate reductase from spinach. Biochim Biophys Acta. 1965 Sep 27;109(1):79–85. doi: 10.1016/0926-6585(65)90092-0. [DOI] [PubMed] [Google Scholar]
- Paneque A., Losada M. Comparative reduction of nitrate by spinach nitrate reductase with NADH2 and NADPH2. Biochim Biophys Acta. 1966 Oct 17;128(1):202–204. doi: 10.1016/0926-6593(66)90162-7. [DOI] [PubMed] [Google Scholar]
- Ritenour G. L., Joy K. W., Bunning J., Hageman R. H. Intracellular localization of nitrate reductase, nitrite reductase, and glutamic Acid dehydrogenase in green leaf tissue. Plant Physiol. 1967 Feb;42(2):233–237. doi: 10.1104/pp.42.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]


