Abstract
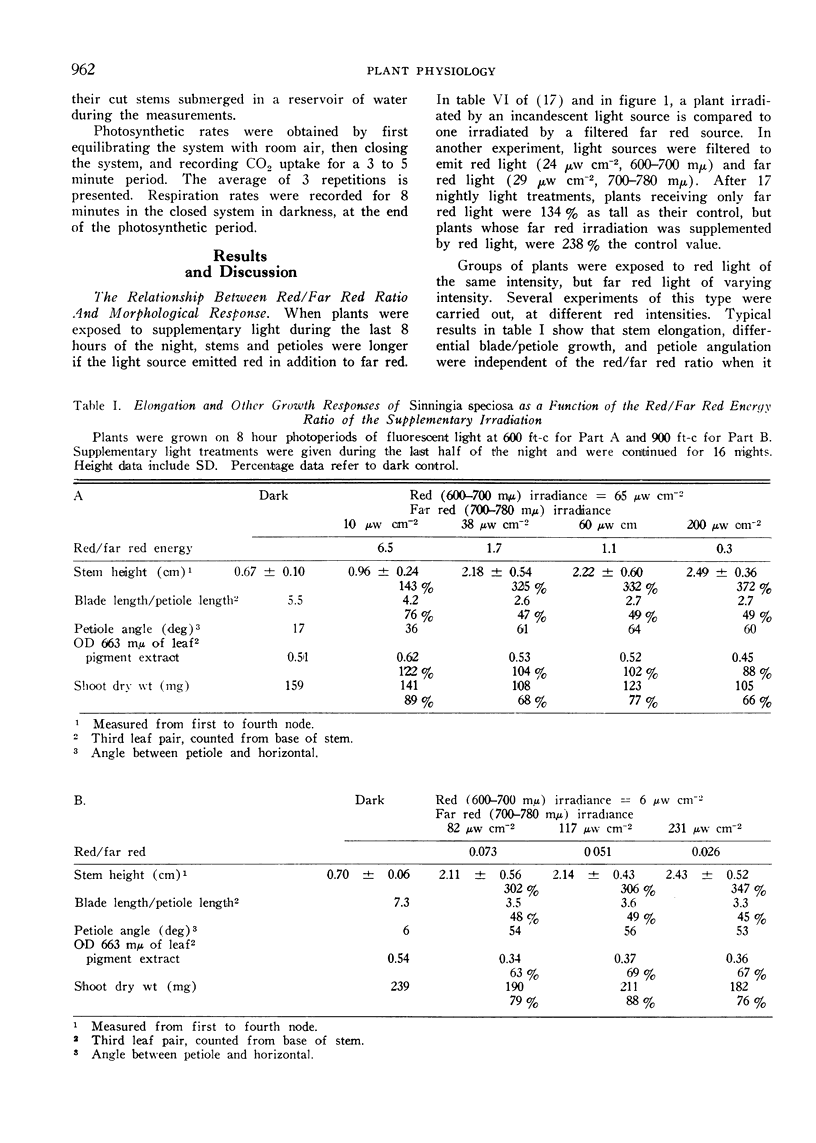
When Sinningia plants were grown with fluorescent light of photosynthetic intensity for 8 hours each day, stems became abnormally elongated when the PFR level was lowered by far red light given during the last half of several consecutive nights. However, plants were even taller if the source also emitted red light. Elongation was independent of the red/far red energy ratio if it was lower than one, but dependent upon irradiance at all values tested.
Elongation of plants irradiated by a well filtered far red source was presumed to be limited by a shortage of respiratory substrate. Enhancement by radiation shorter than 700 mμ was attributed to promotion of processes leading to increased substrate supply. Protochlorophyllide was regarded as the primary photoreceptor. Its photoreduction promoted chlorophyll synthesis which, in turn, increased photosynthetic capacity and thus substrate supply.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EILAM Y., KLEIN S. The effect of light intensity and sucrose feeding on the fine structure in chloroplasts and on the chlorophyll content of etiolated leaves. J Cell Biol. 1962 Aug;14:169–182. doi: 10.1083/jcb.14.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S., Kenney A. L. Chlorophyll and Carotenoid Destruction in the Absence of Light in Seedlings of Zea Mays L. Plant Physiol. 1955 Sep;30(5):413–418. doi: 10.1104/pp.30.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSKI V. M., FRENCH C. S., SMITH J. H. C. The action spectrum for the transformation of protochlorophyll to chlorophyll a in normal and albino corn seedlings. Arch Biochem Biophys. 1951 Mar;31(1):1–17. doi: 10.1016/0003-9861(51)90178-6. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Kasperbauer M. J. Photomorphogenic Responses of Dodder Seedlings. Plant Physiol. 1965 Jan;40(1):109–116. doi: 10.1104/pp.40.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter R. L., Wetherell D. F. Photomorphogenesis in Sinningia speciosa, cv. Queen Victoria I. Characterization of Phytochrome Control. Plant Physiol. 1968 Jun;43(6):953–960. doi: 10.1104/pp.43.6.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelman H. W., Hendricks S. B. Photocontrol of Anthocyanin Formation in Turnip and Red Cabbage Seedlings. Plant Physiol. 1957 Sep;32(5):393–398. doi: 10.1104/pp.32.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelman H. W., Hendricks S. B. Photocontrol of Anthocyanin Synthesis in Apple Skin. Plant Physiol. 1958 May;33(3):185–190. doi: 10.1104/pp.33.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A. I., Epstein H. T., Schiff J. A. Studies of Chloroplast Development in Euglena. VI. Light Intensity as a Controlling Factor in Development. Plant Physiol. 1964 Mar;39(2):226–231. doi: 10.1104/pp.39.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFF J. B., PRICE L. The effect of sugars on chlorophyll biosynthesis in higher plants. J Biol Chem. 1960 Jun;235:1603–1608. [PubMed] [Google Scholar]



