Abstract
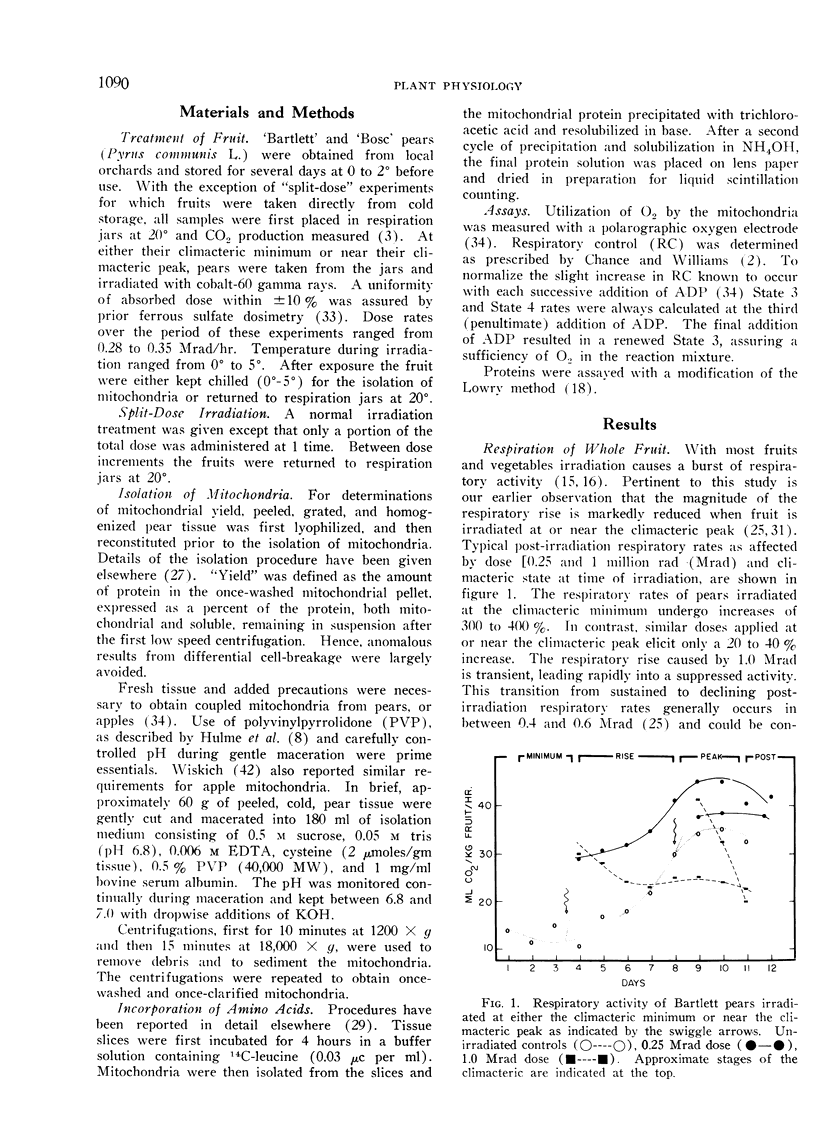
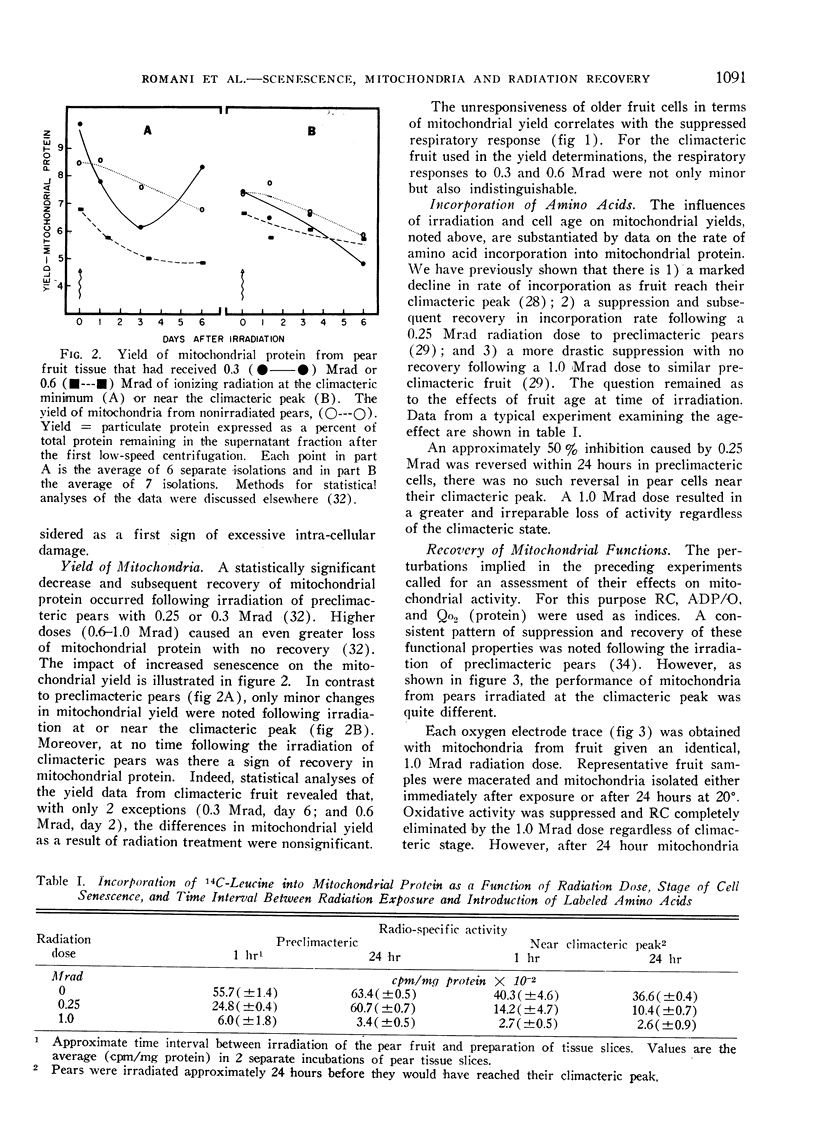
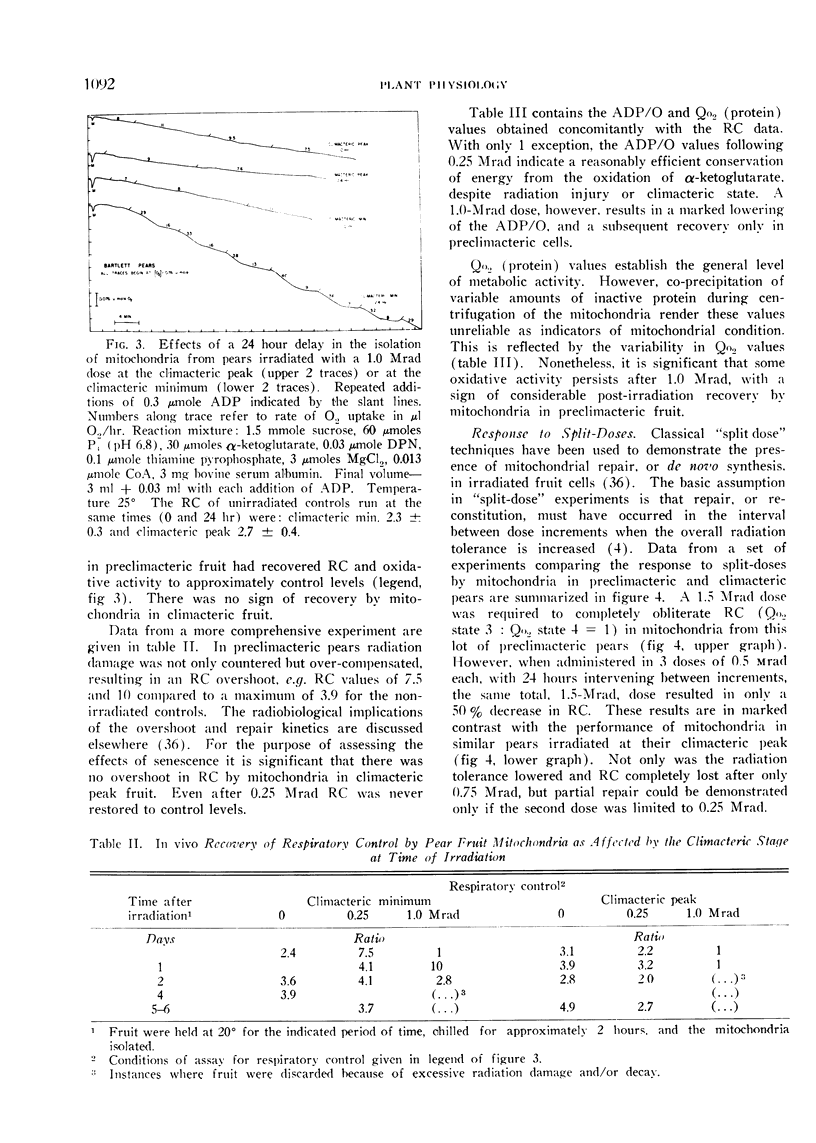
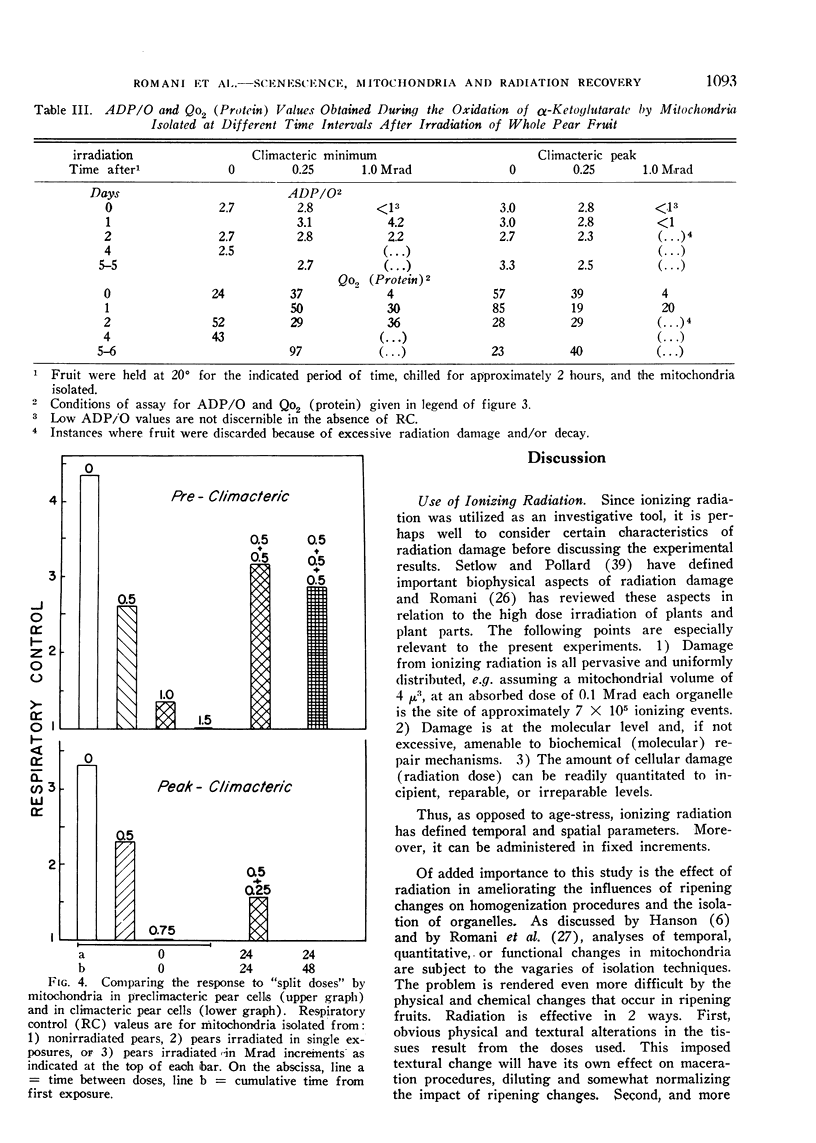
A compensatory response, viz. in vivo recovery from radiation damage to mitochondria, occurs in preclimacteric pear fruits (Pyrus communis L.) treated with ionizing radiation. The compensatory response is absent or markedly impaired in senescent fruits irradiated at or near the climacteric peak. Senescent cells failed to recover from harmful effects of radiation on: 1) mitochondrial yield, 2) in vivo incorporation of amino acids into mitochondrial protein, and 3) mitochondrial respiratory control and ADP/O. A diminished response to “split-dose” irradiation and a delayed rate of recovery confirmed the degeneracy and loss of compensatory power with cell age.
A loss of restorative activity, especially in mitochondria that supply the cell with essential energy, may underlie the more obvious signs of cumulative stress that accompany cellular senescence. Use of ionizing radiation as an investigative tool and the molecular implications of radiation damage, recovery, and cellular senescence are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biale J. B. Growth, Maturation, and Senescence in Fruits: Recent knowledge on growth regulation and on biological oxidations has been applied to studies with fruits. Science. 1964 Nov 13;146(3646):880–888. doi: 10.1126/science.146.3646.880. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- ELKIND M. M., SUTTON H. Radiation response of mammalian cells grown in culture. 1. Repair of X-ray damage in surviving Chinese hamster cells. Radiat Res. 1960 Oct;13:556–593. [PubMed] [Google Scholar]
- Ku L. L., Romani R. J. Ribosomes from pear fruit. Science. 1966 Oct 21;154(3747):408–410. doi: 10.1126/science.154.3747.408. [DOI] [PubMed] [Google Scholar]
- Lance C., Hobson G. E., Young R. E., Biale J. B. Metabolic processes in cytoplasmic particles of the avocado fruit. VII. Oxidative and phosphorylative activities throughout the climacteric cycle. Plant Physiol. 1965 Nov;40(6):1116–1123. doi: 10.1104/pp.40.6.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millerd A., Bonner J., Biale J. B. The Climacteric Rise in Fruit Respiration as Controlled by Phosphorylative Coupling. Plant Physiol. 1953 Jul;28(3):521–531. doi: 10.1104/pp.28.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield D. G. Cytokinetics. Bull Math Biophys. 1967 Sep;29(3):597–603. doi: 10.1007/BF02476596. [DOI] [PubMed] [Google Scholar]
- PEARSON J. A., ROBERTSON R. N. The physiology of growth in apple fruits. VI. The control of respiration rate and synthesis. Aust J Biol Sci. 1954 Feb;7(1):1–17. [PubMed] [Google Scholar]
- Richmond A., Biale J. B. Protein and nucleic acid metabolism in fruits. I. Studies of amino acid incorporation during the climacteric rise in respiration of the avocado. Plant Physiol. 1966 Oct;41(8):1247–1253. doi: 10.1104/pp.41.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond A., Biale J. B. Protein and nucleic acid metabolism in fruits. II. RNA synthesis during the respiratory rise of the avocado. Biochim Biophys Acta. 1967 May 30;138(3):625–627. doi: 10.1016/0005-2787(67)90565-5. [DOI] [PubMed] [Google Scholar]
- Romani R. J., Breidenbach R. W., van Kooy J. G. Isolation, Yield, and Fatty Acid Composition of Intracellular Particles from Ripening Fruits. Plant Physiol. 1965 May;40(3):561–566. doi: 10.1104/pp.40.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani R. J., Ku L. L., Fisher L. K., Van Kooy J. G. Mitochondria: increase in oxidative capacity and quantitative recovery after massive doses of ionizing radiation. Radiat Res. 1966 Jun;28(2):257–265. [PubMed] [Google Scholar]
- Sacher J. A. Permeability Characteristics and Amino Acid Incorporation during Senescence (Ripening) of Banana Tissue. Plant Physiol. 1966 Apr;41(4):701–708. doi: 10.1104/pp.41.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinex F. M. Biochemistry of aging. Perspect Biol Med. 1966 Winter;9(2):208–224. doi: 10.1353/pbm.1966.0006. [DOI] [PubMed] [Google Scholar]
- Young R. E., Biale J. B. Phosphorylation in avocado fruit slices in relation to the respiratory climacteric. Plant Physiol. 1967 Oct;42(10):1357–1362. doi: 10.1104/pp.42.10.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hahn H. P. A model of "regulatory" aging of the cell at the gene level. J Gerontol. 1966 Apr;21(2):291–294. doi: 10.1093/geronj/21.2.291. [DOI] [PubMed] [Google Scholar]


