Abstract
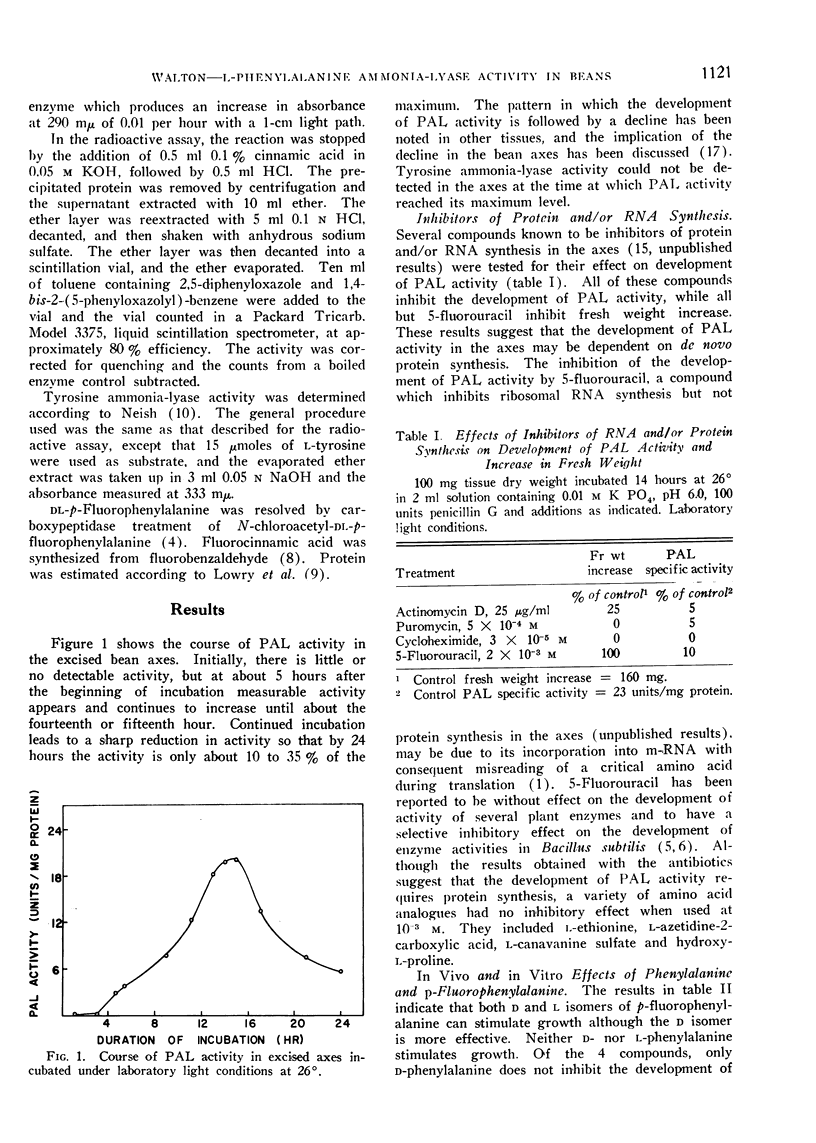
l-Phenylalanine ammonia-lyase (PAL) activity develops in excised bean axes after approximately 5 hours of incubation and reaches a maximum level after 14 hours of incubation. Light does not affect the development of activity, but puromycin, cycloheximide, actinomycin D, and 5-fluorouracil inhibit.
During this period of incubation both d- and l-p-fluorophenylalanine stimulate fresh weight increase and both inhibit the development of PAL activity. Neither l- nor d-phenylalanine stimulates fresh weight increase while the former inhibits development of PAL activity and the latter has no effect. Neither d isomer is deaminated while l-p-fluorophenylalanine is deaminated at about one-half the rate of l-phenylalanine. It is suggested that fluorophenylalanine does not stimulate the fresh weight increase by its effects on the phenolics pathway.
Trans-cinnamic acid was found to inhibit both the development of PAL activity and the in vitro deamination of l-phenylalanine. Various hydroxycinnamic acids, although inhibiting the development of PAL activity, had little or no effect on the in vitro deamination of l-phenylalanine.
No tyrosine ammonia-lyase activity was found in the axes and l-tyrosine had no effect on the in vitro deamination of l-phenylalanine.
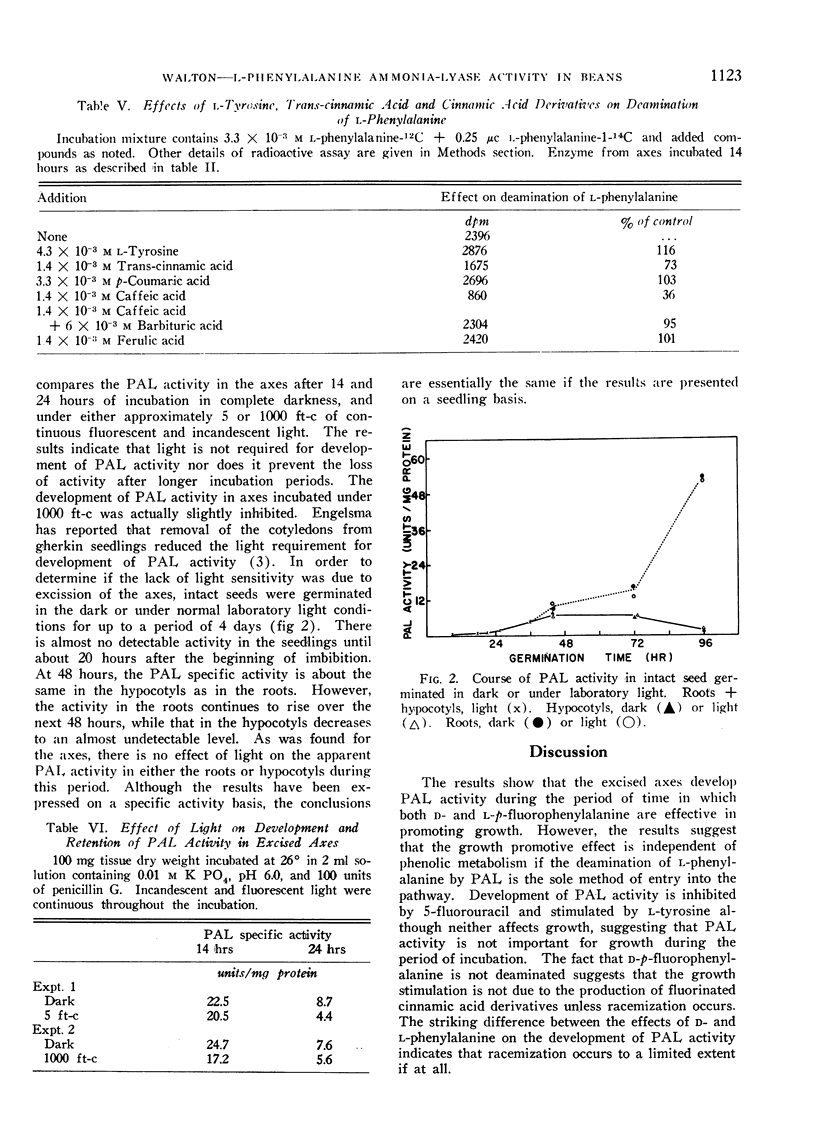
The pattern of PAL development in intact seedlings differs markedly from that which occurs in the excised axes, although light also has no effect on the course of activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAMPE S. P., BENZER S. Reversal of mutant phenotypes by 5-fluorouracil: an approach to nucleotide sequences in messenger-RNA. Proc Natl Acad Sci U S A. 1962 Apr 15;48:532–546. doi: 10.1073/pnas.48.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst F., Mohr H. Half-life of phytochrome-induced phenylalanine deaminase in mustard seedlings (Sinapis alba L.). Naturwissenschaften. 1966 Dec;53(24):707–707. doi: 10.1007/BF00602735. [DOI] [PubMed] [Google Scholar]
- GREENSTEIN J. P. The resolution of racemic alpha-amino acids. Adv Protein Chem. 1954;9:121–202. doi: 10.1016/s0065-3233(08)60206-5. [DOI] [PubMed] [Google Scholar]
- Kadowaki K., Hosoda J., Maruo B. Effects of actinomycin D and 5-fluorouracil on the formation of enzymes in Bacillus subtilis. Biochim Biophys Acta. 1965 Jun 8;103(2):311–318. doi: 10.1016/0005-2787(65)90170-x. [DOI] [PubMed] [Google Scholar]
- Key J. L., Ingle J. REQUIREMENT FOR THE SYNTHESIS OF DNA-LIKE RNA FOR GROWTH OF EXCISED PLANT TISSUE. Proc Natl Acad Sci U S A. 1964 Dec;52(6):1382–1388. doi: 10.1073/pnas.52.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pierpoint W. S. The enzymic oxidation of chlorogenic acid and some reactions of the quinone produced. Biochem J. 1966 Feb;98(2):567–580. doi: 10.1042/bj0980567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader L. E., Hageman R. H. Regulation of Nitrate Reductase Activity in Corn (Zea mays L.) Seedlings by Endogenous Metabolites. Plant Physiol. 1967 Dec;42(12):1750–1756. doi: 10.1104/pp.42.12.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton D. C. Germination of Phaseolus vulgaris II. Stimulation of Axis Growth by dl-Fluorophenylalanines. Plant Physiol. 1966 Nov;41(9):1549–1550. doi: 10.1104/pp.41.9.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]


