Abstract
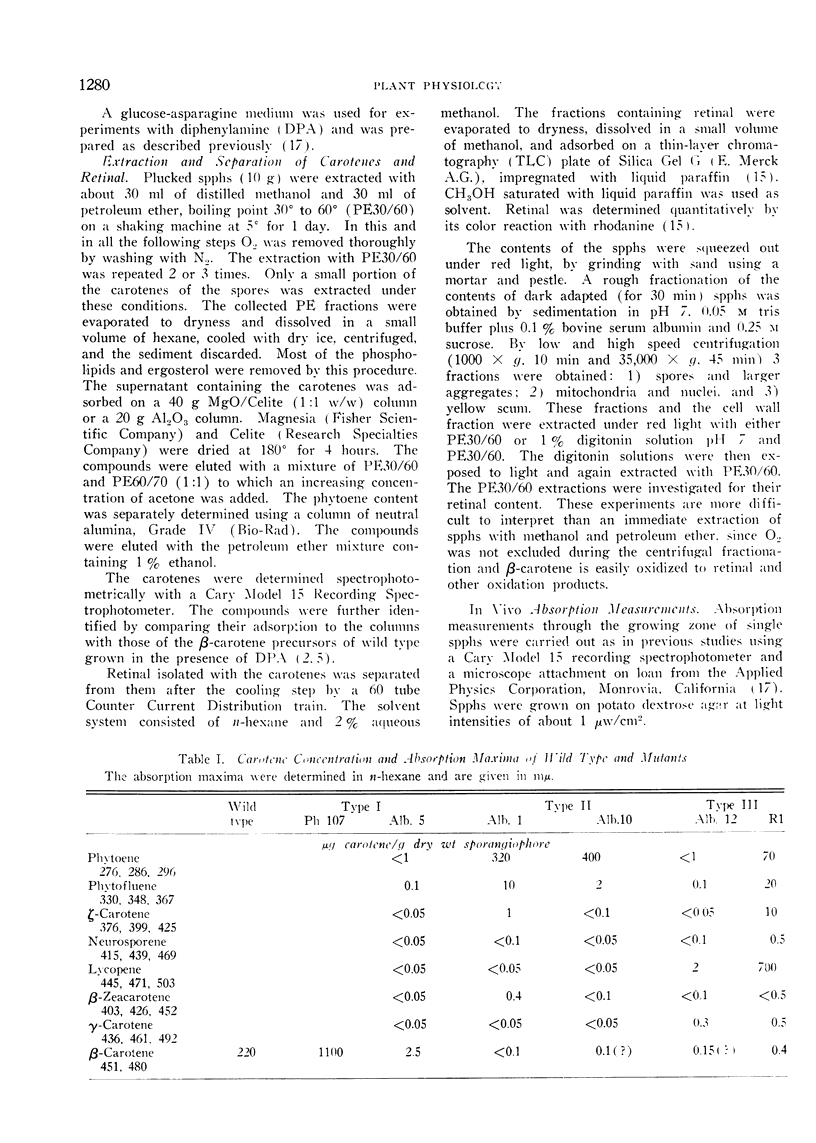
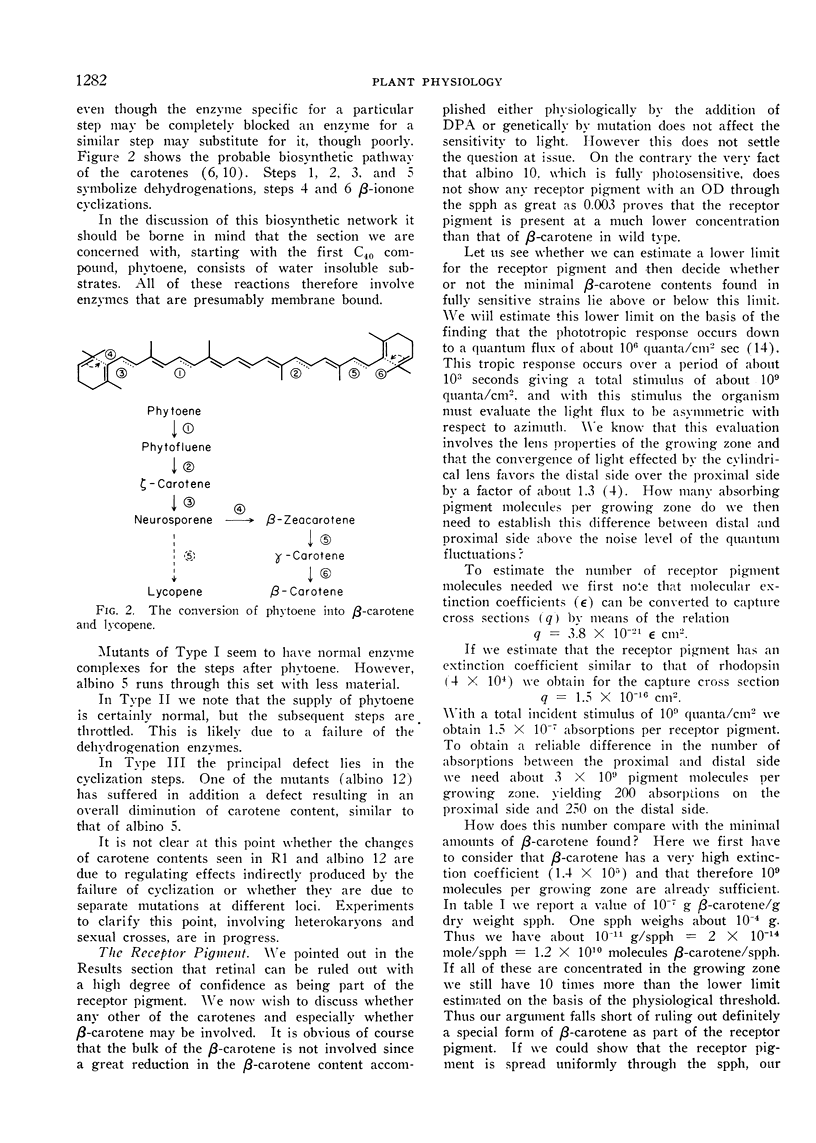
Three different types of β-carotene mutants of Phycomyces have been studied. In 2 mutants (Type I) β-carotene is still the principal carotene but scaled down or up relative to wild type. The carotene mixture of 2 mutants (Type II) consists mainly of phytoene and phytofluene. In Type III (2 mutants) β-carotene is replaced by lycopene.
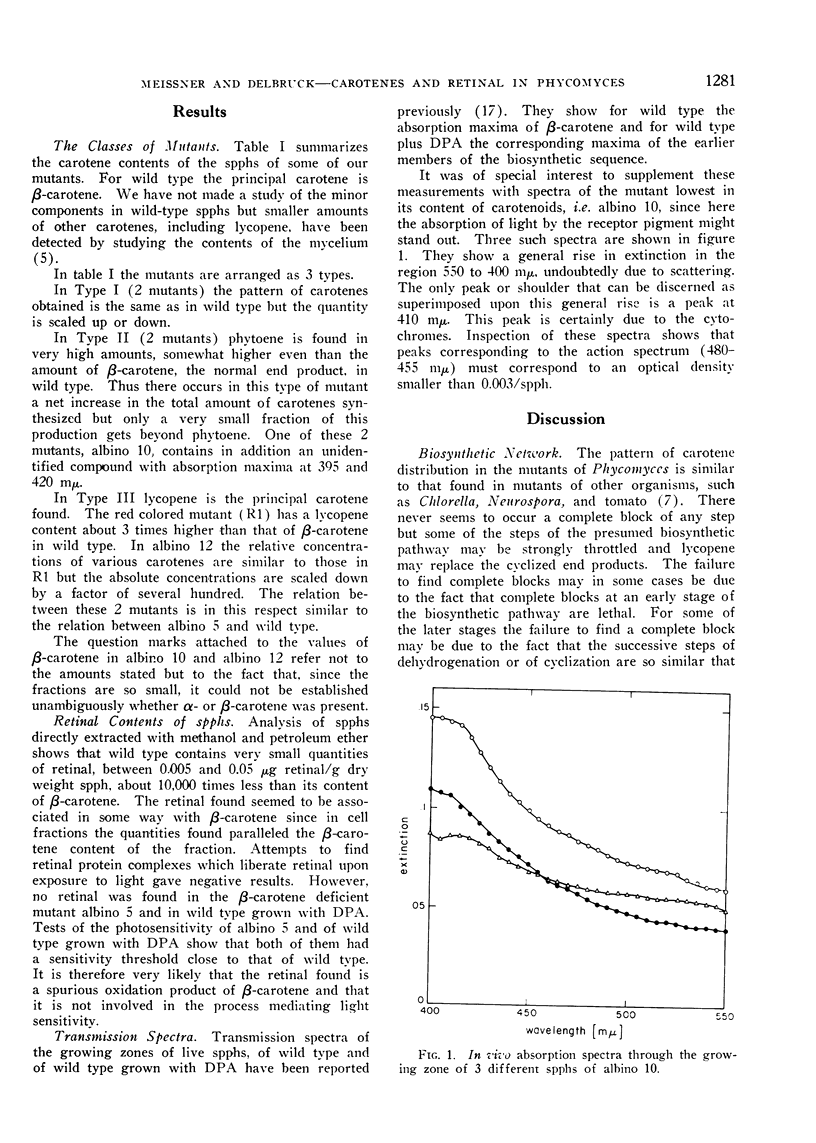
The examination of the mutants reveals that the receptor pigment is very likely neither β-carotene nor retinal. Transmission spectra through the growing zone of live sporangiophores of 1 of these mutants which contains less than one-thousandth of the β-carotene content of wild type show that the receptor pigment extinction is less than 0.003 at its maximum.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Delbrück M., Shropshire W. Action and Transmission Spectra of Phycomyces. Plant Physiol. 1960 Mar;35(2):194–204. doi: 10.1104/pp.35.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN T. W. Studies in carotenogenesis. III. Identification of the minor polyene components of the fungus Phycomyces blakesleeanus and a study of their synthesis under various cultural conditions. Biochem J. 1952 Feb;50(4):550–558. doi: 10.1042/bj0500550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowallik W. Action spectrum for an enhancement of endogenous respiration by light in chlorella. Plant Physiol. 1967 May;42(5):672–676. doi: 10.1104/pp.42.5.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett J. M., French C. S. The action spectrum for blue-light-stimulated oxygen uptake in Chlorella. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1587–1593. doi: 10.1073/pnas.57.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RILLING H. C. ON THE MECHANISM OF PHOTOINDUCTION OF CAROTENOID SYNTHESIS. Biochim Biophys Acta. 1964 May 25;79:464–475. doi: 10.1016/0926-6577(64)90212-8. [DOI] [PubMed] [Google Scholar]
- SHROPSHIRE W., Jr Photoresponses of the fungus, Phycomyces. Physiol Rev. 1963 Jan;43:38–67. doi: 10.1152/physrev.1963.43.1.38. [DOI] [PubMed] [Google Scholar]
- VARJU D., EDGAR L., DELBRUCK M. Interplay between the reactions to light and to gravity in Phycomyces. J Gen Physiol. 1961 Sep;45:47–58. doi: 10.1085/jgp.45.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZALOKAR M. Biosynthesis of carotenoids in Neurospora; action spectrum of photoactivation. Arch Biochem Biophys. 1955 Jun;56(2):318–325. doi: 10.1016/0003-9861(55)90252-6. [DOI] [PubMed] [Google Scholar]
- Zankel K. L., Burke P. V., Delbrück M. Absorption and screening in Phycomyces. J Gen Physiol. 1967 Aug;50(7):1893–1906. doi: 10.1085/jgp.50.7.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]


