Abstract
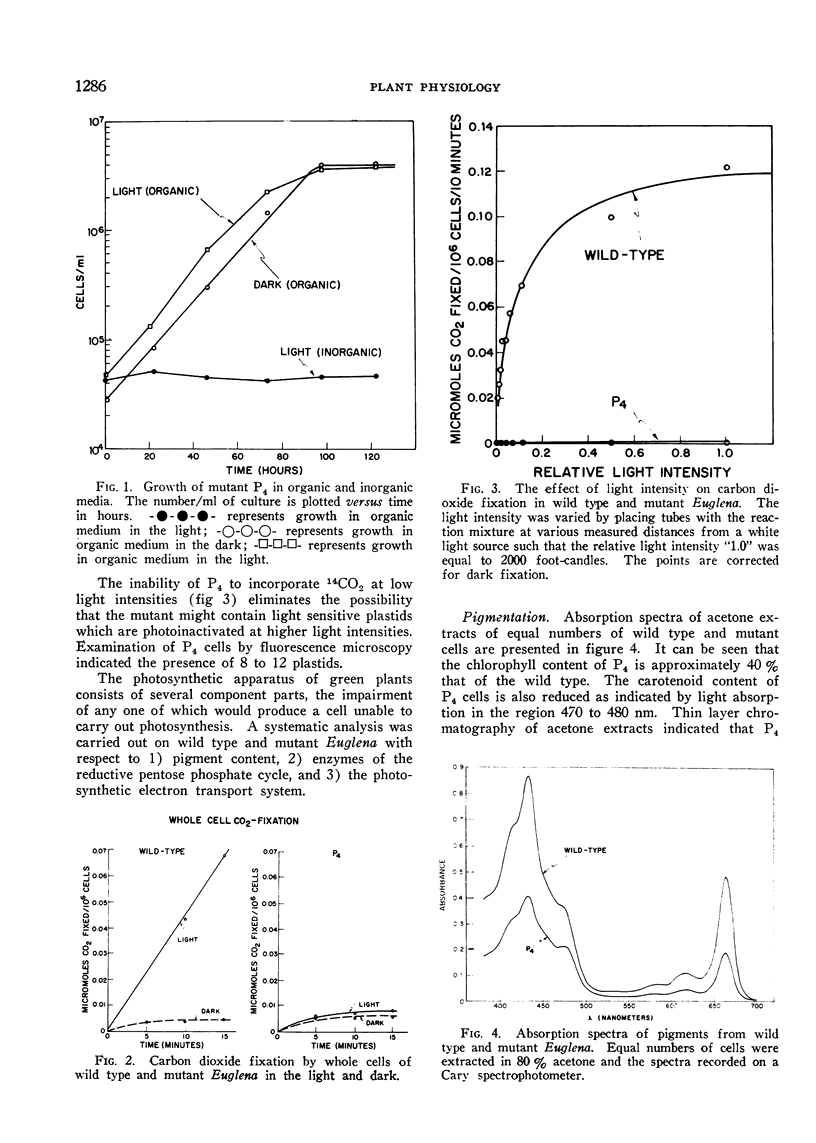
Four mutant strains of Euglena gracilis have been isolated after treatment of wild type cells with ultraviolet light or the chemical mutagen nitrosoguanidine. None of the mutants is capable of autotrophic growth or photosynthetic carbon dioxide fixation.
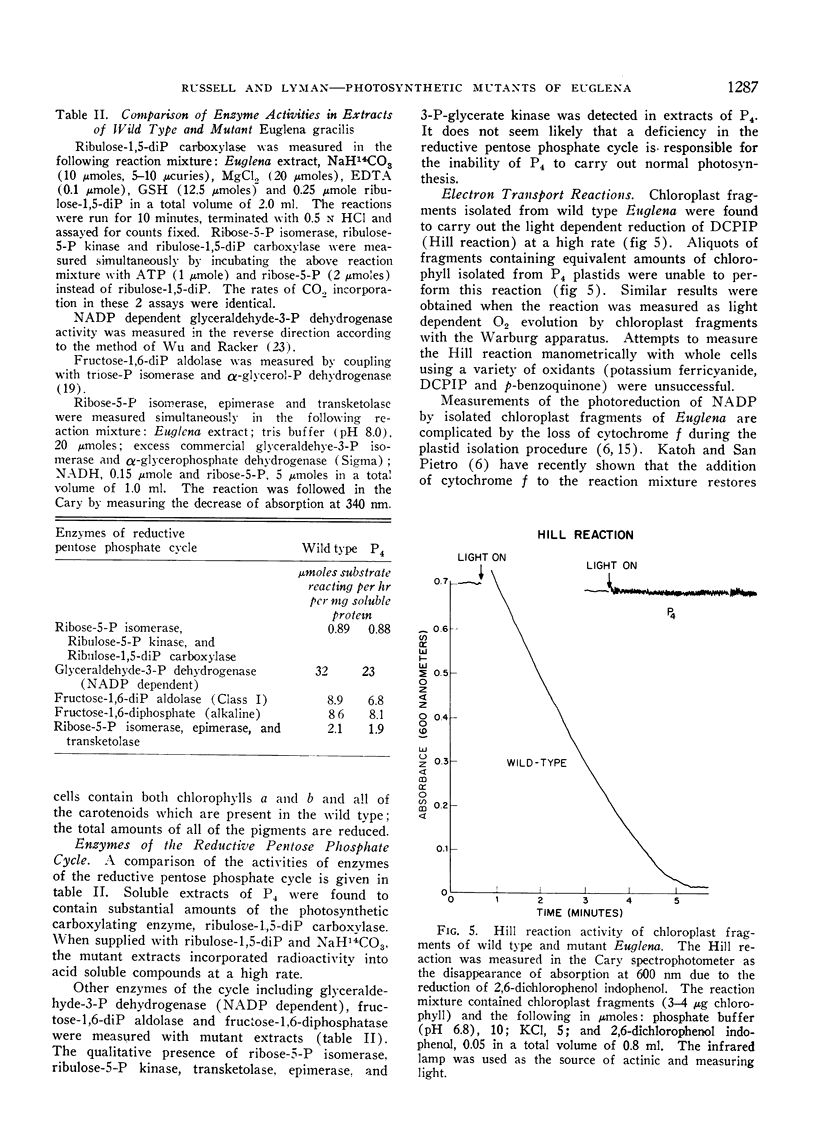
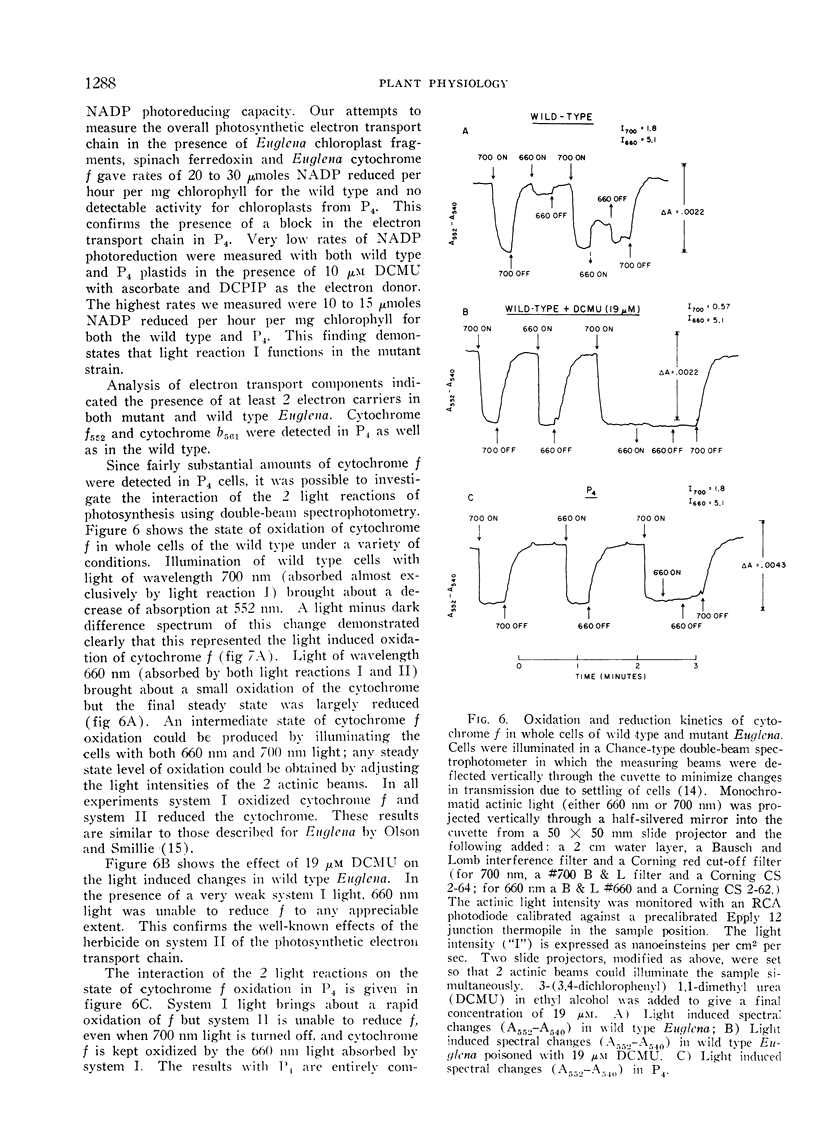
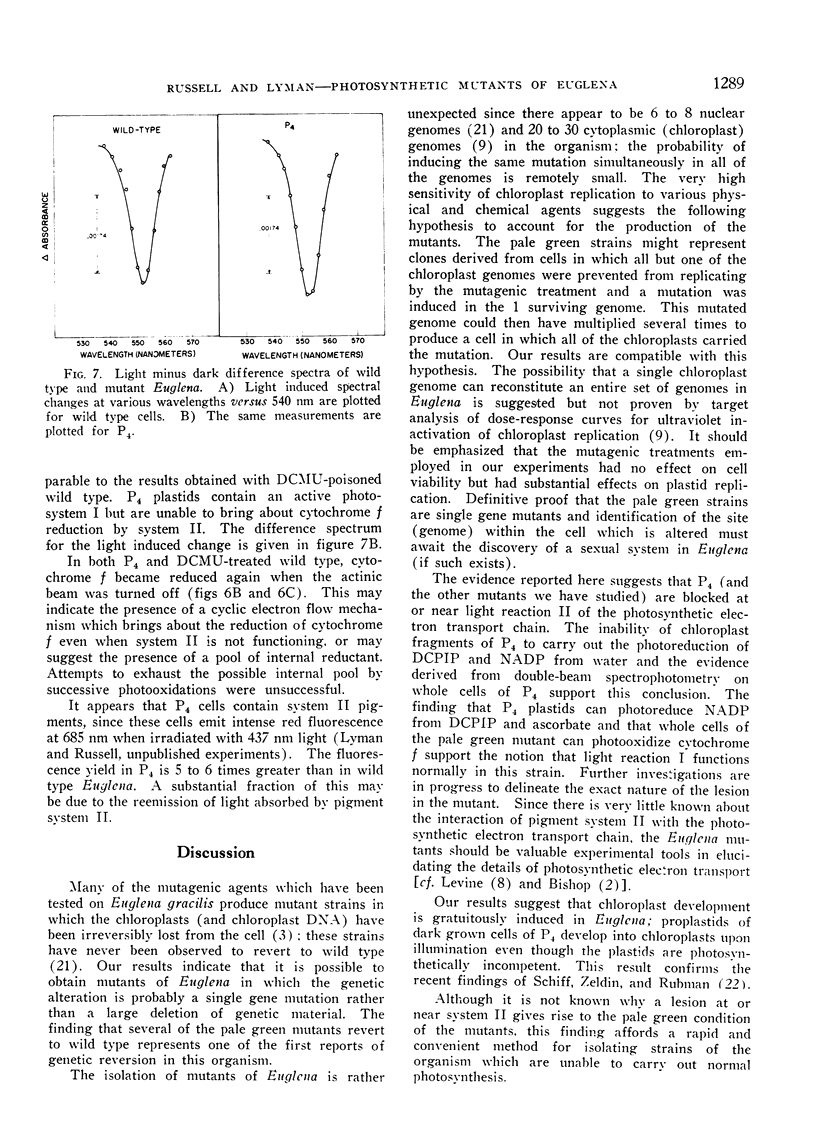
The mutant strains contain normal amounts of the enzymes of the reductive pentose phosphate cycle and are qualitatively similar to the wild type in pigment composition, but are unable to carry out the Hill reaction (light induced reduction of 2,6-dichlorophenol indophenol). Isolated mutant plastids cannot photoreduce NADP with water as the electron donor but can carry out this reaction when the electron donating system is ascorbate and 2,6-dichlorophenol indophenol. Whole cells of the mutants show the light induced oxidation of cytochrome f by light reaction I but are unable to bring about cytochrome f reduction by light reaction II. The mutants appear to be blocked at or near light reaction II in the photosynthetic electron transport chain.
The mutants may represent alterations of the chloroplast genome since the mutation isolation was carried out under conditions where chloroplast viability was severely impaired, but cell viability was unaffected.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN M., SCHIFF J. A., EPSTEIN H. T. STUDIES OF CHLOROPLAST DEVELOPMENT IN EUGLENA. XII. TWO TYPES OF SATELLITE DNA. J Mol Biol. 1965 Apr;11:769–774. doi: 10.1016/s0022-2836(65)80034-1. [DOI] [PubMed] [Google Scholar]
- Katoh S., San Pietro A. The role of C-type cytochrome in the Hill reaction with Euglena chloroplasts. Arch Biochem Biophys. 1967 Feb;118(2):488–496. doi: 10.1016/0003-9861(67)90377-3. [DOI] [PubMed] [Google Scholar]
- MCCALLA D. R. EFFECT OF NITROFURANS ON THE CHLOROPLAST SYSTEM OF EUGLENA GRACILIS. J Protozool. 1965 Feb;12:34–41. doi: 10.1111/j.1550-7408.1965.tb01808.x. [DOI] [PubMed] [Google Scholar]
- MORITA S., OLSON J. M., CONTI S. E. LIGHT-INDUCED REACTIONS OF CYTOCHROMES AND CAROTENOIDS IN RHODOMICROBIUM VANNIELII. Arch Biochem Biophys. 1964 Feb;104:346–353. doi: 10.1016/s0003-9861(64)80025-4. [DOI] [PubMed] [Google Scholar]
- McCalla D. R. Mutation of the Euglena chloroplast system: the mechanism of bleaching by nitrosoguanidine and related compounds. J Protozool. 1967 Aug;14(3):480–482. doi: 10.1111/j.1550-7408.1967.tb02031.x. [DOI] [PubMed] [Google Scholar]
- PERINI F., KAMEN M. D., SCHIFF J. A. IRON-CONTAINING PROTEINS IN EUGLENA. I. DETECTION AND CHARACTERIZATION. Biochim Biophys Acta. 1964 Jul 29;88:74–90. doi: 10.1016/0926-6577(64)90155-x. [DOI] [PubMed] [Google Scholar]
- Russell G. K., Gibbs M. Partial purification and characterization of two fructose diphosphate aldolases from Chlamydomonas mundana. Biochim Biophys Acta. 1967 Jan 11;132(1):145–154. doi: 10.1016/0005-2744(67)90200-8. [DOI] [PubMed] [Google Scholar]


