Abstract
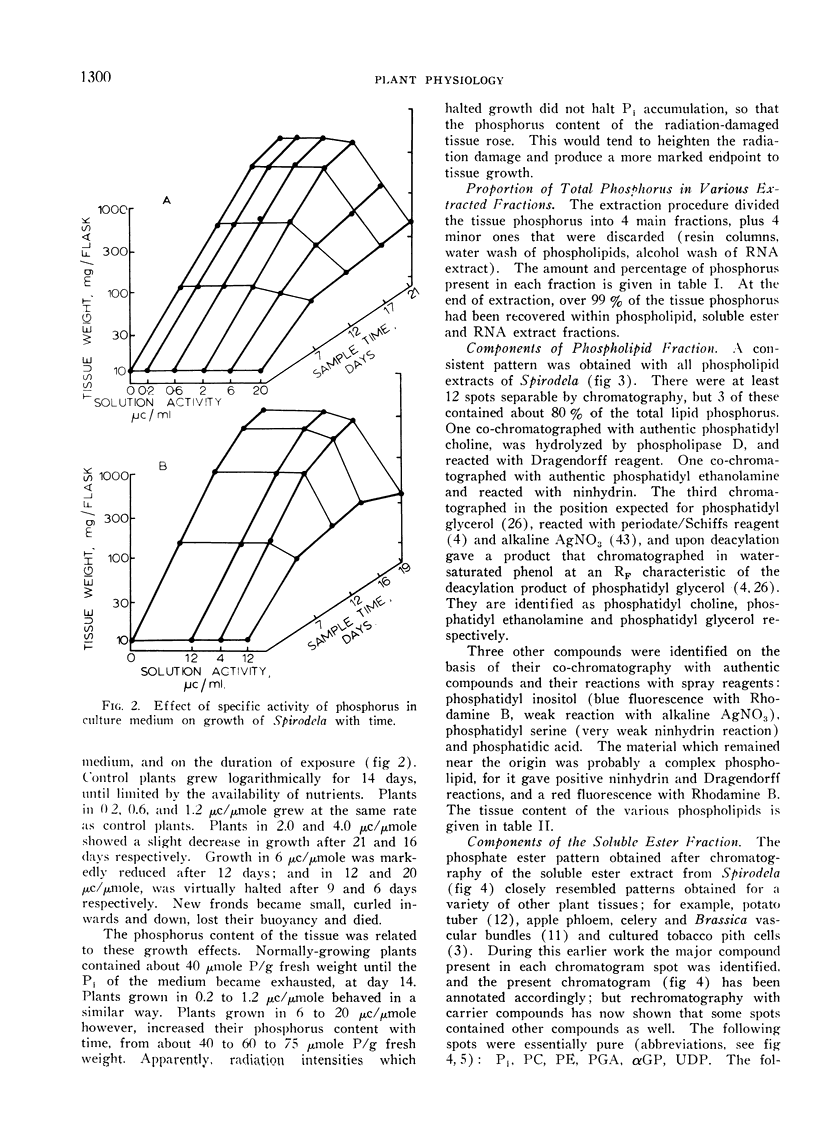
The duckweed Spirodela oligorrhiza was grown in sterile nutrient solutions that contained 1 mm phosphate-32P at various specific activities. In solutions with activities higher than 2 μc per μmole per ml, plant growth was inhibited after a time, and the physical appearance of the plants was affected. The critical level of radiation, at which growth was first affected, corresponded to 5 kilorads.
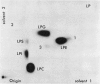
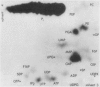
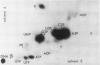
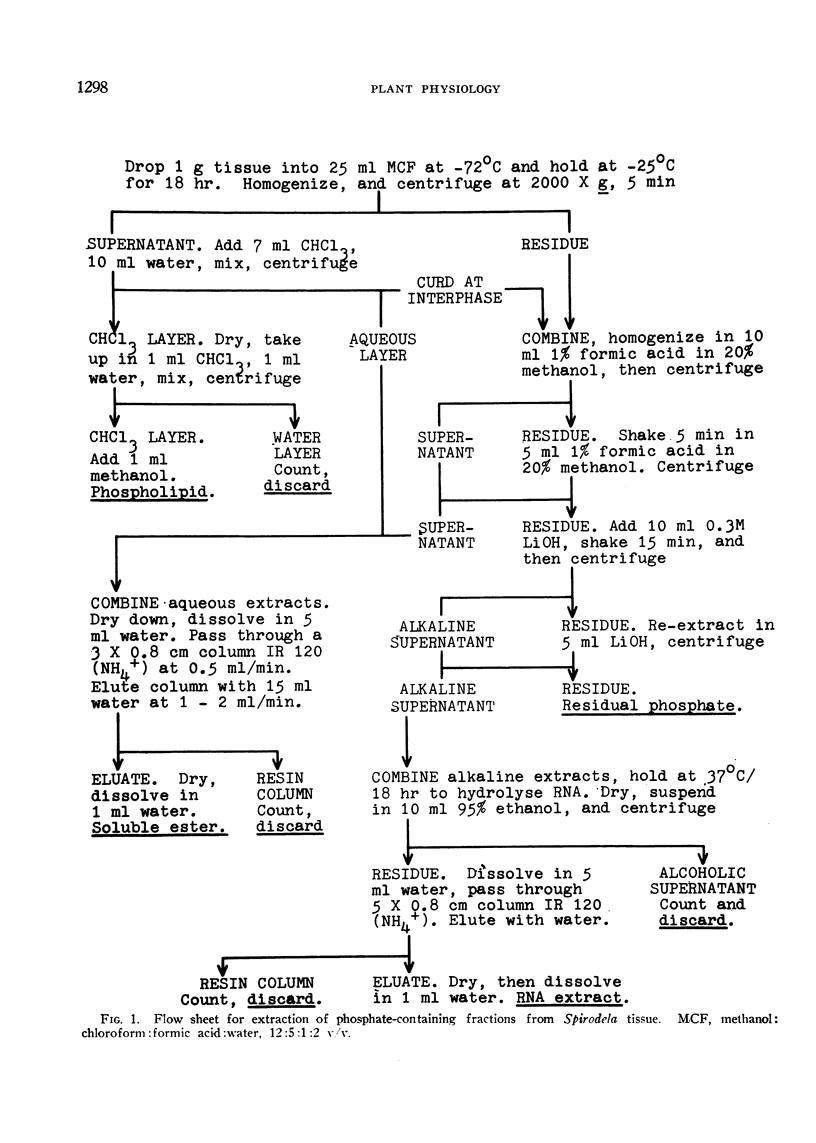
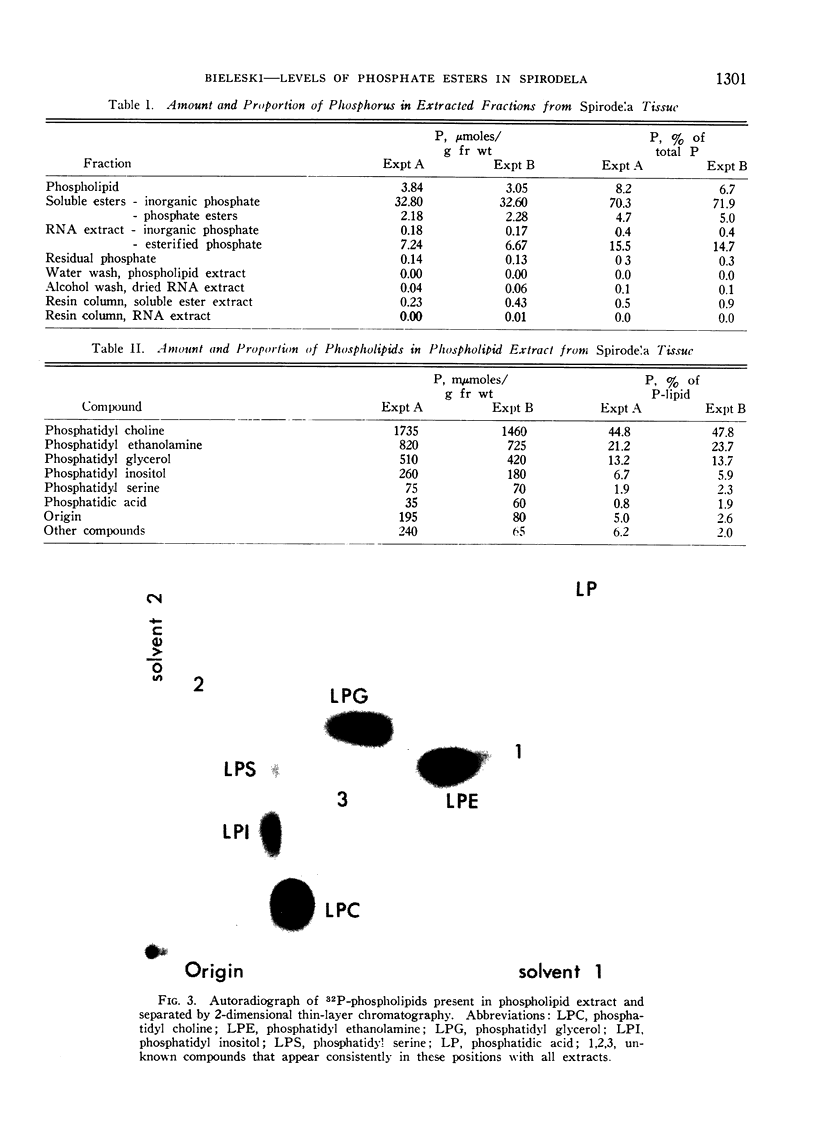
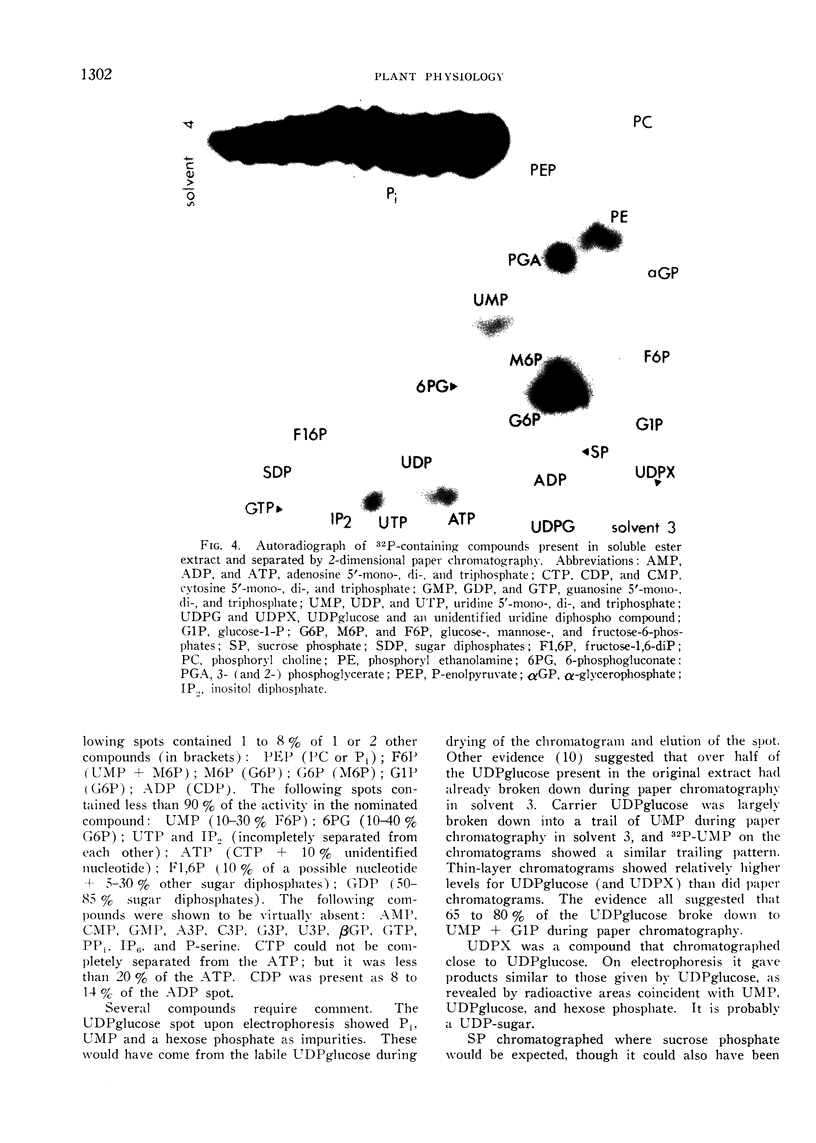
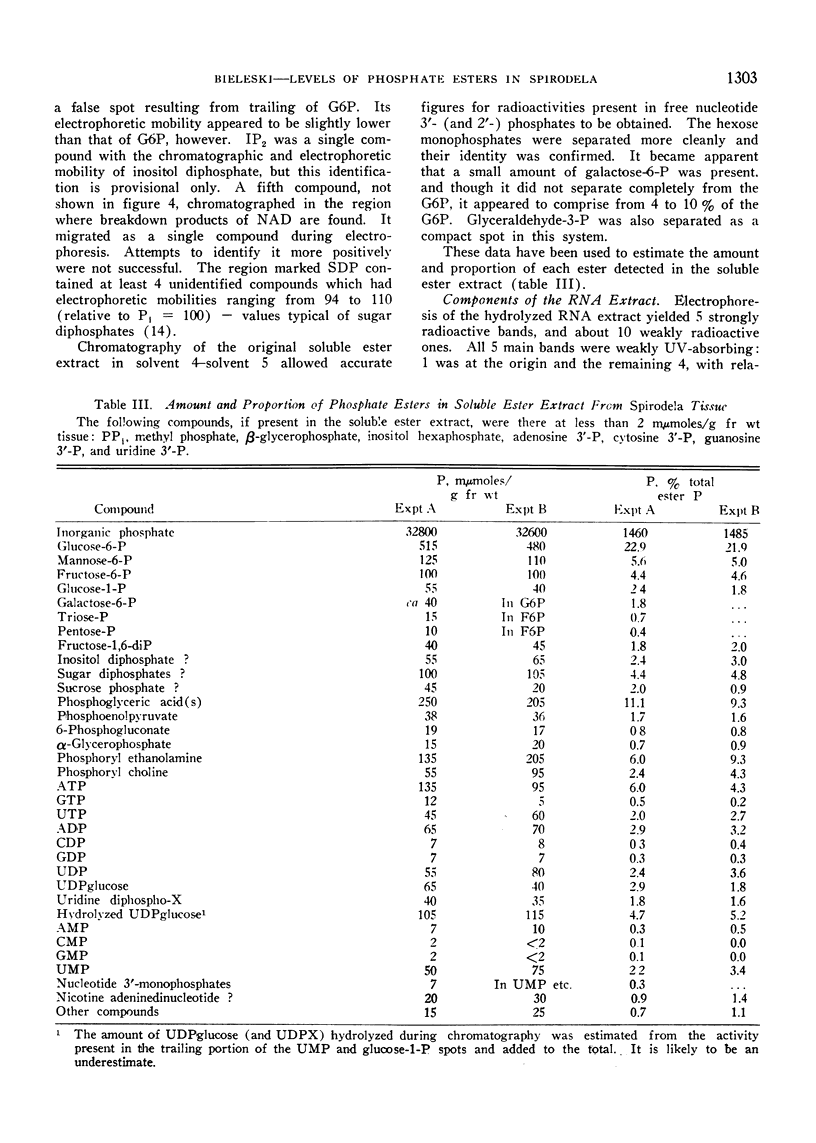
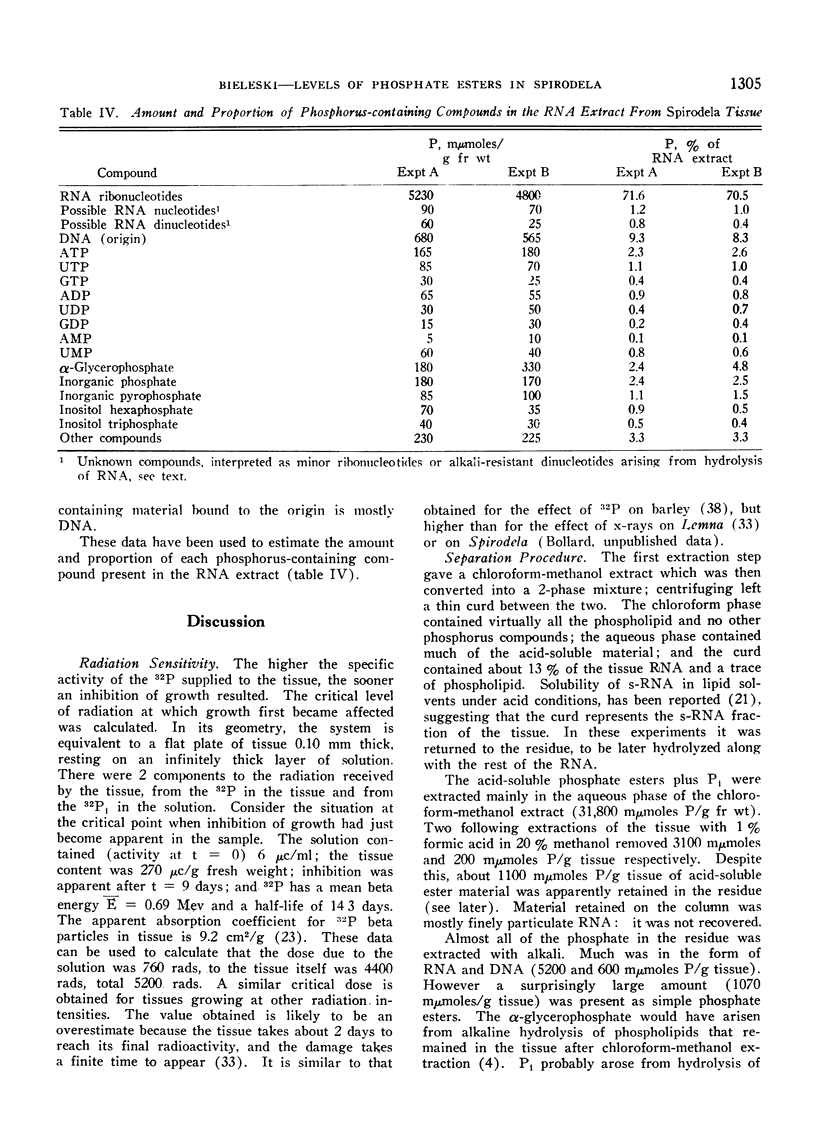
Plants were grown for 9 days (5 generations) in a culture solution containing phosphate at 0.5 μc per μmole per ml (radiation load approx 0.5 kilorads) so that all phosphorus-containing materials in the tissue became uniformly labeled. The various radioactive compounds were extracted, chromatographed, identified, and their radioactivity was measured. From this radioactivity plus the specific activity of the supplied phosphate, the amount of each compound was calculated. The data constitute a complete balance-sheet for phosphorus in a plant tissue. The identity of 98% of the phosphorus in the tissue was determined. Inorganic phosphate (32,700 mμmoles/g fr wt) was the predominant phosphorus-containing compound; RNA (5100 mμmoles P/g fr wt) was the main organic phosphate; phosphatidyl choline (1600 mμmoles/g fr wt) was the main phospholipid, and glucose-6-phosphate (500 mμmoles/g fr wt) the main acid-soluble phosphate ester. Amounts of other phosphorus compounds are given.
Full text
PDF

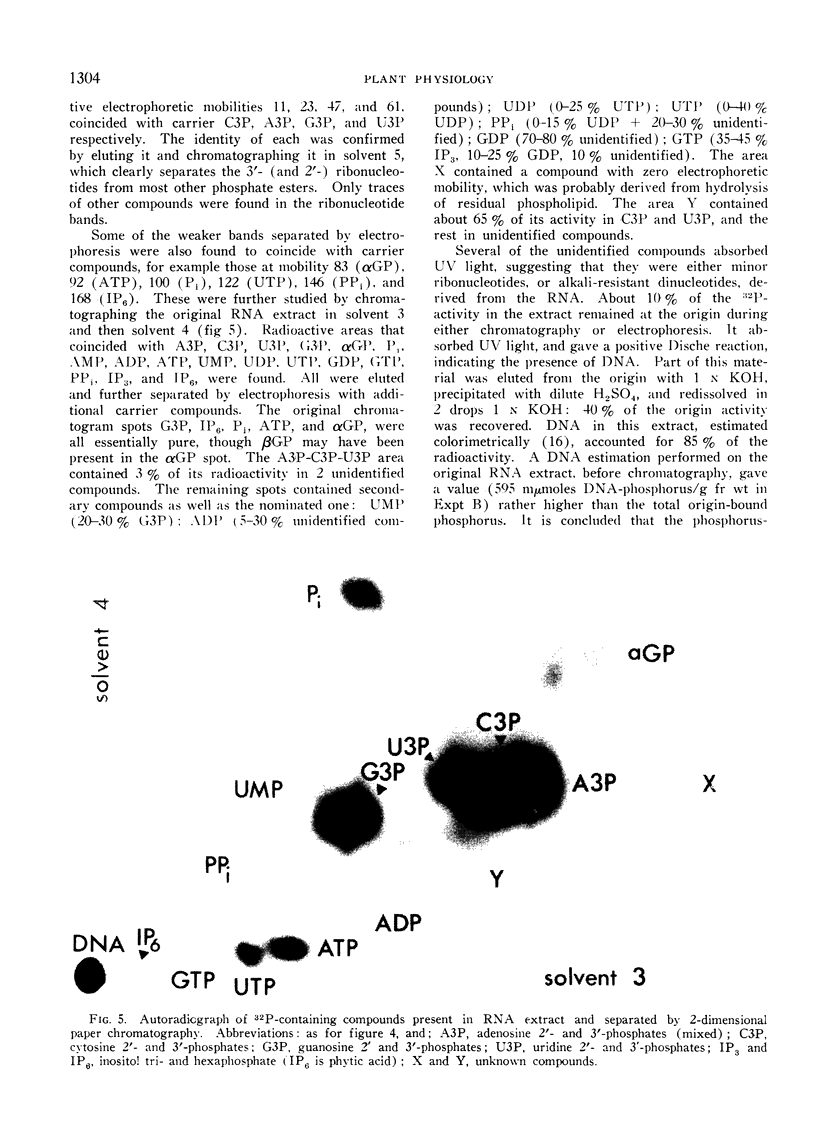



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENSON A. A., STRICKLAND E. H. Plant phospholipids. 3. Identification of diphosphatidyl glycerol. Biochim Biophys Acta. 1960 Jul 1;41:328–333. doi: 10.1016/0006-3002(60)90016-0. [DOI] [PubMed] [Google Scholar]
- BIELESKI R. L. Phosphatase reactions during tissue extractions. Biochim Biophys Acta. 1963 Jul 2;74:135–137. doi: 10.1016/0006-3002(63)91340-4. [DOI] [PubMed] [Google Scholar]
- BIELESKI R. L. SEPARATION OF PHOSPHATE ESTERS BY THIN-LAYER CHROMATOGRAPHY AND ELECTROPHORESIS. Anal Biochem. 1965 Aug;12:230–234. doi: 10.1016/0003-2697(65)90086-2. [DOI] [PubMed] [Google Scholar]
- BIELESKI R. L. THE PROBLEM OF HALTING ENZYME ACTION WHEN EXTRACTING PLANT TISSUES. Anal Biochem. 1964 Dec;9:431–442. doi: 10.1016/0003-2697(64)90204-0. [DOI] [PubMed] [Google Scholar]
- BROWN E. G. CHANGES IN THE FREE NUCLEOTIDE PATTERN OF PEA SEEDS IN RELATION TO GERMINATION. Biochem J. 1965 May;95:509–514. doi: 10.1042/bj0950509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN E. G. PURINE AND PYRIMIDINE DERIVATIVES IN MATURE PEA SEEDS. Biochem J. 1963 Sep;88:498–504. doi: 10.1042/bj0880498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN E. G. The acid-soluble nucleotides of mature pea seeds. Biochem J. 1962 Dec;85:633–640. doi: 10.1042/bj0850633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski R. L. Accumulation of phosphate, sulfate and sucrose by excised Phloem tissues. Plant Physiol. 1966 Mar;41(3):447–454. doi: 10.1104/pp.41.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski R. L., Laties G. G. Turnover Rates of Phosphate Esters in Fresh and Aged Slices of Potato Tuber Tissue. Plant Physiol. 1963 Sep;38(5):586–594. doi: 10.1104/pp.38.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski R. L., Turner N. A. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal Biochem. 1966 Nov;17(2):278–293. doi: 10.1016/0003-2697(66)90206-5. [DOI] [PubMed] [Google Scholar]
- Cherry J. H., Hageman R. H. Separation and Identification of Soluble Nucleotides from Etiolated Corn Seedlings as Function of Growth. Plant Physiol. 1960 May;35(3):343–352. doi: 10.1104/pp.35.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON F. M., LONG C. The structure of the naturally occurring phosphoglycerides. 4. Action of cabbage-leaf phospholipase D on ovolecithin and related substances. Biochem J. 1958 Jul;69(3):458–466. doi: 10.1042/bj0690458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson R. G., Rowan K. S. Phosphate Metabolism and Induced Respiration in Washed Carrot Slices. Plant Physiol. 1965 Nov;40(6):1247–1250. doi: 10.1104/pp.40.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON H. T., WERUM L. N., THORNBURG W. W. USE OF 2-(O-HYDROXYPHENYL)-BENZOXAZOLE IN DETECTION OF PHOSPHATE ESTERS AND OTHER FERRIC-COMPLEXING COMPOUNDS ON PAPER CHROMATOGRAMS. J Chromatogr. 1964 Jan;13:272–273. doi: 10.1016/s0021-9673(01)95115-5. [DOI] [PubMed] [Google Scholar]
- HALLINAN T., FLECK A., MUNRO H. N. Loss of ribonucleic acid into lipid solvents after acid precipitation. Biochim Biophys Acta. 1963 Jan 29;68:131–133. doi: 10.1016/0006-3002(63)90121-5. [DOI] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Compartmentation and reduction of pyridine nucleotides in relation to photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):390–408. doi: 10.1016/0926-6585(65)90166-4. [DOI] [PubMed] [Google Scholar]
- KATES M. Chromatographic and radioisotopic investigations of the lipid components of runner bean leaves. Biochim Biophys Acta. 1960 Jul 1;41:315–328. doi: 10.1016/0006-3002(60)90015-9. [DOI] [PubMed] [Google Scholar]
- LEPAGE M. THE SEPARATION AND IDENTIFICATION OF PLANT PHOSPHOLIPIDS AND GLYCOLIPIDS BY TWO-DIMENSIONAL THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1964 Jan;13:99–103. doi: 10.1016/s0021-9673(01)95078-2. [DOI] [PubMed] [Google Scholar]
- LYTTLETON J. W., PETERSEN G. B. THE ISOLATION OF DEOXYRIBONUCLEIC ACID FROM PLANT TISSUES. Biochim Biophys Acta. 1964 Mar 23;80:391–398. doi: 10.1016/0926-6550(64)90141-0. [DOI] [PubMed] [Google Scholar]
- MURATA T., MINAMIKAWA T., AKAZAWA T., SUGIYAMA T. ISOLATION OF ADENOSINE DIPHOSPHATE GLUCOSE FROM RIPENING RICE GRAINS AND ITS ENZYMIC SYNTHESIS. Arch Biochem Biophys. 1964 Jul 20;106:371–378. doi: 10.1016/0003-9861(64)90202-4. [DOI] [PubMed] [Google Scholar]
- RALPH R. K., BELLAMY A. R. ISOLATION AND PURIFICATION OF UNDEGRADED RIBONUCLEIC ACIDS. Biochim Biophys Acta. 1964 May 18;87:9–16. doi: 10.1016/0926-6550(64)90041-6. [DOI] [PubMed] [Google Scholar]
- ROWAN K. S. Uridine diphosphoglucose in banana fruit. Biochim Biophys Acta. 1959 Jul;34:270–271. doi: 10.1016/0006-3002(59)90266-5. [DOI] [PubMed] [Google Scholar]
- Rowan K. S. Phosphorus metabolism in plants. Int Rev Cytol. 1966;19:301–390. doi: 10.1016/s0074-7696(08)60571-9. [DOI] [PubMed] [Google Scholar]
- SUGINO Y., MIYOSHI Y. THE SPECIFIC PRECIPITATION OF ORTHOPHOSPHATE AND SOME BIOCHEMICAL APPLICATIONS. J Biol Chem. 1964 Jul;239:2360–2364. [PubMed] [Google Scholar]
- SUNDERLAND D. W., MERRETT M. J. NICOTINAMIDE-ADENINE DINUCLEOTIDES, ADENOSINE DIPHOSPHATE AND ADENOSINE TRIPHOSPHATE CONTENT OF TISSUES INFECTED BY TOBACCO MOSAIC VIRUS. Nature. 1963 Sep 14;199:1116–1117. doi: 10.1038/1991116a0. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- TURNER D. H., TURNER J. F. The use of perchloric acid in the extraction of phosphoric compounds from plant tissues. Biochim Biophys Acta. 1961 Aug 19;51:591–593. doi: 10.1016/0006-3002(61)90621-7. [DOI] [PubMed] [Google Scholar]
- WINTERMANS J. F. Concentrations of phosphatides and glycolipids in leaves and chloroplasts. Biochim Biophys Acta. 1960 Oct 21;44:49–54. doi: 10.1016/0006-3002(60)91521-3. [DOI] [PubMed] [Google Scholar]
- Wilson A. M., Huffaker R. C. Effects of Moisture Stress on Acid-Soluble Phosphorus Compounds in Trifolium subterraneum. Plant Physiol. 1964 Jul;39(4):555–560. doi: 10.1104/pp.39.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]