Abstract
Purpose:
In the CASPIAN trial, first-line durvalumab plus platinum-etoposide (EP) significantly improved overall survival (OS) versus EP alone in extensive-stage small cell lung cancer (ES-SCLC). We report exploratory analyses of CASPIAN outcomes by programmed cell death ligand-1 (PD-L1) expression and tissue tumor mutational burden (tTMB).
Experimental Design:
Patients were randomized (1:1:1) to durvalumab (1,500 mg) plus EP, durvalumab plus tremelimumab (75 mg) plus EP, or EP alone. Treatment effects in PD-L1 and tTMB subgroups were estimated using an unstratified Cox proportional hazards model.
Results:
The PD-L1 and tTMB biomarker-evaluable populations (BEP) comprised 54.4% (438/805) and 35.2% (283/805) of the intention-to-treat population, respectively. PD-L1 prevalence was low: 5.7%, 25.8%, and 28.3% had PD-L1 expression on ≥1% tumor cells (TC), ≥1% immune cells (IC), and ≥1% TCs or ICs, respectively. OS benefit with durvalumab plus EP versus EP was similar across PD-L1 subgroups, with HRs all falling within the 95% confidence interval (CI) for the PD-L1 BEP (0.47‒0.79). OS benefit with durvalumab plus tremelimumab plus EP versus EP was greater in PD-L1 ≥1% versus <1% subgroups, although CIs overlapped. There was no evidence of an interaction between tTMB and treatment effect on OS (durvalumab plus EP vs. EP, P = 0.916; durvalumab plus tremelimumab plus EP vs. EP, P = 0.672).
Conclusions:
OS benefit with first-line durvalumab plus EP in patients with ES-SCLC was observed regardless of PD-L1 or tTMB status. PD-L1 expression may prove to be a useful biomarker for combined treatment with PD-(L)1 and CTLA-4 inhibition, although this requires confirmation with an independent dataset.
Translational Relevance.
Addition of durvalumab to first-line platinum-etoposide (EP) prolongs overall survival (OS) in patients with extensive-stage small cell lung cancer (ES-SCLC). Some reports suggest programmed cell death ligand-1 (PD-L1) expression and tissue tumor mutational burden (tTMB) are predictive biomarkers of outcomes with PD-(L)1 inhibitors in other tumor types, but no evidence clearly supports their validity in SCLC. Exploratory analyses from the phase III CASPIAN trial showed low prevalence of PD-L1 expression, particularly on tumor cells. OS benefit with durvalumab plus EP versus EP was not linked to PD-L1 expression or tTMB, suggesting neither is useful in selecting patients or predicting outcomes with durvalumab plus EP in ES-SCLC. With durvalumab plus tremelimumab and EP, OS benefit versus EP appeared greater in subgroups with PD-L1 expression on ≥1% tumor or immune cells, suggesting PD-L1 may be a useful biomarker for treatment with anti-PD-(L)1 and anti-CTLA-4; however, confirmation from a prospective dataset is required.
Introduction
After many years with little improvement in outcomes for extensive-stage small cell lung cancer (ES-SCLC), the phase III CASPIAN and IMpower133 studies showed that the addition of immune checkpoint inhibitors targeting programmed cell death ligand-1 (PD-L1) to first-line platinum doublet chemotherapy prolonged overall survival (OS) in patients with this aggressive and rapidly fatal tumor (1–5).
Durvalumab is a selective, high-affinity human IgG1 mAb that targets PD-L1, blocking its binding to programmed cell death-1 (PD-1) and CD80 (6). The phase III CASPIAN study investigated the efficacy and safety of first-line durvalumab, with or without the cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) inhibitor tremelimumab, in combination with etoposide plus either carboplatin or cisplatin (EP), compared with EP alone in patients with treatment-naïve ES-SCLC (2). At a planned interim analysis (data cutoff: March 11, 2019), durvalumab plus EP significantly improved OS compared with EP alone, with a HR of 0.73 [95% confidence interval (CI), 0.59–0.91; P = 0.0047]: median OS was 13.0 and 10.3 months in the durvalumab plus EP and EP arms, respectively (2). This survival benefit was sustained at updated analyses after approximately 2 and 3 years of follow-up, establishing durvalumab plus EP as a global standard of care in this setting (3, 5). However, durvalumab plus tremelimumab plus EP did not significantly improve OS versus EP (HR, 0.82; 95% CI, 0.68–1.00; P = 0.0451; ref. 3).
Although PD-L1 inhibition combined with chemotherapy represents a meaningful advance in the management of ES-SCLC, only a proportion of patients derive long-term benefit. The majority of patients progress or relapse after treatment, highlighting the need to identify those most likely to benefit from this therapeutic strategy. In other tumor types and in non–small cell lung cancer (NSCLC) in particular, benefit from PD-1 and PD-L1 inhibitors has in some cases been associated with increased PD-L1 expression on tumor cells (TC) and on immune cells (IC; ref. 7). In addition, it has been reported that tumor mutational burden (TMB), measured either from circulating tumor DNA (ctDNA) in blood or in tumor tissue samples, may be a predictive biomarker of efficacy outcomes in advanced solid tumors (8–12). To date, however, there is no clear, consistent evidence supporting the validity of any biomarkers for predicting outcomes with immunotherapy in combination with chemotherapy in SCLC.
The prevalence of tumor PD-L1 expression is substantially lower in SCLC compared with NSCLC, and data about the relevance of PD-L1 expression in this setting are conflicting (13, 14). Among solid tumors, SCLC tumors have one of the highest burdens of somatic nonsynonymous mutations, probably related to exposure to tobacco smoke (15–17). High TMB is hypothesized to be associated with the presence of neoantigens that are able to trigger a tumor-specific T-cell response, such as may be promoted by treatment with immune checkpoint inhibitors (13, 18, 19). Current data exploring the relationship between TMB and efficacy outcomes with immune checkpoint inhibitors in SCLC are inconsistent (4, 20, 21).
Here, we report the results of exploratory analyses of PD-L1 expression and tissue TMB (tTMB), and the relationship of these biomarkers with outcomes, in patients with treatment-naïve ES-SCLC in the CASPIAN study. A plain language summary of this article can be found in the Supplementary Data.
Materials and Methods
Study design and patients
CASPIAN (NCT03043872) is an open-label, sponsor-blind, multicenter, randomized, international phase III study, for which the primary analyses have been published previously (2, 3). In brief, eligible patients were aged ≥18 years and had treatment-naïve, histologically or cytologically documented ES-SCLC; World Health Organization (WHO) performance status score of 0 or 1; measurable disease according to RECIST, version 1.1 (22); and a life expectancy of at least 12 weeks. Patients with asymptomatic or treated and stable brain metastases were permitted. All patients provided signed informed consent for participation in the study. The study protocol and all amendments were approved by the relevant ethics committees and regulatory authorities, and the study was run in accordance with the International Conference on Harmonization good clinical practice guidelines, the Declaration of Helsinki, and applicable local regulations.
Treatment
Patients were randomized (1:1:1) to receive durvalumab plus EP, durvalumab plus tremelimumab plus EP, or EP; randomization was stratified according to planned platinum agent (carboplatin or cisplatin). In all arms, chemotherapy comprised etoposide 80‒100 mg/m2, administered on days 1 to 3 of each 21-day cycle, and investigator's choice of either carboplatin AUC 5‒6 mg/mL/minute or cisplatin 75‒80 mg/m2, given on day 1 of each cycle. In the immunotherapy arms, patients received four cycles of EP plus durvalumab 1,500 mg with or without tremelimumab 75 mg every 3 weeks, followed by maintenance durvalumab 1,500 mg every 4 weeks; patients in the durvalumab plus tremelimumab plus EP arms also received one further dose of tremelimumab 75 mg after EP. In the EP arm, patients could receive an additional two cycles of EP (up to six cycles maximum) and prophylactic cranial irradiation administered at the investigator's discretion after chemotherapy. Treatment continued until disease progression, unacceptable toxicity, or other discontinuation criteria were met. Study treatment beyond disease progression was allowed if there was evidence of clinical benefit.
Endpoints and assessments
The two primary endpoints were OS for durvalumab plus EP versus EP and for durvalumab plus tremelimumab plus EP versus EP. Secondary endpoints included progression-free survival (PFS) and objective response rate (ORR; including unconfirmed responses) based on investigator assessment according to RECIST v1.1, as well as safety and tolerability graded per NCI Common Terminology Criteria for Adverse Events, version 4.03. Confirmed ORR was analyzed post hoc. Assessment of efficacy based on biomarkers, including PD-L1 expression on TCs and ICs, and tTMB, was an exploratory endpoint. Provision of an archival (<3 years old) tumor tissue block (or ≥15 newly cut unstained slides) was mandatory for inclusion in the study, if such samples were available. Because there were no established prognostic or predictive biomarkers in SCLC at the time of designing the study, neither measurement of PD-L1 expression nor TMB was required during patient screening.
PD-L1 testing
PD-L1 expression on TCs and ICs was assessed using the VENTANA SP263 IHC assay (Ventana Medical Systems; ref. 23).
The percentage of tumor-associated ICs with PD-L1 staining intensity above background determined the PD-L1 IC expression status (IC ≥1%), and the percentage of TCs with any membrane staining above background determined the PD-L1 TC expression status (TC ≥1%). Cutoffs for PD-L1 expression at ≥1% of ICs, ≥1% of TCs, and ≥1% of ICs or TCs were explored. Testing was done in a central laboratory by pathologists trained and qualified by Ventana to score the samples at specific cutoffs.
TMB assessment
TMB was assessed using the FoundationOne CDx assay (Foundation Medicine) in tissue biopsy samples remaining after PD-L1 testing; the algorithm has been described previously (24). Because this was an exploratory analysis, no cut-off points were predefined; instead, a range of cutoffs were selected on the basis of the distribution of tTMB scores. A cutoff of 10 mutations per megabase (mut/Mb) was selected for more detailed analysis, as this threshold was shown to be predictive of PFS and response to nivolumab + ipilimumab in NSCLC (9, 10). In addition, tTMB ≥10 mut/Mb was associated with higher ORR in patients with advanced solid tumors (including SCLC) treated with pembrolizumab, which led to the FDA approval of the FoundationOne CDx assay as a companion diagnostic for pembrolizumab in the treatment of solid tumors with tTMB ≥10 mut/Mb (12, 25).
Statistical analysis
The biomarker analyses reported here were based on data from the updated analysis of CASPIAN with a median follow-up of 25.1 months (data cutoff: January 27, 2020). The PD-L1 and tTMB biomarker-evaluable population (BEP) included all randomized patients who had a tumor sample available for PD-L1 and tTMB testing, respectively, and an evaluable result. Within each subset, safety was assessed in all patients who received at least one dose of treatment.
For both the overall PD-L1 BEP and the overall tTMB BEP, OS and PFS were analyzed as for the intention-to-treat (ITT) population using a stratified log-rank test adjusted for planned platinum therapy at cycle 1, with HRs and 95% CI estimated using a Cox proportional hazards model. OS and PFS were estimated using the Kaplan–Meier method. PD-L1 and tTMB subgroup analyses (based on different cut-off thresholds) used an unstratified Cox proportional hazards model with treatment as the only covariate. The ORR was analyzed using a logistic regression model stratified for planned platinum at cycle 1 for the overall PD-L1 and tTMB BEPs, and an unstratified model for subgroup analyses, to calculate the ORs and associated 95% CIs comparing the response rate between treatment arms. An unstratified Cox proportional hazards or logistic regression model (for ORR) was used to test for any evidence of an interaction between treatment effect and tTMB; the model in each case included covariates for treatment effect, tTMB score (as a continuous variable), and a treatment by tTMB score interaction. A χ2 statistic from each model with and without the interaction term was used to calculate the P value. Because the analyses of PD-L1 expression and TMB were exploratory, no adjustments were made for multiple comparisons. All analyses used SAS (version 9.4).
Data availability
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. The AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
Results
PD-L1 and tTMB BEPs
A total of 805 patients were randomized in the CASPIAN study (ITT population) and were assigned to durvalumab plus EP (n = 268), durvalumab plus tremelimumab plus EP (n = 268), or EP alone (n = 269; ref. 2). Tumor samples were available for assessment of PD-L1 expression from 497 patients, although results could not be obtained for 59 patients (mainly due to insufficient number of slides or insufficient viable tumor for testing). The PD-L1 BEP therefore comprised 438 patients (54.4% of ITT; Supplementary Fig. S1): 152 (56.7%), 157 (58.6%), and 129 (48.0%) in the durvalumab plus EP, durvalumab plus tremelimumab plus EP, and EP arms, respectively. All patients in the two immunotherapy arms of the PD-L1 BEP received treatment, but 2 patients in the EP arm did not receive any treatment (one patient died before being treated and another withdrew from the study) and were excluded from the PD-L1 safety population. Tumor samples for tTMB assessment were available from 355 patients; testing was not performed on samples from 72 patients (mainly due to sample quality control failure). The tTMB BEP therefore comprised samples from 283 patients (35.2% of ITT; Supplementary Fig. S1) that were evaluable for TMB status: 107 (39.9%) in the durvalumab plus EP arm, 105 (39.2%) in the durvalumab plus tremelimumab plus EP arm, and 71 (26.4%) in the EP arm. All patients received treatment and were included in both efficacy and safety analyses. Patient demographics and baseline disease characteristics were largely balanced across treatment arms within each BEP, and generally consistent with the ITT population (Supplementary Table S1). Representativeness of study participants is described in Supplementary Table S2.
In the PD-L1 BEP, the OS benefit appeared greater than that in the ITT population for both treatment comparisons (Supplementary Fig. S2), with similar trends seen for PFS and ORR (Supplementary Fig. S3 and S4). For durvalumab plus EP versus EP, the HR for OS in the PD-L1 BEP was 0.61 (95% CI, 0.47–0.79), compared with 0.75 (95% CI, 0.62–0.91) in the ITT population. For durvalumab plus tremelimumab plus EP versus EP, the HRs for OS were 0.73 (95% CI, 0.57–0.95) in the PD-L1 BEP and 0.82 (95% CI, 0.68–1.00) in the ITT population.
In the tTMB BEP, OS benefit was comparable to the ITT population for both treatment comparisons (Supplementary Fig. S5). For durvalumab plus EP versus EP, the HR for OS in the tTMB BEP was 0.71 (95% CI, 0.51–0.99), while for durvalumab plus tremelimumab plus EP versus EP, the HR for OS was 0.83 (95% CI, 0.60–1.16) in the tTMB BEP. Clinical benefit for PFS and confirmed ORR in the tTMB BEP were also generally similar to the ITT population (Supplementary Fig. S6 and S7).
Prevalence of PD-L1 expression
The prevalence of PD-L1 expression on TCs and ICs was low; 5.7% of patients had PD-L1 expression on ≥1% of TCs, 25.8% had PD-L1 expression on ≥1% of ICs, and 28.3% had PD-L1 expression on ≥1% of TCs or ICs (Supplementary Fig. S8; Supplementary Table S1). Because of the low levels of PD-L1 expression, a 1% cutoff in ICs and separately in TCs was chosen for exploratory analysis of PD-L1 positivity, as well as one combining TC and IC expression (either TC or IC ≥1% vs. TC and IC <1%). Although randomization was not stratified by PD-L1 status, the proportion of patients with PD-L1 expression above and below the 1% cutoff was generally well balanced on TCs across treatment arms; however, the proportion of patients with IC ≥1% was higher in the durvalumab plus tremelimumab plus EP arm compared with the other two arms (Supplementary Table S1).
Relationship between PD-L1 expression and efficacy outcomes
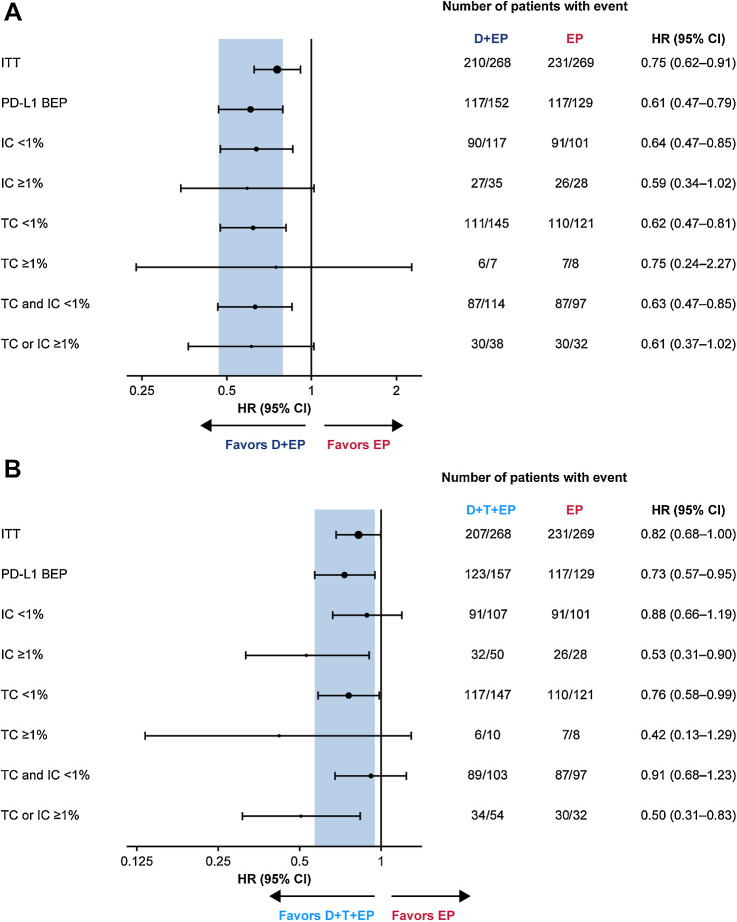
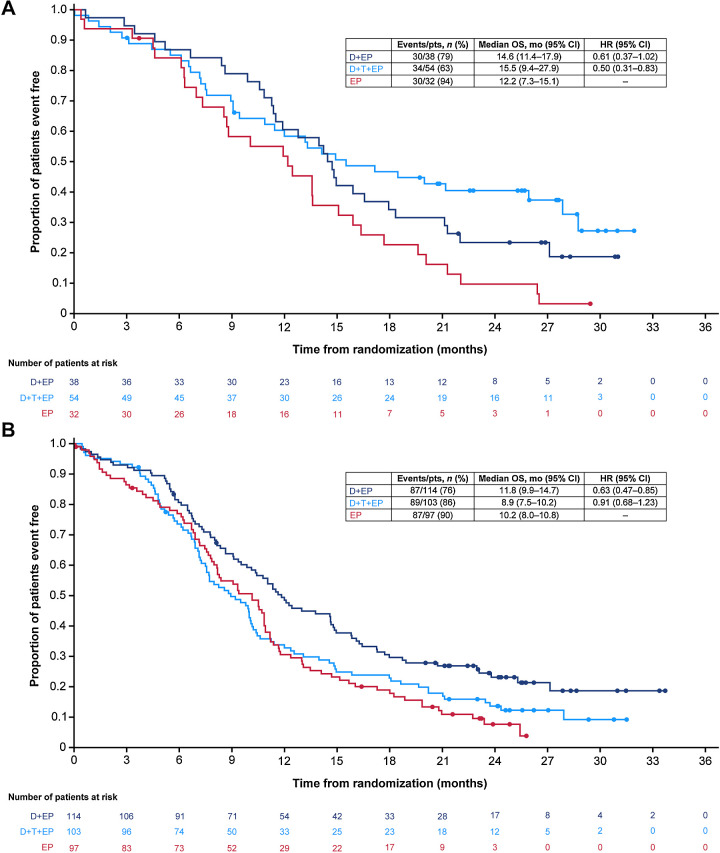
For durvalumab plus EP versus EP, the OS benefit was broadly similar regardless of PD-L1 expression on either TCs or ICs, with HRs of 0.75, 0.59, and 0.61, respectively, for the PD-L1 ≥1% subgroups based on TC, IC, and TC or IC expression, and 0.62, 0.64, and 0.63, respectively, for the PD-L1 < 1% subgroups based on TC, IC, or TC and IC expression (Fig. 1A). For durvalumab plus tremelimumab plus EP versus EP, OS HRs appeared improved in the PD-L1 ≥1% subgroups based on TC, IC, and TC or IC expression (HRs 0.42, 0.53, and 0.50, respectively) compared with the PD-L1 <1% subgroups (HRs 0.76, 0.88, and 0.91, respectively), suggesting that PD-L1 expression based on a 1% cutoff may predict OS benefit (Fig. 1B). In particular, the treatment effect on OS was more favorable in the subgroup of patients with TC or IC ≥1% (HR, 0.50; 95% CI, 0.31–0.83) than in the subgroup with TC and IC <1% (HR, 0.91; 95% CI, 0.68–1.23; Fig. 2A and B).
Figure 1.
Subgroup analysis of OS by PD-L1 TC and IC expression for durvalumab plus EP versus EP (A) and durvalumab plus tremelimumab plus EP versus EP (B). In each panel, the shaded band shows the CI for the ITT population; circle sizes are proportional to the number of events. CI, confidence interval; D, durvalumab; EP, platinum-etoposide; HR, hazard ratio; IC, immune cell; ITT, intention-to-treat; OS, overall survival; PD-L1, programmed cell death ligand-1; T, tremelimumab; TC, tumor cell.
Figure 2.
Kaplan–Meier analysis of OS for the PD-L1 TC or IC ≥1% subgroup (A) and the PD-L1 TC and IC <1% subgroup (B). CI, confidence interval; D, durvalumab; EP, platinum-etoposide; HR, hazard ratio; IC, immune cell; OS, overall survival; PD-L1, programmed cell death ligand-1; T, tremelimumab; TC, tumor cell.
Forest plots of HRs for PFS and ORs for confirmed ORR in the PD-L1 subgroups are shown in Supplementary Fig. S3 and S4. PFS benefit was improved in the PD-L1 TC or IC ≥1% subgroup compared with the PD-L1 TC and IC <1% subgroup, both for durvalumab plus EP versus EP (HR, 0.55; 95% CI, 0.33–0.93 vs. HR, 0.75; 95% CI, 0.56–1.00) and for durvalumab plus tremelimumab plus EP versus EP (HR, 0.62; 95% CI, 0.38–1.01 vs. HR, 0.83; 95% CI, 0.61–1.11). ORs for confirmed ORR were largely similar across PD-L1 subgroups.
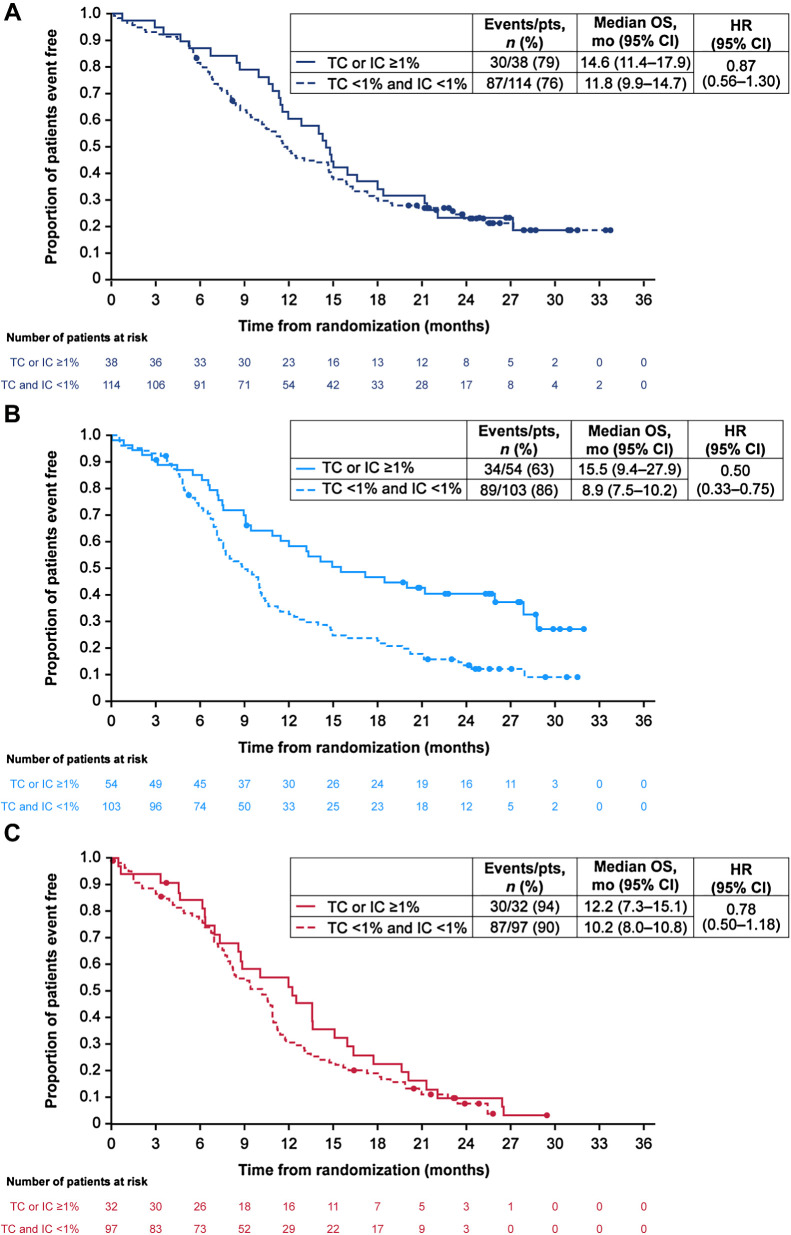
To further investigate the apparent association of PD-L1 expression with OS benefit for durvalumab plus tremelimumab plus EP versus EP, we examined baseline disease characteristics in the PD-L1 subgroups (Table 1). This revealed some imbalances between the treatment arms in key factors. In the TC or IC ≥1% subgroup, 24.1% of patients in the durvalumab plus tremelimumab plus EP arm had a WHO performance status score of 0 versus 37.5% of patients in the EP arm; 29.6% versus 37.5% of patients, respectively, had baseline liver metastases; and 18.5% versus 12.5%, respectively, had baseline brain metastases. A post hoc exploratory analysis adjusted to account for the imbalances in these characteristics within this subgroup produced an HR of 0.53 (95% CI, 0.31–0.90) for OS benefit with durvalumab plus tremelimumab plus EP versus EP, which was similar to the OS benefit observed in the unadjusted analysis (HR, 0.50; 95% CI, 0.31–0.83). Calculation of HRs between the PD-L1 subgroups within each treatment arm indicated longer OS in the TC or IC ≥1% subgroup compared with the TC and IC <1% subgroup in the durvalumab plus tremelimumab plus EP arm (HR, 0.50; 95% CI, 0.33–0.75); the difference between PD-L1 subgroups was less pronounced in the durvalumab plus EP arm (HR, 0.87; 95% CI, 0.56–1.30) and the EP arm (HR, 0.78; 95% CI, 0.50–1.18; Fig. 3A, B, and C).
Table 1.
Baseline patient demographics and disease characteristics by PD-L1 subgroup.
| Durvalumab + EP (n = 152) | Durvalumab + tremelimumab + EP (n = 157) | EP (n = 129) | ||||
|---|---|---|---|---|---|---|
| TC and IC <1% (n = 114) | TC or IC ≥1% (n = 38) | TC and IC <1% (n = 103) | TC or IC ≥1% (n = 54) | TC and IC <1% (n = 97) | TC or IC ≥1% (n = 32) | |
| Median age (range), years | 62.5 (46–81) | 66.0 (40–78) | 63.0 (43–84) | 60.5 (36–83) | 63.0 (38–78) | 61.5 (42–80) |
| Age group, n (%) | ||||||
| <65 | 72 (63.2) | 17 (44.7) | 61 (59.2) | 34 (63.0) | 56 (57.7) | 21 (65.6) |
| ≥65 | 42 (36.8) | 21 (55.3) | 42 (40.8) | 20 (37.0) | 41 (42.3) | 11 (34.4) |
| Sex, n (%) | ||||||
| Men | 83 (72.8) | 28 (73.7) | 75 (72.8) | 38 (70.4) | 68 (70.1) | 21 (65.6) |
| Women | 31 (27.2) | 10 (26.3) | 28 (27.2) | 16 (29.6) | 29 (29.9) | 11 (34.4) |
| Race, n (%) | ||||||
| White | 97 (85.1) | 36 (94.7) | 87 (84.5) | 46 (85.2) | 79 (81.4) | 27 (84.4) |
| Asian | 14 (12.3) | 2 (5.3) | 15 (14.6) | 8 (14.8) | 14 (14.4) | 5 (15.6) |
| Black | 2 (1.8) | 0 | 0 | 0 | 2 (2.1) | 0 |
| Other or missing data | 1 (0.9) | 0 | 1 (1.0) | 0 | 2 (2.1) | 0 |
| Disease stage, n (%) | ||||||
| III | 13 (11.4) | 5 (13.2) | 5 (4.9) | 4 (7.4) | 8 (8.2) | 2 (6.3) |
| IV | 101 (88.6) | 33 (86.8) | 98 (95.1) | 50 (92.6) | 89 (91.8) | 30 (93.8) |
| WHO PS, n (%) | ||||||
| 0 | 36 (31.6) | 13 (34.2) | 42 (40.8) | 13 (24.1) | 29 (29.9) | 12 (37.5) |
| 1 | 78 (68.4) | 25 (65.8) | 61 (59.2) | 41 (75.9) | 68 (70.1) | 20 (62.5) |
| Smoking history, n (%) | ||||||
| Never smoker | 10 (8.8) | 4 (10.5) | 5 (4.9) | 6 (11.1) | 5 (5.2) | 2 (6.3) |
| Former smoker | 55 (48.2) | 15 (39.5) | 54 (52.4) | 28 (51.9) | 41 (42.3) | 12 (37.5) |
| Current smoker | 49 (43.0) | 19 (50.0) | 44 (42.7) | 20 (37.0) | 51 (52.6) | 18 (56.3) |
| Brain or CNS metastases, n (%) | 8 (7.0) | 4 (10.5) | 15 (14.6) | 10 (18.5) | 8 (8.2) | 4 (12.5) |
| Liver metastases, n (%) | 46 (40.4) | 9 (23.7) | 57 (55.3) | 16 (29.6) | 40 (41.2) | 12 (37.5) |
Abbreviations: CNS, central nervous system; EP, platinum-etoposide; IC, immune cell; PD-L1, programmed cell death ligand-1; PS, performance status; TC, tumor cell; WHO, World Health Organization.
Figure 3.
Kaplan–Meier analysis of OS by PD-L1 subgroup in durvalumab plus EP arm (A), durvalumab plus tremelimumab plus EP arm (B), and EP arm (C). CI, confidence interval; EP, platinum-etoposide; HR, hazard ratio; IC, immune cell; OS, overall survival; PD-L1, programmed cell death ligand-1; TC, tumor cell.
tTMB score distribution
The overall distribution of tTMB scores was broadly similar across treatment arms within the tTMB BEP (Supplementary Fig. S9). On the basis of the observed tTMB scores, cut-off points ranging from 6 to 14 mut/Mb were chosen for exploratory analysis; for each selected cut-off point, one or more of the treatment arms had at least 25% of the tTMB BEP in the smallest category of the cutoff.
Relationship between tTMB and efficacy outcomes
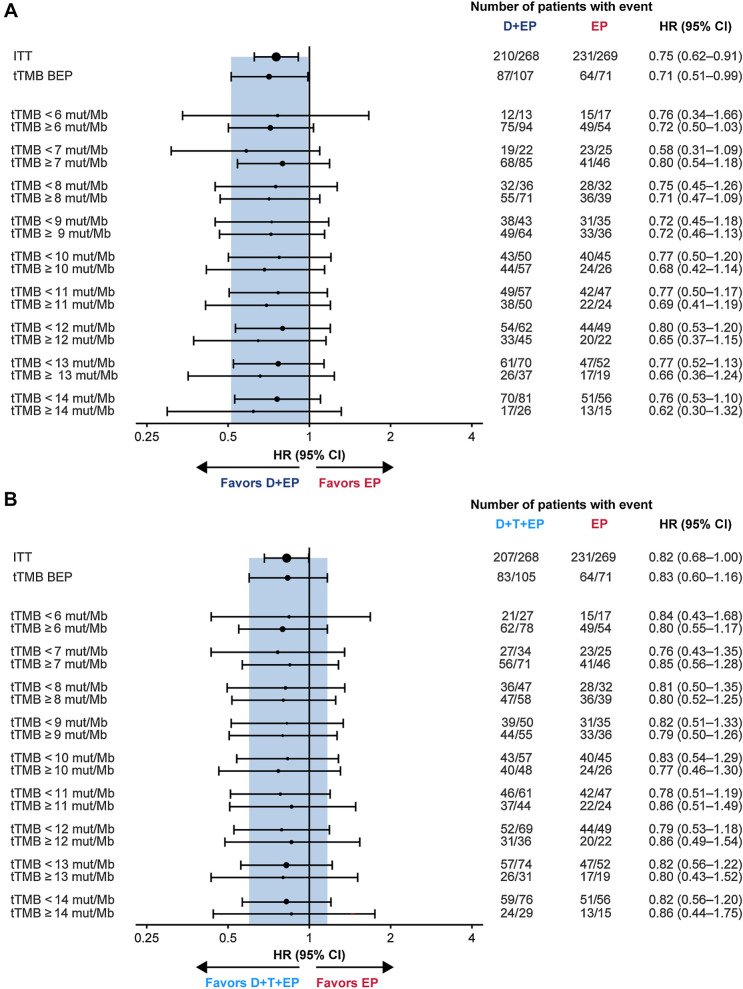
Exploratory analysis of OS at tTMB cutoffs ranging from 6 to 14 mut/Mb showed that HRs for durvalumab plus EP versus EP, and for durvalumab plus tremelimumab plus EP versus EP, all fell within the CI associated with the HR in the tTMB BEP and favored the immunotherapy regimen across the tTMB subgroups, suggesting that tTMB did not predict treatment benefit (Fig. 4A and B). When considering tTMB score on a continuous scale, there was no evidence of an interaction between treatment effect and tTMB for OS (durvalumab plus EP vs. EP, P = 0.916; durvalumab plus tremelimumab plus EP vs. EP, P = 0.672). Kaplan–Meier curves for OS in subgroups using a tTMB cutoff of 10 mut/Mb (selected on the basis of previously published data with other immunotherapies; see Materials and Methods) are shown in Supplementary Fig. S10. For durvalumab plus EP versus EP, the HR for OS was 0.77 (95% CI, 0.50–1.20) in the tTMB <10 mut/Mb subgroup and 0.68 (95% CI, 0.42–1.14) in the tTMB ≥10 mut/Mb subgroup. For durvalumab plus tremelimumab plus EP versus EP, the HR for OS was 0.83 (95% CI, 0.54–1.29) in the tTMB <10 mut/Mb subgroup and 0.77 (95% CI, 0.46–1.30) in the tTMB ≥10 mut/Mb subgroup.
Figure 4.
Subgroup analysis of OS by tTMB for durvalumab plus EP versus EP (A) and durvalumab plus tremelimumab plus EP versus EP (B). In each panel, the shaded band shows the CI for the ITT population; circle sizes are proportional to the number of events. BEP, biomarker evaluable population; CI, confidence interval; D, durvalumab; EP, platinum-etoposide; HR, hazard ratio; ITT, intention-to-treat; OS, overall survival; mut/Mb, mutations per megabase; T, tremelimumab; tTMB, tissue tumor mutational burden.
Forest plots of HRs for PFS and ORs for confirmed ORR at tTMB cutoffs ranging from 6 to 14 mut/Mb are shown in Supplementary Fig. S6 and S7. For PFS, there was no evidence of an interaction between treatment and tTMB as a continuous variable when comparing durvalumab plus EP versus EP (P = 0.613); however, a significant interaction was observed for durvalumab plus tremelimumab plus EP versus EP (P = 0.044), with greater PFS improvements in the lower tTMB subgroups. There was no evidence of an interaction between treatment and tTMB on the continuous scale for confirmed ORR with either treatment comparison (durvalumab plus EP vs. EP, P = 0.115; durvalumab plus tremelimumab plus EP vs. EP, P = 0.114). For durvalumab plus tremelimumab plus EP versus EP, a notable difference in ORs between tTMB-high versus low subgroups was observed at cutoffs of 10 mut/Mb and higher, where ORs favored EP in the tTMB-high subgroups and favored durvalumab plus tremelimumab plus EP in the tTMB-low subgroups.
Relationship between PD-L1 expression and tTMB
A post hoc analysis revealed no difference in tTMB score according to PD-L1 expression (P = 0.959). The overlap of patients with tTMB ≥10 mut/Mb and PD-L1 TC or IC ≥1% was limited (11.6% of patients who had both a PD-L1 and a tTMB result), suggesting that presence of these biomarkers is not correlated (Supplementary Fig. S11).
Safety and treatment exposure
Safety in the PD-L1 and tTMB safety populations was similar to the overall safety population, although the rate of adverse events leading to treatment discontinuation in the EP arm was numerically higher in the PD-L1 and tTMB (vs. the overall) safety populations (Supplementary Table S3). Analysis of exposure by PD-L1 subgroup showed that, in both immunotherapy arms, exposure to durvalumab was higher in the TC or IC ≥1% subgroups than in the TC and IC <1% subgroups, with the highest median number of durvalumab doses and longest median duration of durvalumab treatment among patients with TC or IC ≥1% in the durvalumab plus EP arm (Supplementary Table S4). Safety was generally balanced between the TC and IC <1% subgroup and TC or IC ≥1% subgroup within each treatment arm, taking into account the small patient numbers of the subgroups (Supplementary Table S5).
Discussion
In this exploratory biomarker analysis from the CASPIAN study, tumor samples were collected from 64% of patients in the ITT population; PD-L1 and tTMB were evaluable in 54% and 35% of the ITT population, respectively. On the basis of the low prevalence of PD-L1 expression in the CASPIAN population, we explored thresholds of 1% expression on TCs, ICs, or TCs and ICs combined. We observed a similar benefit in OS with durvalumab plus EP versus EP regardless of PD-L1 expression. However, OS benefit with durvalumab plus tremelimumab plus EP versus EP appeared greater in the PD-L1 ≥1% subgroups, driven particularly by benefit in the IC ≥1% subgroup, suggesting potential predictive value of PD-L1 for this combination regimen. Because the predictive value of TMB has not been established in SCLC, there was no predefined tTMB cutoff in this study. However, analysis using a range of tTMB cutoffs that gave a meaningful sample size indicated that tTMB was not predictive of OS benefit with either durvalumab plus EP or with durvalumab plus tremelimumab plus EP, versus EP.
Consistent with previously published data in SCLC (4, 26–28), the overall prevalence of PD-L1 expression in CASPIAN was low, particularly on TCs. The PD-L1 BEP had better efficacy outcomes than the ITT population for both treatment comparisons; this increased clinical benefit appeared to be driven by a relatively poor performance in the EP arm, as indicated by a higher proportion of deaths and progression events, and lower confirmed ORR in patients from this treatment arm in the BEP when compared with the ITT population. While there were no obvious differences in baseline characteristics of the EP arm in each BEP, the higher rate of adverse events leading to treatment discontinuation in the EP arm of the PD-L1 safety population compared with the EP arm of the overall safety population may have contributed to the comparatively poor survival outcomes in the EP arm in the PD-L1 BEP.
The OS benefit was greater with durvalumab plus tremelimumab plus EP versus EP in the TC or IC ≥1% subgroup than in the TC and IC <1% subgroup. Randomization was not stratified by PD-L1 expression and there were some imbalances in baseline characteristics between treatment arms within the TC or IC ≥1% subgroup that could have influenced prognosis. However, the OS benefit remained after adjustment for these imbalances in a post hoc analysis. Although total exposure to durvalumab was greater in the durvalumab plus EP arm than in the durvalumab plus tremelimumab plus EP arm in the TC or IC ≥1% population, treatment with durvalumab plus tremelimumab plus EP had a more pronounced effect on OS. These results suggest that CTLA-4 inhibition may better complement the effect of PD-L1 inhibition to maintain antitumor activity in patients with PD-L1 expression, but this finding would require prospective validation.
The lack of association between PD-L1 expression and efficacy outcomes with anti-PD-(L)1 in the absence of coinhibition of CTLA-4 in CASPIAN is consistent with findings from the phase III IMpower133 study of first-line atezolizumab plus chemotherapy and the phase III KEYNOTE-604 study of first-line pembrolizumab plus chemotherapy, both in patients with ES-SCLC (4, 28). Whether or not the relationship between PD-L1 expression and treatment effect might be influenced by the presence of chemotherapy is currently unclear. Conflicting results were reported from earlier phase studies of PD-1/PD-L1 inhibitors as monotherapy in pretreated SCLC without an active comparator arm (26, 29, 30). However, comparison of results across earlier phase studies is complicated by the use of different assays and scoring systems to evaluate PD-L1, as well as the exploratory nature of these analyses, and the potential confounding prognostic effects in single-arm studies.
For the CASPIAN tTMB BEP, baseline characteristics as well as efficacy and safety outcomes were generally consistent with the ITT population, suggesting that these patients are likely to be a representative sample. tTMB did not seem to predict OS with either immunotherapy regimen, when assessed either based on binary cutoffs or as a continuous variable. Overall, the value of TMB as a predictive biomarker of outcomes in SCLC is currently unclear, with inconsistent results across different studies. In the phase III IMpower133 study, treatment benefit with first-line atezolizumab plus chemotherapy in ES-SCLC was not associated with TMB measured in blood (bTMB) using a cutoff of either 10 or 16 mut/Mb (1, 4). Consistent with both CASPIAN and IMpower133, results from the phase III KEYNOTE-604 study showed that tTMB was not positively associated with OS or PFS in patients treated with first-line pembrolizumab plus EP (31). However, analyses of the phase I/II CheckMate 032 study, in patients with SCLC previously treated with chemotherapy, have suggested an association of higher tTMB levels with improved outcomes to treatment with nivolumab monotherapy or nivolumab plus ipilimumab (20, 32), and a small retrospective analysis has also suggested the possible value of tTMB as a biomarker in SCLC treated with immune checkpoint inhibitors (21). Indications that TMB is predictive of response to immunotherapy, but not of response to the combination of immunotherapy with chemotherapy, have also been observed in NSCLC: tTMB was associated with OS, PFS, and ORR with first-line pembrolizumab monotherapy in the KEYNOTE-010 and KEYNOTE-042 studies (33, 34), while tTMB was not significantly associated with efficacy of first-line pembrolizumab plus platinum-based chemotherapy in the KEYNOTE-021, 189, and 407 studies (35, 36). Ultimately, comparison of findings across studies is complicated by significant variability in TMB measurement techniques, and there is a need for standardization of TMB assessment methodology across different assays, platforms, and centers to improve insights from future research (37).
The impact of tTMB on PFS and ORR in CASPIAN was less clear-cut: for durvalumab plus tremelimumab plus EP versus EP, the HRs for PFS and ORs for ORR appeared more favorable in the lower tTMB subgroups, with a significant interaction observed for PFS with tTMB as a continuous variable. However, it should be noted that these results were associated with wide CIs, and PFS and ORR were secondary endpoints in CASPIAN. Because the trend was weak and inconsistent with the OS findings, and is also contrary to current knowledge indicating that tTMB ≥10 mut/Mb predicts better outcomes with immunotherapy in NSCLC (9, 10, 12), we believe this could be a chance finding. Similar results were reported in KEYNOTE-604, with lower TMB predicting higher PFS and OS benefit with pembrolizumab plus EP versus placebo plus EP. However, this finding is likely to have been impacted by the statistically significant positive association of TMB with OS in the placebo plus EP arm and given the exploratory nature of the analyses, should be interpreted with caution (31).
PD-L1 and TMB have been shown to identify different, but overlapping populations in SCLC (4) and NSCLC (8, 11), and to be independent biomarkers in an analysis of nearly 10,000 clinical samples across a broad range of cancer types (27). In CASPIAN, there was no clear association between tTMB score and PD-L1 expression on either TC or IC, and it is conceivable that a combination of these two biomarkers may have greater predictive power for response to checkpoint inhibitors than either biomarker alone in SCLC. However, other biomarkers, such as SCLC molecular subtypes (38–40) or HLA genotype (41, 42), may have a more important role than PD-L1 or tTMB in this setting, given the differences in immune biology compared with NSCLC. Exploration of such emerging biomarkers using genomic and transcriptomic profiling in the CASPIAN dataset is ongoing (43, 44). Liquid biopsies can provide a rapid, noninvasive alternative to tumor biopsies to assess the molecular profile of a disease and to monitor its on-treatment clonal evolution. Previous studies in various tumor types have demonstrated that reduction in ctDNA during immunotherapy treatment may be associated with patient outcomes, possibly indicating a decrease in disease burden in response to treatment (45, 46), including in several studies in SCLC (47). In future, it is possible that ctDNA dynamics could be a useful tool to enable early clinical decision-making by identifying patients who are responding to or resistant to treatment for SCLC.
Limitations of this analysis primarily relate to its exploratory nature; CASPIAN was not designed to evaluate outcomes based on PD-L1 expression or tTMB, and although provision of archival tissue (if such samples were available) was mandatory, only half of patients were evaluable for PD-L1 and one-third for tTMB. This led to relatively small sample sizes in some of the biomarker-defined subgroups as indicated by the wide CIs. Furthermore, there was an imbalance in sample availability, with a smaller proportion of patients being evaluable for PD-L1 and tTMB in the EP arm compared with the durvalumab plus EP and durvalumab plus tremelimumab plus EP arms. Although the CASPIAN study was open label, the pretreatment withdrawal rate was low. Thus, there appears to be no obvious reason that fewer patients should have known biomarker status in the EP arm, and we assume the data were missing at random. In addition, the PD-L1 BEP appeared to have better treatment outcomes compared with the ITT population, which could have confounded the analysis. However, both treatment comparisons displayed this difference in efficacy, suggesting that it was unlikely to explain the differential effect observed for durvalumab plus tremelimumab plus EP based on PD-L1 expression. It should also be noted that that no samples were collected in CASPIAN for analysis of bTMB, so no comparison with tTMB data in the same patients could be performed. It is possible that assessment of bTMB in ES-SCLC could provide a more accurate representation of the overall disease than tTMB, because it captures total tumor TMB, including both primary and metastatic sites.
In conclusion, these results support treatment benefit with first-line durvalumab plus EP in patients with ES-SCLC irrespective of biomarker status; there was no evidence that either PD-L1 expression or tTMB can be used to select patients or predict outcomes with durvalumab plus EP in this disease setting. However, our observations in the durvalumab plus tremelimumab plus EP arm suggest that PD-L1 expression may yet prove to be a useful biomarker for combined treatment with PD-(L)1 and CTLA-4 inhibition, although this requires confirmation with a prospective and independent dataset.
Supplementary Material
Plain Language Summary
Sample flow for biomarker analysis.
Kaplan-Meier analysis of OS in the ITT population and the PD-L1 BEP.
Subgroup analysis of PFS by PD-L1 TC and IC expression for (A) durvalumab plus EP versus EP and (B) durvalumab plus tremelimumab plus EP versus EP. In each panel, the shaded band shows the confidence interval for the ITT population; circle sizes are proportional to the number of events.
Subgroup analysis of confirmed ORR by PD-L1 TC and IC expression for (A) durvalumab plus EP versus EP and (B) durvalumab plus tremelimumab plus EP versus EP. In each panel, the shaded band shows the confidence interval for the ITT population; circle sizes are proportional to the number of responses. Groups are not plotted when there are ≤5 responses across both arms.
Kaplan-Meier analysis of OS in the ITT population and the tTMB BEP.
Subgroup analysis of PFS by tTMB for (A) durvalumab plus EP versus EP and (B) durvalumab plus tremelimumab plus EP versus EP. In each panel, the shaded band shows the confidence interval for the ITT population; circle sizes are proportional to the number of events.
Subgroup analysis of confirmed ORR by tTMB for (A) durvalumab plus EP versus EP and (B) durvalumab plus tremelimumab plus EP versus EP. In each panel, the shaded band shows the confidence interval for the ITT population; circle sizes are proportional to the number of responses.
Prevalence of PD-L1 expression on TC and IC in the PD-L1 BEP. *Includes patients whose tumors had <1% PD-L1 expression on TCs or ICs, but not absolute zero.
Distribution of tTMB scores by treatment arm in the tTMB BEP.
Kaplan-Meier analysis of OS for (A) the tTMB <10 mut/Mb subgroup and (B) the tTMB ≥10 mut/Mb subgroup.
Relationship between PD-L1 expression and tTMB score. (A) Box plots showing the distribution of tTMB score in patients by PD-L1 subgroup; (B) Venn diagram showing overlap between the tTMB ≥10 mut/Mb and PD-L1 TC or IC ≥1% subgroups. Percentages are calculated from the PD-L1 and tTMB BEP, i.e., patients who had both a PD-L1 and a tTMB result (n=275); samples from 171 patients had tTMB ≥10 mut/Mb and/or PD-L1 TC or IC ≥1%, while samples from 104 patients (37.8%) had both tTMB <10 mut/Mb and PD-L1 TC and IC <1%.
Baseline patient demographics and disease characteristics for the PD-L1 and tTMB BEPs and the ITT population.
Representativeness of study participants.
Summary of adverse events of any cause in the PD-L1, tTMB, and overall safety populations.
Treatment exposure by PD-L1 subgroup (PD-L1 safety population).
Summary of adverse events of any cause by PD-L1 subgroup (PD-L1 safety population).
Acknowledgments
The study was funded by AstraZeneca.
The authors would like to thank the patients, their families and caregivers, and all investigators involved in this study. Medical writing support for the development of this article, under the direction of the authors, was provided by Helen Kitchen and Samantha Holmes of Ashfield MedComms, an Inizio company, and was funded by AstraZeneca.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
L. Paz-Ares reports grants or contracts from MSD, AstraZeneca, Pfizer, and BMS (to institution); consulting fees from Lilly, MSD, Roche, Pharmamar, Merck KGaA, AstraZeneca, Novartis, Servier, Amgen, Pfizer, Sanofi, Bayer, BMS, Mirati, GlaxoSmithKline, Janssen, Takeda, and Daiichi-Sankyo (to self); honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca, Janssen, Merck, and Mirati (to self); board of directors membership at Altum Sequencing and STAb Therapeutics; service as principal investigator for Alkermes, Amgen, AstraZeneca, BMS, Daiichi-Sankyo, IO Biotech, Janssen-Cilag, Lilly, MSD, Novartis, Pfizer, Pharmamar, Roche, Sanofi, Takeda, and Tesaro; institution: MSD, AstraZeneca, Pfizer, BMS. M.C. Garassino reports personal fees from AstraZeneca during the conduct of the study as well as grants and personal fees from Merck and Eli Lilly and personal fees from Roche, Daiichi-Sankyo, Takeda, BMS, AbbVie, Bayer, Celgene, Novartis, Pfizer, Blueprint, Mirati, Sanofi, Abion, and Beigenius outside the submitted work. Y. Chen reports other support from AstraZeneca, BMS, Amgen, Takeda, Guardant Health, Jazz Pharmaceuticals, and Pfizer outside the submitted work. N. Reinmuth reports personal fees from AstraZeneca, Amgen, BMS, Boehringer Ingelheim, Daiichi-Sankyo, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Merck, Pfizer, Takeda, and Sanofi outside the submitted work. K. Hotta reports grants from AstraZeneca during the conduct of the study as well as personal fees from Pfizer, Takeda, Ono, Boehringer Ingelheim, Nippon Kayaku, and AbbVie and grants and personal fees from AstraZeneca, Chugai, Lilly, MSD, and BMS outside the submitted work. M.J. Hochmair reports personal fees from BMS, Roche, Lilly, Boehringer Ingelheim, and AstraZeneca outside the submitted work. G. Losonczy reports personal fees from AstraZeneca during the conduct of the study; in addition, G. Losonczy has a patent for clinical trial of new drug licensed and has received financial support for participating in this clinical study. M. Xie reports personal fees from AstraZeneca outside the submitted work. Z. Lai is a full-time employee and stock owner of AstraZeneca. H. Mann reports personal fees from AstraZeneca during the conduct of the study as well as personal fees from AstraZeneca outside the submitted work. H. Jiang reports other support from AstraZeneca outside the submitted work. Y. Shrestha reports other support from AstraZeneca during the conduct of the study. J.W. Goldman reports grants and personal fees from AstraZeneca, Genentech, and Pfizer; personal fees from Jazz Pharmaceuticals; and grants from GlaxoSmithKline outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
L. Paz-Ares: Conceptualization, resources, supervision, investigation, writing–review and editing. M.C. Garassino: Investigation, writing–review and editing. Y. Chen: Supervision, validation, investigation, writing–review and editing. N. Reinmuth: Resources, data curation, investigation, writing–review and editing. K. Hotta: Investigation, writing–review and editing. A. Poltoratskiy: Investigation, methodology, writing–review and editing. D. Trukhin: Resources, data curation, investigation, writing–review and editing. M.J. Hochmair: Conceptualization, supervision, investigation, writing–review and editing. M. Özgüroğlu: Resources, data curation, formal analysis, investigation, writing–review and editing. J.H. Ji: Supervision, investigation, writing–review and editing. G. Statsenko: Data curation, investigation, writing–review and editing. N. Conev: Investigation, writing–review and editing. I. Bondarenko: Supervision, writing–review and editing. L. Havel: Resources, data curation, investigation, writing–review and editing, data collection, selection and treatment of subjects. G. Losonczy: Resources, investigation, writing–review and editing. M. Xie: Formal analysis, validation, visualization, writing–review and editing. Z. Lai: Resources, data curation, validation, visualization, methodology, writing–review and editing. N. Godin-Heymann: Conceptualization, resources, funding acquisition, writing–review and editing. H. Mann: Formal analysis, validation, methodology, writing–review and editing. H. Jiang: Conceptualization, supervision, investigation, writing–review and editing. Y. Shrestha: Conceptualization, data curation, formal analysis, supervision, funding acquisition, investigation, visualization, writing–review and editing. J.W. Goldman: Resources, investigation, writing–review and editing.
References
- 1. Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018;379:2220–9. [DOI] [PubMed] [Google Scholar]
- 2. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929–39. [DOI] [PubMed] [Google Scholar]
- 3. Goldman JW, Garassino MC, Chen Y, Ozguroglu M, Dvorkin M, Trukhin D, et al. Patient-reported outcomes with first-line durvalumab plus platinum-etoposide versus platinum-etoposide in extensive-stage small-cell lung cancer (CASPIAN): a randomized, controlled, open-label, phase III study. Lung Cancer 2020;149:46–52. [DOI] [PubMed] [Google Scholar]
- 4. Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol 2021;39:619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paz-Ares L, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open 2022;7:100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stewart R, Morrow M, Hammond SA, Mulgrew K, Marcus D, Poon E, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res 2015;3:1052–62. [DOI] [PubMed] [Google Scholar]
- 7. Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity 2018;48:434–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 2018;24:1441–8. [DOI] [PubMed] [Google Scholar]
- 9. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018;378:2093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, Gainor JF, et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol 2019;37:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol 2020;6:661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21:1353–65. [DOI] [PubMed] [Google Scholar]
- 13. Gelsomino F, Lamberti G, Parisi C, Casolari L, Melotti B, Sperandi F, et al. The evolving landscape of immunotherapy in small-cell lung cancer: a focus on predictive biomarkers. Cancer Treat Rev 2019;79:101887. [DOI] [PubMed] [Google Scholar]
- 14. Saltos A, Shafique M, Chiappori A. Update on the biology, management, and treatment of small cell lung cancer (SCLC). Front Oncol 2020;10:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peifer M, Fernandez-Cuesta L, Sos ML, George J, Seidel D, Kasper LH, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. George J, Lim JS, Jang SJ, Cun Y, Ozretic L, Kong G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 2012;482:400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 2018;33:853–61.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ricciuti B, Kravets S, Dahlberg SE, Umeton R, Albayrak A, Subegdjo SJ, et al. Use of targeted next generation sequencing to characterize tumor mutational burden and efficacy of immune checkpoint inhibition in small cell lung cancer. J Immunother Cancer 2019;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 23. Rebelatto MC, Midha A, Mistry A, Sabalos C, Schechter N, Li X, et al. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol 2016;11:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Foundation Medicine press release. FoundationOne®CDx Receives FDA Approval as the First Companion Diagnostic to Identify Advanced Cancer Patients with Solid Tumors that are Tumor Mutational Burden-High (TMB-H) and Appropriate for Immunotherapy Treatment with KEYTRUDA® (pembrolizumab). Available from: https://www.foundationmedicine.com/press-releases/2bac198e-d31b-4e95-bfbf-28a55093b8e8.
- 26. Antonia SJ, Lopez-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883–95. [DOI] [PubMed] [Google Scholar]
- 27. Yarchoan M, Albacker LA, Hopkins AC, Montesion M, Murugesan K, Vithayathil TT, et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 2019;4:e126908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csoszi T, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol 2020;38:2369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ready N, Farago AF, de Braud F, Atmaca A, Hellmann MD, Schneider JG, et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol 2019;14:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chung HC, Lopez-Martin JA, Kao SC-H, Miller WH, Ros W, Gao B, et al. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J Clin Oncol 36: 15s, 2018(suppl; abstr 8506). [Google Scholar]
- 31. Rudin CM, Kim HR, Navarro A, Gottfried M, Peters S, Csoszi T, et al. Exploratory biomarker analysis of the phase 3 KEYNOTE-604 study of pembrolizumab plus etoposide for extensive-stage SCLC. J Clin Oncol 41: 16s, 2023(suppl; abstr 8503). [Google Scholar]
- 32. Ready NE, Ott PA, Hellmann MD, Zugazagoitia J, Hann CL, de Braud F, et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the CheckMate 032 randomized cohort. J Thorac Oncol 2020;15:426–35. [DOI] [PubMed] [Google Scholar]
- 33. Herbst RS, Lopes G, Kowalski DM, Nishio M, Wu YL, de Castro Junior G, et al. Association between tissue TMB (tTMB) and clinical outcomes with pembrolizumab monotherapy (pembro) in PD-L1-positive advanced NSCLC in the KEYNOTE-010 and -042 trials. Ann Oncol 2019;30:v916–v7. [Google Scholar]
- 34. Mok TSK, Lopes G, Cho BC, Kowalski DM, Kasahara K, Wu YL, et al. Associations of tissue tumor mutational burden and mutational status with clinical outcomes in KEYNOTE-042: pembrolizumab versus chemotherapy for advanced PD-L1-positive NSCLC. Ann Oncol 2023;34:377–88. [DOI] [PubMed] [Google Scholar]
- 35. Paz-Ares L, Langer CJ, Novello S, Halmos B, Cheng Y, Gadgeel SM, et al. Pembrolizumab (pembro) plus platinum-based chemotherapy (chemo) for metastatic NSCLC: Tissue TMB (tTMB) and outcomes in KEYNOTE-021, 189, and 407. Ann Oncol 2019;30:v917–v8. [Google Scholar]
- 36. Garassino MC, Gadgeel S, Novello S, Halmos B, Felip E, Speranza G, et al. Associations of tissue tumor mutational burden and mutational status with clinical outcomes with pembrolizumab plus chemotherapy versus chemotherapy for metastatic NSCLC. JTO Clin Res Rep 2023;4:100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stenzinger A, Allen JD, Maas J, Stewart MD, Merino DM, Wempe MM, et al. Tumor mutational burden standardization initiatives: Recommendations for consistent tumor mutational burden assessment in clinical samples to guide immunotherapy treatment decisions. Genes Chromosomes Cancer 2019;58:578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer 2019;19:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Owonikoko TK, Dwivedi B, Chen Z, Zhang C, Barwick B, Ernani V, et al. YAP1 expression in SCLC defines a distinct subtype with T-cell-inflamed phenotype. J Thorac Oncol 2021;16:464–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gay CM, Stewart CA, Park EM, Diao L, Groves SM, Heeke S, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 2021;39:346–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 2018;359:582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chowell D, Krishna C, Pierini F, Makarov V, Rizvi NA, Kuo F, et al. Evolutionary divergence of HLA class I genotype impacts efficacy of cancer immunotherapy. Nat Med 2019;25:1715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Garassino MC, Shrestha Y, Xie M, Lai Z, Spencer S, Dalvi T, et al. MA16.06 Durvalumab ± tremelimumab + platinum-etoposide in 1L ES-SCLC: exploratory analysis of HLA genotype and survival in CASPIAN. J Thorac Oncol 2021;16:S939. [Google Scholar]
- 44. Xie M, Chugh P, Broadhurst H, Lai Z, Whitston D, Paz-Ares L, et al. Durvalumab (D) + platinum-etoposide (EP) in 1L extensive-stage small-cell lung cancer (ES-SCLC): Exploratory analysis of SCLC molecular subtypes in CASPIAN [abstract]. In:Proceedings of the American Association for Cancer Research Annual Meeting 2022; 2022Apr 8–13. Philadelphia (PA): AACR; Cancer Res 2022;82(12_Suppl):Abstract nr CT024. [Google Scholar]
- 45. Sivapalan L, Murray JC, Canzoniero JV, Landon B, Jackson J, Scott S, et al. Liquid biopsy approaches to capture tumor evolution and clinical outcomes during cancer immunotherapy. J Immunother Cancer 2023;11:e005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang Q, Luo J, Wu S, Si H, Gao C, Xu W, et al. Prognostic and predictive impact of circulating tumor DNA in patients with advanced cancers treated with immune checkpoint blockade. Cancer Discov 2020;10:1842–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pizzutilo EG, Pedrani M, Amatu A, Ruggieri L, Lauricella C, Veronese SM, et al. Liquid biopsy for small cell lung cancer either de novo or transformed: systematic review of different applications and meta-analysis. Cancers 2021;13:2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plain Language Summary
Sample flow for biomarker analysis.
Kaplan-Meier analysis of OS in the ITT population and the PD-L1 BEP.
Subgroup analysis of PFS by PD-L1 TC and IC expression for (A) durvalumab plus EP versus EP and (B) durvalumab plus tremelimumab plus EP versus EP. In each panel, the shaded band shows the confidence interval for the ITT population; circle sizes are proportional to the number of events.
Subgroup analysis of confirmed ORR by PD-L1 TC and IC expression for (A) durvalumab plus EP versus EP and (B) durvalumab plus tremelimumab plus EP versus EP. In each panel, the shaded band shows the confidence interval for the ITT population; circle sizes are proportional to the number of responses. Groups are not plotted when there are ≤5 responses across both arms.
Kaplan-Meier analysis of OS in the ITT population and the tTMB BEP.
Subgroup analysis of PFS by tTMB for (A) durvalumab plus EP versus EP and (B) durvalumab plus tremelimumab plus EP versus EP. In each panel, the shaded band shows the confidence interval for the ITT population; circle sizes are proportional to the number of events.
Subgroup analysis of confirmed ORR by tTMB for (A) durvalumab plus EP versus EP and (B) durvalumab plus tremelimumab plus EP versus EP. In each panel, the shaded band shows the confidence interval for the ITT population; circle sizes are proportional to the number of responses.
Prevalence of PD-L1 expression on TC and IC in the PD-L1 BEP. *Includes patients whose tumors had <1% PD-L1 expression on TCs or ICs, but not absolute zero.
Distribution of tTMB scores by treatment arm in the tTMB BEP.
Kaplan-Meier analysis of OS for (A) the tTMB <10 mut/Mb subgroup and (B) the tTMB ≥10 mut/Mb subgroup.
Relationship between PD-L1 expression and tTMB score. (A) Box plots showing the distribution of tTMB score in patients by PD-L1 subgroup; (B) Venn diagram showing overlap between the tTMB ≥10 mut/Mb and PD-L1 TC or IC ≥1% subgroups. Percentages are calculated from the PD-L1 and tTMB BEP, i.e., patients who had both a PD-L1 and a tTMB result (n=275); samples from 171 patients had tTMB ≥10 mut/Mb and/or PD-L1 TC or IC ≥1%, while samples from 104 patients (37.8%) had both tTMB <10 mut/Mb and PD-L1 TC and IC <1%.
Baseline patient demographics and disease characteristics for the PD-L1 and tTMB BEPs and the ITT population.
Representativeness of study participants.
Summary of adverse events of any cause in the PD-L1, tTMB, and overall safety populations.
Treatment exposure by PD-L1 subgroup (PD-L1 safety population).
Summary of adverse events of any cause by PD-L1 subgroup (PD-L1 safety population).
Data Availability Statement
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. The AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.