Abstract
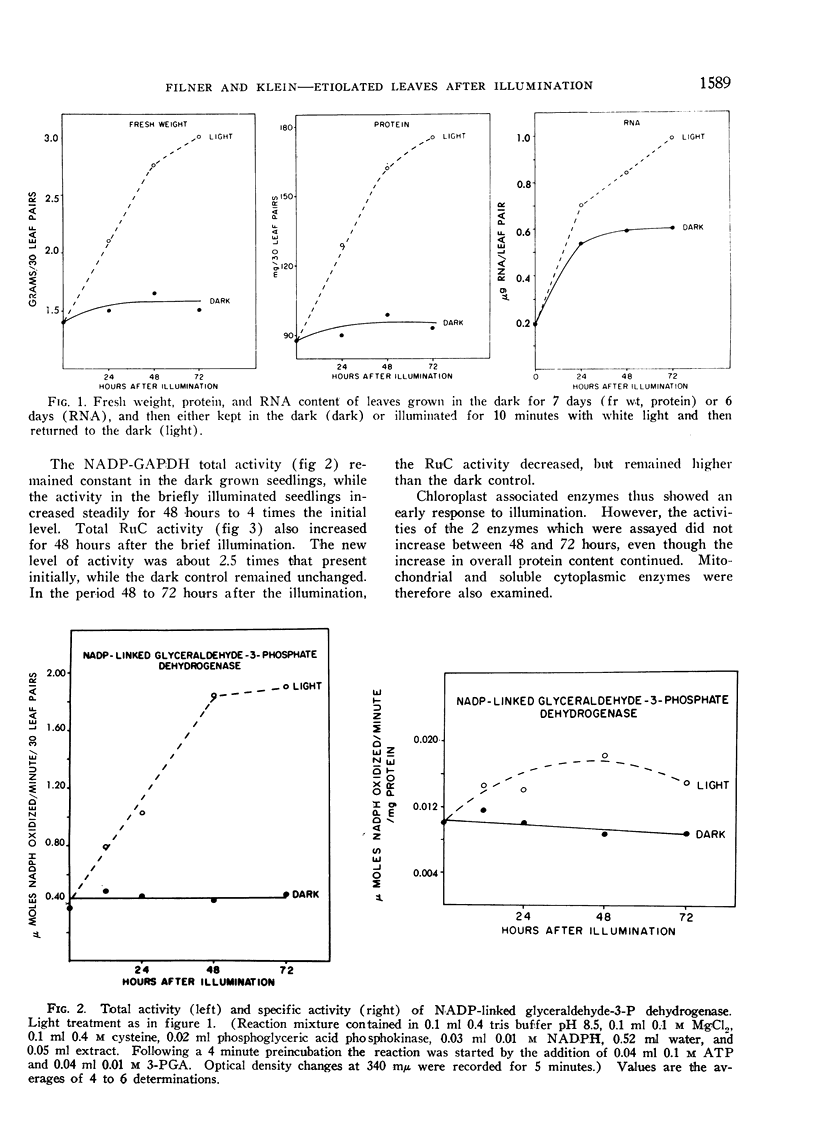
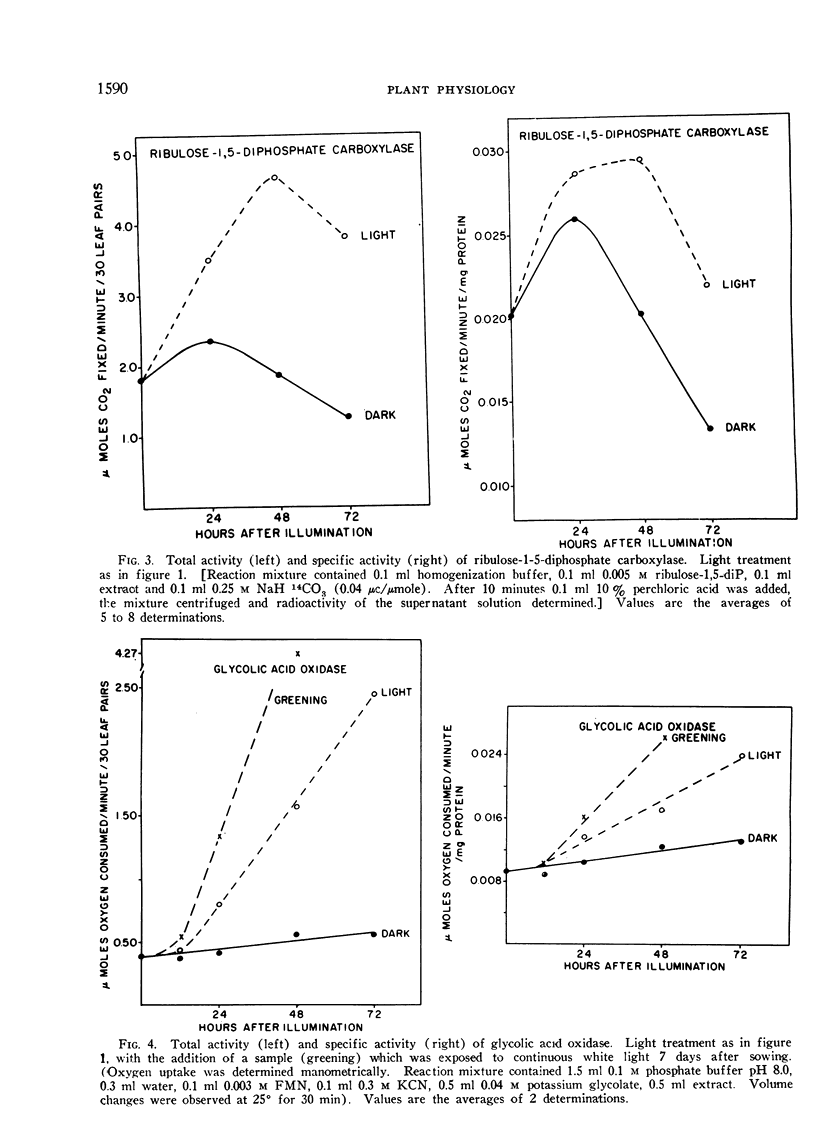
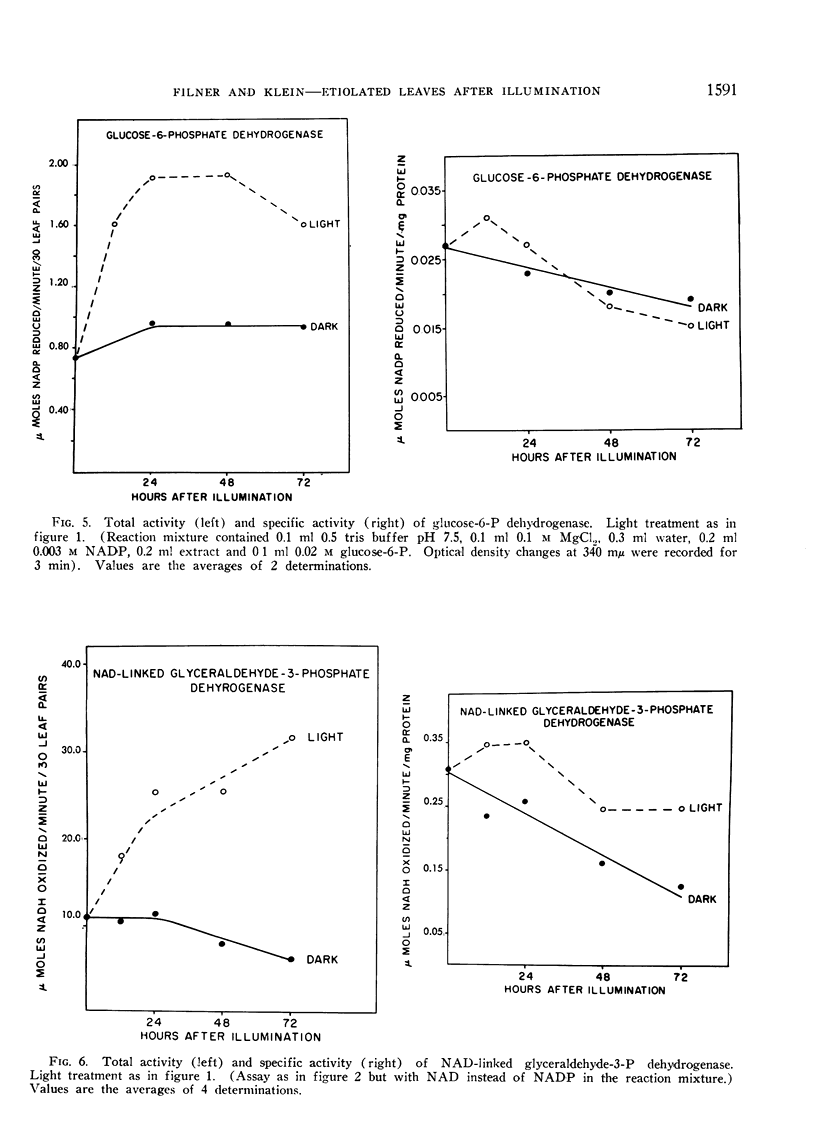
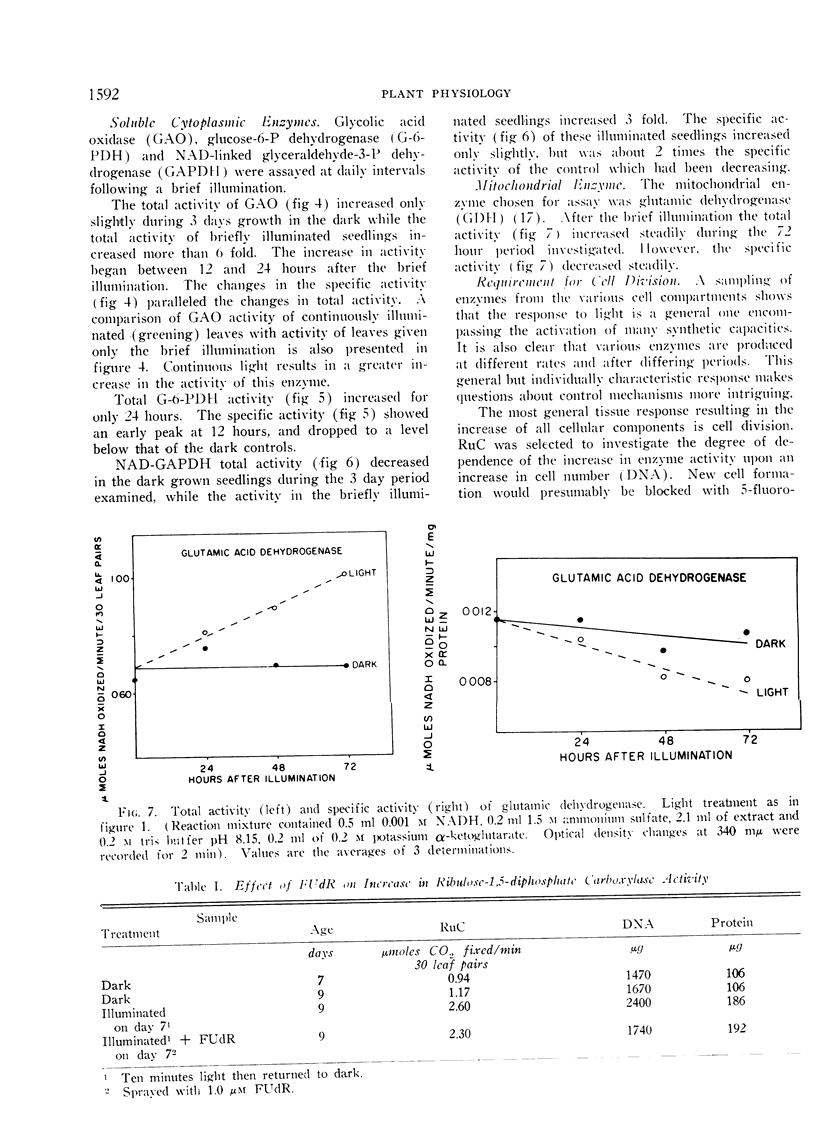
The phytochrome controlled increase in total protein in the primary leaf pair of etiolated bean (Phaseolus vulgaris var. Black Valentine) seedlings, which occurs during growth in the dark subsequent to a brief illumination, was investigated. Enzymes from the chloroplasts, the mitochondria, and the soluble cytoplasm all increase in total activity after the illumination.
The total protein and the ribulose carboxylase increases are not inhibited by FUdR, an inhibitor of DNA synthesis. Cycloheximide, an inhibitor of protein synthesis, applied at a time when the ribulose carboxylase activity increase has already commenced, blocks further increase. It was concluded that the total protein and the enzyme increases in the leaf are the result of increases in the per cell levels.
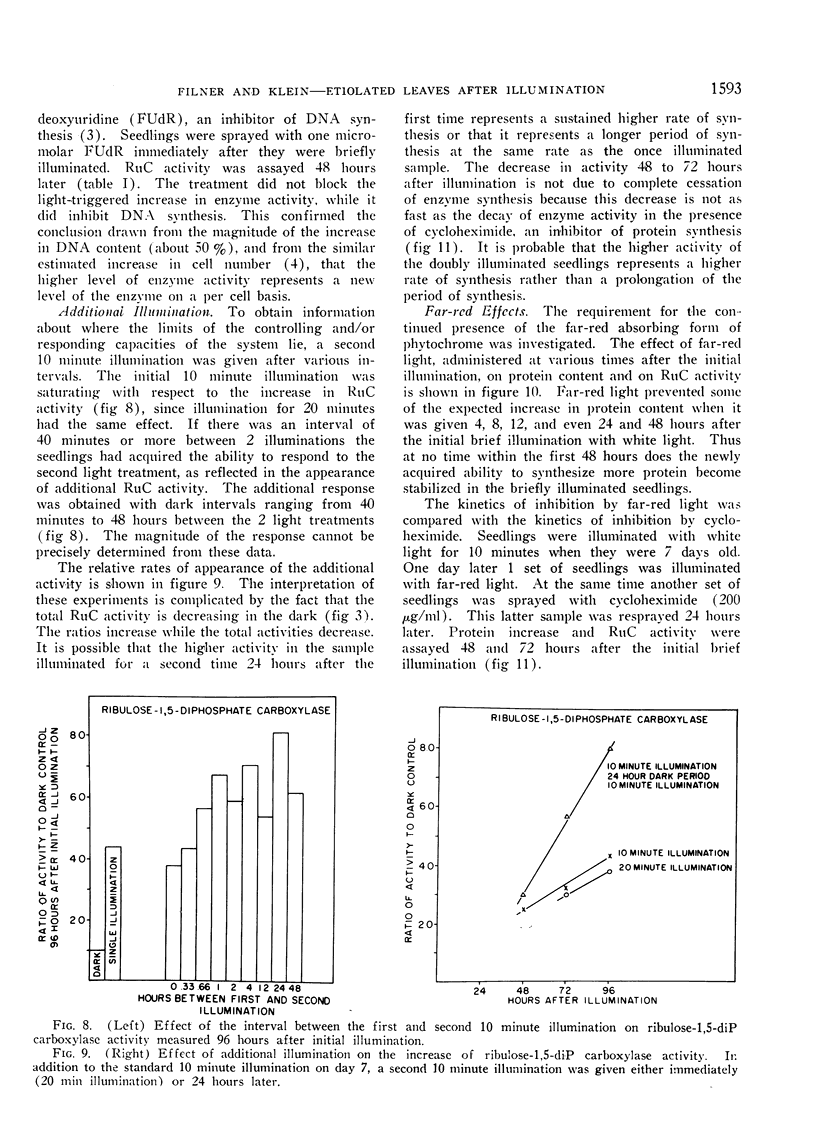
The initial brief illumination is saturating, but 40 minutes later the seedlings have acquired the ability to respond to a second brief illumination. The rate of increase in ribulose carboxylase activity in seedlings that have been illuminated twice is greater than the rate in seedlings that have been illuminated only once.
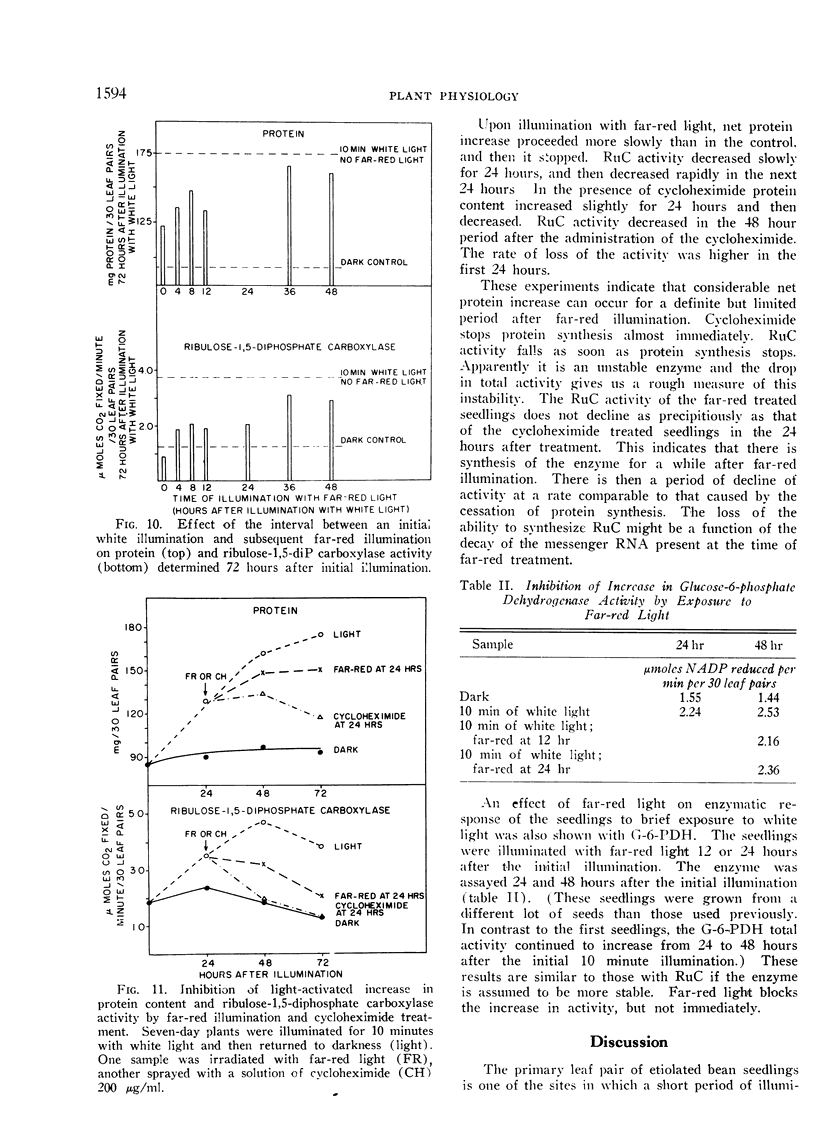
Far-red light prevents further increase in enzyme activity 48 hours after the initial illumination. There is a lag period interposed between the time of illumination with far-red light and the time at which the seedlings show the greatest effect of far-red light. It was concluded that the phytochrome influence on protein synthesis is not at the terminal steps.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULEN W. A. The isolation and characterization of glutamic dehydrogenase from corn leaves. Arch Biochem Biophys. 1956 May;62(1):173–183. doi: 10.1016/0003-9861(56)90100-x. [DOI] [PubMed] [Google Scholar]
- Chen S., McMahon D., Bogorad L. Early effects of illumination on the activity of some photosynthetic enzymes. Plant Physiol. 1967 Jan;42(1):1–5. doi: 10.1104/pp.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Pon N. G., Heber M. Localization of Carboxydismutase & Triosephosphate Dehydrogenases in Chloroplasts. Plant Physiol. 1963 May;38(3):355–360. doi: 10.1104/pp.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W. H., Edwards J. L., Shropshire W. Spectrophotometric Measurements of Phytochrome in vivo and Their Correlation with Photomorphogenic Responses of Phaseolus. Plant Physiol. 1967 Feb;42(2):264–270. doi: 10.1104/pp.42.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W. H., Withrow R. B., Elstad V. B. Response of the Hypocotyl Hook of Bean Seedlings to Radiant Energy and Other Factors. Plant Physiol. 1956 Jul;31(4):289–294. doi: 10.1104/pp.31.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEGO J. L., JAGENDORF A. T. Effect of light on growth of Black Valentine bean plastids. Biochim Biophys Acta. 1961 Oct 28;53:237–254. doi: 10.1016/0006-3002(61)90437-1. [DOI] [PubMed] [Google Scholar]
- Marcus A. Photocontrol of Formation of Red Kidney Bean Leaf Triphosphopyridine Nucleotide Linked Triosephosphate Dehydrogenase. Plant Physiol. 1960 Jan;35(1):126–128. doi: 10.1104/pp.35.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLBERT N. E., BURRIS R. H. Light activation of the plant enzyme which oxidizes glycolic acid. J Biol Chem. 1950 Oct;186(2):791–804. [PubMed] [Google Scholar]


