Abstract
Background:
Coronary artery calcium (CAC) and left ventricular diastolic dysfunction (LVDD) are strong predictors of cardiovascular events and share common risk factors. However, their independent association remains unclear.
Methods:
In the Project Baseline Health Study (PBHS), 2082 participants underwent cardiac-gated, non-contrast chest computed tomography (CT) and echocardiography. The association between left ventricular (LV) diastolic function and CAC was assessed using multidimensional network and multivariable-adjusted regression analyses. Multivariable analysis was conducted on continuous LV diastolic parameters and categorical classification of LVDD and adjusted for traditional cardiometabolic risk factors. LVDD was defined using reference limits from a low-risk reference group without established cardiovascular disease, cardiovascular risk factors or evidence of CAC, (n = 560). We also classified LVDD using the American Society of Echocardiography recommendations.
Results:
The mean age of the participants was 51 17 years with 56.6% female and 62.6% non-Hispanic White. Overall, 38.1% had hypertension; 13.7% had diabetes; and 39.9% had CAC >0. An intertwined network was observed between diastolic parameters, CAC score, age, LV mass index, and pulse pressure. In the multivariable-adjusted analysis, e’, E/e’, and LV mass index were independently associated with CAC after adjustment for traditional risk factors. For both e’ and E/e’, the effect size and statistical significance were higher across increasing CAC tertiles. Other independent correlates of e’ and E/e’ included age, female sex, Black race, height, weight, pulse pressure, hemoglobin A1C, and HDL cholesterol. The independent association with CAC was confirmed using categorical analysis of LVDD, which occurred in 554 participants (26.6%) using population-derived thresholds.
Conclusion:
In the PBHS study, the subclinical coronary atherosclerotic disease burden detected using CAC scoring was independently associated with diastolic function.
Keywords: Coronary artery calcium, Left ventricular diastolic dysfunction, Heart failure, Coronary artery disease, Cardiovascular disease, Blood pressure, Hypertension
1. Introduction
Left ventricular diastolic dysfunction (LVDD) is common with an estimated prevalence of 25–30%.1–3 Its presence identifies individuals at greater risk of major cardiovascular (CV) events, including CV mortality, heart failure, atrial fibrillation, and atherosclerotic events.1–5 Impairment in active myocardial relaxation or a decrease in chamber distensibility can both contribute to LVDD.6,7 These features may be captured by several echocardiographic parameters, including early and late diastolic peak velocities of mitral inflow (E/A ratio), early diastolic mitral annular velocities (e’), the mitral inflow to annular velocity ratio (E/e’), left atrial volume index (LAVI), and surrogates of pulmonary hypertension, such as maximal tricuspid regurgitation velocities (TRVmax).3,8 Among diastolic parameters, e’ reflects the velocity of early myocardial relaxation while E/e’ is considered a surrogate for ventricular filling pressures.8
Coronary artery disease (CAD) represents one of the major causes of LVDD along with systemic hypertension and diabetes mellitus.9 In recent years, there has been great interest to better understand the relationship between LVDD and subclinical CAD. In the Coronary Artery Risk Development in Young Adults (CARDIA) study, Yared et al. found independent associations between diastolic function and coronary artery calcium (CAC) score.10,11 In contrast, in the Framingham Heart study by Castro-Diehl et al., the association between LVDD and CAC did not persist after multivariable adjustment.12 One challenge, however, in studying the association between LVDD and CAC is that they share several common risk factors. In recent years, different tools have been developed to better visualize multidimensional associations between these risk factors and subclinical phenotypes; among them, network graphs have emerged as particularly useful in clinical and translational medicine.13–15
In this Project Baseline Health Study (PBHS) analysis, we sought to determine the association between LV diastolic function and CAC using both network graphs and multivariable regression analysis. Our first objective was to understand the multidimensional relationship between LV diastolic function, CAC, and traditional cardiometabolic risk factors using network graphs. We then analyzed the relationship between LV diastolic function and CAC using multivariable regression analysis. This analysis was applied to both continuous parameters of LV diastolic function (E/A, e’, E/e’, LAVI, and LV mass index [LVMI]) and categorical classification of LVDD.
2. Methods
2.1. The Project Baseline Health Study
The PBHS design and data collection have been previously described.16 The PBHS study has completed intensive measurement of 2502 individuals who were enrolled through a virtual online registry and selected to produce a diverse cohort. The sampling method was designed to over-represent people at risk of heart disease or cancer. The study was conducted at Stanford University (Stanford, California), Duke University (Durham, North Carolina), and the California Health and Longevity Institute (Westlake Village, California) with enrolling sites in Durham, North Carolina; Kannapolis, North Carolina; Los Angeles, California; and Palo Alto, California. The substudies have a governance approach, and the academic researchers have scientific independence for data analysis. The study was approved by the Institutional Review Boards at Stanford University and Duke University, and participants gave written informed consent for participating.
Of the 2502 participants enrolled, 2082 underwent both non-contrast computed tomography (CT) for CAC score and resting echocardiography and represent the sample population for this substudy (Fig. 1A). We defined a low-risk reference group to derive limits of reference for diastolic parameters (Fig. 1B). Individuals included in the low-risk reference group were (1) free of cardiovascular, pulmonary, or kidney disease; (2) free from major risk factors for atherosclerotic cardiovascular disease (ASCVD); (3) free from enhanced cardiometabolic risk factors; and (4) had no evidence of subclinical CAD defined by a CAC score >0. More specifically, we excluded individuals with established disease based on the following criteria: cardiovascular disease defined as a previous history of myocardial infarction, stroke, heart failure, percutaneous coronary intervention, coronary artery bypass surgery, or atrial fibrillation; asymptomatic reduction in LV ejection fraction (LVEF) < 50%; valvular heart disease (more than moderate valvular regurgitation or any valvular stenosis); pericardial disease; hypertrophic cardiomyopathy; a history of pulmonary hypertension or a right ventricular systolic pressure >35 mmHg; a history of aortic dissection; chronic pulmonary disease; or chronic kidney disease with an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2. For major risk factors, we excluded participants with a diagnosis of systemic hypertension (>140/90 mmHg or anti-hypertensive medication use),2,4,17 a history of diabetes mellitus (type 1 or 2 or HbA1C >6.5%), dyslipidemia defined by a total cholesterol level >240 mg/dL or a low-density lipoprotein (LDL) level >160 mg/dL, and current smoker status. In addition, we excluded participants who had a combination of enhanced metabolic factors: waist ≥102 cm (men) and ≥88 cm (women),18 triglycerides ≥200 mg/dL or high-density lipoprotein (HDL) < 40 mg/dL (men) and <50 mg/dL (women), and prediabetes (HbA1C ≥ 5.7%). Additionally, 10 individuals were excluded after outlier analysis because of a cancer history or the presence of inflammatory disease.
Fig. 1.
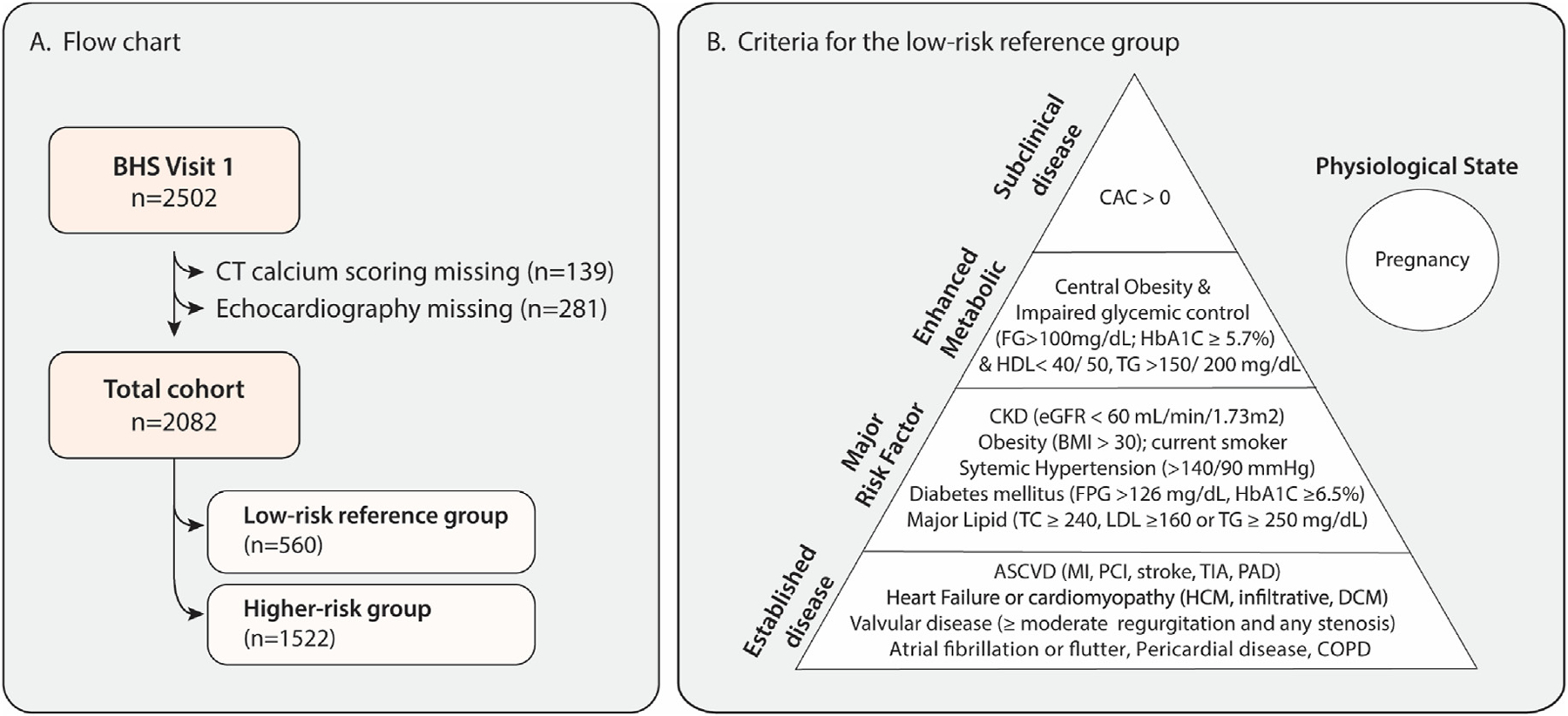
Project Baseline Health Substudy. (A) Patients with available CT coronary calcium score and echocardiography were included in the study. (B) The healthy reference group (n = 560) included participants without evidence of established disease, major cardiovascular risk factors, or metabolic syndrome, and with a CAC score of 0. In addition, pregnant women were excluded. BHS, Baseline Health Study; CAC, coronary artery calcium; CKD, chronic kidney disease; CT, computed tomography; DCM, dilated cardiomyopathy; FG, fasting glucose; FPG, fasting plasma glucose; HCM, hypertrophic cardiomyopathy; TC, total cholesterol; TG, triglycerides.
2.2. Cardiovascular Imaging protocols
Echocardiography was acquired at each site with real-time quality control provided by the Duke Imaging Core Laboratory. Stanford University and Duke University used either Philips EPIQ 7 or iE33 while the California Health & Longevity Institute used General Electric Vivid 7. Images were analyzed for chamber quantification and evaluation of diastolic dysfunction according to the American Society of Echocardiography (ASE) recommendations and core laboratory best practices.8,19,20 The Imaging Core Laboratory personnel performed all measurements and analyses using the Digisonics software platform without knowledge of clinical, laboratory, or other imaging or physiological data. Recorded echocardiographic parameters included LV linear dimensions, wall thickness, biplane end-diastolic and end-systolic volumes, LV mass, relative wall thickness (RWT), E/A ratio, average of septal and lateral e’ velocity, E/e’ ratio, left atrial maximal volumes, TRVmax, estimated right ventricular systolic pressure (RVSP), and aortic root diameters. LV volume was measured using the biplane Simpson method with boundaries traced at the compacted and non-compacted myocardial interface. Left atrial volume (LAV) was measured using the biplane Simpson method. Ventricular and atrial volumes were indexed to body surface area using the Dubois formula.
Coronary calcium was assessed by non-contrast CT of the chest using 64-detector (or greater) CT systems with cardiac gating according to a standardized protocol and read as part of usual clinical care at each institution. The scanners included Siemens (Somatom Definition Edge or Definition Flash) or General Electric (GE Discovery 750HD, GE Light-speed 16, GE VCT). The CAC score was determined using the Agatston method, which registers each calcium lesion scaled by an attenuation factor and summed over all lesions.21
The Core Laboratory reproducibility assessments were performed annually throughout the study and yielded intraclass correlation coefficients (ICCs) of 0.94 for LV end-diastolic volume, 0.96 for LV end-systolic volume, 0.96 for LVEF, 0.99 for LV mass, 0.94 for LA volume, and 0.92 for right ventricular fractional area change. The ICC was 0.95 for CAC score.
2.3. Grading system for LV diastolic dysfunction
In keeping with previous population-based studies, we graded diastolic dysfunction using thresholds derived from a low-risk reference group.1–3 We also graded diastolic dysfunction according to ASE recommendations for the evaluation of left ventricular diastolic function by echocardiography.8
For the population-based thresholds, LVDD was defined using age-adjusted E/A or e’, E/e’ ratio (single threshold), and supporting criteria for diastolic dysfunction.1,3 The 2.5th and 97.5th percentiles were used to define the reference limits. Grade 1 LVDD was defined as the presence of low age-specific E/A or e’ and a normal average E/e’ ratio. Grade 2 LVDD was defined as the presence of a high average E/e’ ratio and at least one supporting criteria for diastolic dysfunction. Isolated E/e’ was not considered significant for diastolic dysfunction. Supporting criteria for grade 2 LVDD include low e’ or E/e’, evidence of concentric remodeling, left ventricular hypertrophy (LVH), left atrial enlargement (LAE), or elevated RVSP. LAE was defined using the 97.5th percentile from the low-risk reference group with LA volume indexed to body surface area or stroke volume. RVSP >30 mmHg or TRVmax >2.5 m/s were used as criteria to define pulmonary hypertension to align with recent guidelines.22,23 LVDD was also graded according to ASE recommendations.8 In the absence of LVH, low LVEF (<50%) or a prior history of cardiac disease, LVDD was graded according to e’ velocity (septal e’ <7 cm/s, lateral e’ <10 cm/s), average E/e’ >14, maximal TR velocity >2.8 cm/s, or LAVI >34 mL/m2. LVDD was present if >50% of criteria were present (assigned grade 2), indeterminate if 50% of criteria were present, and no diastolic dysfunction if <50% of criteria were present. In the presence of structural heart disease or LVEF <50%, grades of diastolic dysfunction were classified according to E/A ratio, E/e’, maximal TR velocity, and LAVI.8
2.4. 10-Year atherosclerotic cardiovascular disease (ASCVD) risk
The 10-year ASCVD risk was calculated based on established equations endorsed by American cardiology societies.18
2.5. Statistical methods
Data were accessed and analyzed through the online Verily Terra platform (https://terra.bio/). We applied the Python 3 environment incorporated in the platform. We first created a multidimensional analysis of LV diastolic function parameters and CAC using network graphs. We then used multivariable regression analysis for the association of continuous diastolic parameters with CAC followed by categorical analysis of LVDD. We used the low-risk reference group to derive limits of reference for diastolic parameters. To account for the skewed distribution, CAC was categorized into 4 groups, ie, CAC = 0 or tertiles when CAC >0. For the analysis, we also categorized CAC groups according to previously published categories of CAC score (0, 1–100, 101–300, and ≥300).24
The continuous parameters are presented as the mean ± standard deviation or the median and interquartile range as appropriate according to their distribution. Categories are presented as the number and percentage. For the multidimensional analysis, we constructed a network from the clinical characteristics, echocardiographic indexes, and CAC score with edges weighted by the maximal information coefficient (MIC). MIC is a measure of association strength that ranges between 0 (statistical independence) and 1 (noise-free relationship).15 MIC was preferred over other association measures given its better generality (eg, ability to capture linear, non-linear, or periodic relationships) and its better equitability (ie, the MIC statistic should give similar scores to equally noisy relationships of different types).15 MIC was calculated for all node pairs using the compute score function from the minepy 1.2.5 library.25 MIC-based networks were visualized with the Fruchterman Reingold layout using Gephi 0.9.2 software. The Fruchterman Reingold layout uses an analogy of physical springs as edges that attract connected vertices toward each other and a competing repulsive force that pushes all vertices away from one another; this usually results in graphs with edges of more or less equal length with as few crossing edges as possible.14 For the continuous multivariable regression analysis, we used a stepwise approach first to analyze the associations between LV diastolic function indexes and traditional risk factors followed by a multivariable adjusted analysis with CAC. Variables considered in the stepwise linear model included age, age,2 sex, race, smoking status, height, weight, pulse pressure, heart rate, diabetes, chronic kidney disease, chronic obstructive pulmonary disease (COPD), a history of cardiovascular disease, type of antihypertensive medication, lipid-lowering drugs, total cholesterol, HDL cholesterol, LDL cholesterol, HbA1c, and triglycerides. The limit for inclusion in the stepwise models was a P value of 0.05. We considered both age and age2 because diastolic parameters do not relate linearly with age, with a steeper change at increasing age. For the categorical LVDD analysis, we first defined limits of reference for diastolic parameters in our healthy reference group followed by a similar multivariable analysis for the continuous parameters. In the low-risk reference group, we determined age-specific percentiles of the LV diastolic indexes from their bootstrap distribution obtained from 1000 random samples.26 Based on these percentiles, we defined limits of reference for low E/A, e’ (2.5th percentile), and high E/e’ (97.5th percentile), and subsequently graded LV diastolic function (normal, isolated high E/e’, grade 1 dysfunction, grade 2 dysfunction) as previously detailed. We also analyzed the correlates of LVDD as defined by ASE criteria. We also compared the prevalence of LVDD according to 10-year ASCVD risk and CAC subgroups. Finally, we conducted a sensitivity analysis based on median age to analyze the potential confounding factor of age.
3. Results
3.1. Study population
The mean age of the 2082 PBHS participants was 50.6 ± 17.0 years with 56.6% female, 62.6% non-Hispanic White, 17.0% Black or African American, and 10.3% Asian (Table 1). At the time of the examination, 14.1% of the participants were active smokers; 38.4% had hypertension with 21.2% on antihypertensive treatment; 13.7% had diabetes mellitus; and 2.9% had known coronary artery disease. The distribution of CAC scores in the population was skewed (Figure S1) with 1251 (60.1%) individuals having a CAC score of 0, 472 (22.7%) having a CAC score between 0 and 100, 199 (9.6%) having a CAC score between 100 and 300, and 160 (7.8%) having a CAC score above or equal to 300.
Table 1.
General characteristics of the study population.
| Characteristics | Entire cohort (n = 2082) | Low-risk reference group (n = 560) |
|---|---|---|
| General clinical data | ||
| Age, y | 50.6 ± 17.0 | 39.2 ± 13.5 |
| Female, n (%) | 1178 (56.6) | 344 (61.4) |
| Body mass index, kg/m2 | 28.4 ± 6.7 | 23.8 ± 2.9 |
| Systolic BP, mm Hg | 123.3 ± 16.2 | 112.8 ± 11.0 |
| Diastolic BP, mm Hg | 75.8 ± 10.0 | 70.9 ± 7.6 |
| Heart rate, bpm | 67.1 ± 11.5 | 65.5 ± 10.3 |
| Race | ||
| White, n (%) | 1303 (62.6) | 331 (59.1) |
| Black or African American, n (%) | 354 (17.0) | 97 (9.1) |
| Asian, n (%) | 214 (10.3) | 67 (17.3) |
| Other, n (%) | 211 (10.1) | 65 (14.4) |
| Questionnaire and medical history | ||
| Current smoker, n (%) | 293 (14.1) | – |
| Hypertension, n (%) | 799 (38.4) | – |
| Diabetes mellitus type 2, n (%) | 276 (13.7) | – |
| History of CAD, n (%) | 60 (2.9) | – |
| Medication history at enrollment | ||
| Antihypertensive treatment, n (%) | 441 (21.2) | – |
| Beta-blockers | 116 (5.6) | – |
| ACE-I or ARB | 265 (12.7) | – |
| Diuretics | 148 (7.1) | – |
| Anti-diabetic agents | 165 (7.9) | – |
| Lipid-lowering agents | 340 (16.3) | – |
| Statin therapy | 299 (14.4) | – |
| Biochemistry | ||
| HbA1c, % | 5.67 ± 0.98 | 5.25 ± 0.35 |
| Total cholesterol, mmol/L | 4.78 ± 1.00 | 4.62 ± 0.77 |
| LDL cholesterol, mmol/L | 2.58 ± 0.85 | 2.46 ± 0.64 |
| HDL cholesterol, mmol/L | 1.52 ± 0.50 | 1.66 ± 0.47 |
| Triglycerides, mmol/L | 1.20 (0.66–2.71) | 0.98 (0.56–2.20) |
| eGFR, mL/min/1.73 m2 | 87.8 ± 19.6 | 92.8 ± 16.7 |
| Conventional echocardiography | ||
| LV mass index, g/m2 | 70.0 ± 17.1 | 64.9 ± 15.5 |
| Relative wall thickness | 0.37 ± 0.08 | 0.34 ± 0.06 |
| LV ejection fraction, % | 58.7 ± 4.0 | 58.4 ± 2.9 |
| LV diastolic function | ||
| E/A ratio | 1.28 ± 0.50 | 1.59 ± 0.51 |
| e’ septal-lateral, cm/s | 10.3 ± 3.1 | 12.8 ± 2.7 |
| E/e’ ratio | 8.04 ± 2.72 | 6.43 ± 1.46 |
| LA volume index, mL/m2 | 28.0 ± 7.5 | 27.0 ± 5.86 |
| Coronary artery calcium | ||
| CAC score = 0, n (%) | 1251 (60.1) | 560 (100) |
| CAC score 0–100, n (%) | 472 (22.7) | – |
| CAC score 100–300, n (%) | 199 (9.6) | – |
| CAC score ≥300, n (%) | 160 (7.8) | – |
Values are mean ± SD, number of subjects (%), or median (10–90 percentile). ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BP, blood pressure; CAC, coronary artery calcium; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; LA, left atrial; LV, left ventricular.
The low-risk reference group included 560 (27%) participants. Compared with the entire cohort, the low-risk reference sample was younger (39.2 ± 13.5 vs. 50.6 ± 17.0 years, P < 0.001), had a slightly higher percentage of women (61.4% vs. 56.6%, P = 0.04), and was free of cardiovascular disease and major risk factors by definition and had a CAC score = 0 (Table 1). The low-risk reference group had multiethnic and multiracial representation, although the proportion of Black participants was lower, whereas the proportion of Asian participants was higher (P = 0.03).
3.2. Multidimensional network between diastolic parameters, CAC, and clinical variables
To better visualize the relationship between diastolic parameters, CAC, and traditional risk factors, we constructed a network graph weighted by the MIC where relationships with MIC >0.10 are shown (Fig. 2). In addition, CAC score and its neighboring nodes (MIC >0.15) are marked in red. A network graph allows for visualization of pairwise associations between variables. The different features or nodes are positioned near neighbors that share similar connections. In addition, we weighted connections by the strength of their associations (MIC). Compared with a correlation matrix, network graphs allow for easier visualization of complex relationships where one can focus on subnetworks of interest. The MIC roughly equals the coefficient of determination (R2) relative to each respective noiseless function; for linear relationships, MIC >0.1 would correspond approximately to a correlation >0.32, and MIC >0.15 would correspond approximately to a correlation >0.40.
Fig. 2.
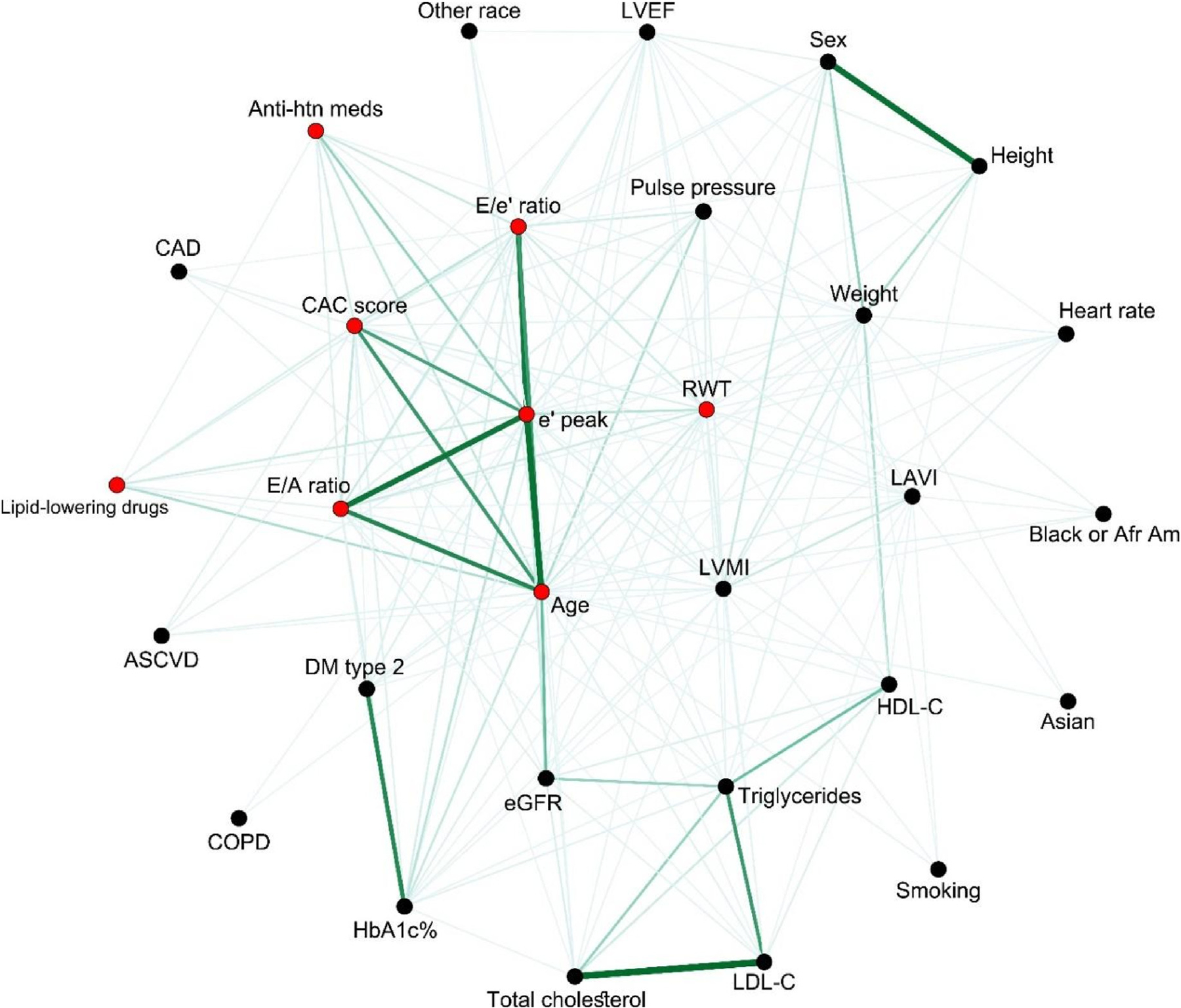
Multidimensional clinical network, including echocardiographic parameters and CAC score. Edges with a Maximal Information Coefficient (MIC) above 0.10 are shown. Thicker and darker lines imply higher MIC. CAC score and its neighboring nodes (MIC above 0.15) are marked in red. ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcium; COPD, chronic pulmonary obstructive disease; DM, diabetes mellitus; E, mitral inflow velocity; E/A ratio, early and late diastolic peak velocities of mitral inflow; E/e’, mitral inflow to annular velocity ratio; e’, early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein-C; LAVI, left atrial volume index; LDL-C, low-density lipoprotein-C; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; RWT, relative wall thickness. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
A first observation from the network is that CAC, e’, E/e’, E/A, and age are closely related to each other and have stronger weighted relationships. This highlights the relationship between CAC and diastolic parameters, especially with e’ velocity. Second, the topology of the network is also informative. In fact, traditional risk factors, such as systemic hypertension, pulse pressure, diabetes mellitus, HbA1C, dyslipidemia (treated), and triglycerides, were connected to both CAC and diastolic parameters. This finding suggests that in order to identify independent relationships between CAC and diastolic parameters, a wide range of CAC scores needs to be present. Third, the central position of e’ velocity also hints to potential stronger independent relationships with traditional risk factors, as well as demographic and anthropomorphic measures.
3.3. Multivariable regression analysis of LV diastolic function
We first analyzed the multivariable-adjusted association between diastolic parameters and traditional risk factors (Table 2). The coefficient of determination was strongest for e’ velocity (67%) followed by E/e’ (44%), E/A (41%), and LAVI (18%). Both e’ and E/e’ parameters shared several significant covariables, including age, Black and Asian race (negative effect size for e’ and positive for E/e’), body height and weight, pulse pressure, HbA1C, and lipid parameters. Female sex was primarily associated with E/e’ velocity while heart rate and eGFR were independently associated with LAVI. We then analyzed the multivariable-adjusted association with CAC groups (CAC =0 and tertiles) (Table 3). Among the four diastolic parameters, e’ velocity and E/e’ ratio were independently associated with CAC groups with an effect size that was higher with increasing CAC tertiles. In contrast, no significant relationship was found with E/A ratio or LAVI. When categorized according to CAC score thresholds (0, <100, 100–300, and ≥300), an independent association was also noted with e’ and E/e’ in the groups with a CAC score between 1 and 100 and ≥ 300 (Table S1). Among other echocardiographic variables, independent associations were also observed between CAC and LVMI, RWT, and aortic root size, but only the association with LVMI would withstand significance after accounting for multiple comparisons (Table S2).
Table 2.
Multivariable-adjusted association between LV diastolic function indexes and traditional risk factors.
| Correlate | Average e’ (cm/s) |
Average E/e’ ratio |
E/A ratio |
LA volume index (mL/m2) |
||||
|---|---|---|---|---|---|---|---|---|
| Effect size ± SE | P value | Effect size ± SE | P value | Effect size ± SE | P value | Effect size ± SE | P value | |
| Age, +15 years | −0.23 ± 0.015 | <0.0001 | −0.58 ± 0.045 | <0.0001 | ||||
| Age2 +15 years | 0.20 ± 0.090 | <0.0001 | 0.14 ± 0.010 | <0.0001 | 0.045 ± 0.007 | <0.0001 | 1.80 ± 0.23 | <0.0001 |
| Female sex | 0.66 ± 0.13 | <0.0001 | ||||||
| Asian | −0.55 ± 0.14 | <0.0001 | 0.50 ± 0.16 | 0.002 | −0.071 ± 0.029 | 0.013 | ||
| Black or African American | −0.56 ± 0.11 | <0.0001 | 0.27 ± 0.13 | 0.032 | 1.53 ± 0.41 | <0.0001 | ||
| Body height, + 10 cm | 0.32 ± 0.052 | <0.0001 | −0.38 ± 0.071 | <0.0001 | 0.041 ± 0.010 | <0.0001 | ||
| Body weight, + 20 kg | −0.51 ± 0.043 | <0.0001 | 0.41 ± 0.065 | <0.0001 | −0.072 ± 0.002 | <0.0001 | ||
| Pulse pressure, +10 mmHg | −0.13 ± 0.046 | 0.003 | 0.46 ± 0.058 | <0.0001 | 0.91 ± 0.16 | <0.0001 | ||
| Heart rate, +10 bpm | −1.56 ± 0.14 | <0.0001 | ||||||
| Use of beta blockers | −0.64 ± 0.18 | <0.0001 | 1.65 ± 0.70 | 0.018 | ||||
| Use of diuretics | 0.80 ± 0.18 | <0.0001 | −1.25 ± 0.62 | 0.043 | ||||
| Use of ACE inhibitors | −0.079 ± 0.036 | 0.028 | ||||||
| Cardiovascular disease | 0.10 ± 0.034 | 0.003 | 2.64 ± 0.63 | <0.0001 | ||||
| COPD | −1.87 ± 0.88 | 0.033 | ||||||
| HbA1c, +1% | −0.16 ± 0.044 | <0.0001 | 0.28 ± 0.050 | <0.0001 | −0.021 ± 0.010 | 0.023 | −0.58 ± 0.17 | 0.001 |
| Total cholesterol, +1 mmol/L | −0.14 ± 0.046 | 0.002 | ||||||
| LDL-C, +0.85 mmol/L | −0.020 ± 0.002 | <0.0001 | ||||||
| Triglycerides, per doubling | −0.10 ± 0.019 | <0.0001 | −0.015 ± 0.004 | 0.018 | ||||
| eGFR, +20 mL/min/1.73 m2 | 0.40 ± 0.18 | 0.024 | ||||||
Average e’ velocity represents the average of septal and lateral mitral annular velocity; average E/e’ ratio represents the early maximal inflow velocity divided by the average e’ velocity. Empty cells represent parameters not selected in the models or not applicable (such as height and weight for indexed LA volume). Adjusted R2 was 41.1% for E/A, 67.3% for e’, 44.1% for E/e’, and 18.1% for LA volume index. Variables considered included age, age2, sex, race, smoking status (never smoker, past smoker and current smoker), body height and weight, pulse pressure, heart rate, diabetes, chronic kidney disease, COPD, eGFR, cardiovascular disease, type of anti-hypertensive medication (beta-blockers, diuretics, calcium channel blockers, ACE inhibitors, angiotensin receptor blocker and alpha antagonists), lipid-lowering drugs, total cholesterol, HDL cholesterol, LDL cholesterol, HbA1c and triglycerides. COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate.
Table 3.
Multivariable-adjusted association between LV diastolic function indexes and CAC score.
| CAC group | Average e’ (cm/s) |
Average E/e’ ratio |
E/A ratio |
LAVI |
||||
|---|---|---|---|---|---|---|---|---|
| Effect size ± SE | P value | Effect size ± SE | P value | Effect size ± SE | P value | Effect size ± SE | P value | |
| CAC = 0 (n = 1251) | reference | reference | ||||||
| CAC >0 and ≤ 17.5 (n = 277) | −0.33 ± 0.12 | 0.008 | 0.17 ± 0.14 | 0.23 | −0.025 ± 0.026 | 0.35 | 0.84 ± 0.47 | 0.073 |
| CAC >17.5 and ≤ 176 (n = 277) | −0.44 ± 0.13 | 0.002 | 0.46 ± 0.15 | 0.003 | –0.016 ± 0.029 | 0.59 | 0.32 ± 0.51 | 0.52 |
| CAC >176 (n = 277) | −0.52 ± 0.15 | <0.0001 | 0.86 ± 0.17 | <0.0001 | −0.030 ± 0.032 | 0.36 | 0.21 ± 0.57 | 0.72 |
The subgrouping of individuals with CAC >0 was based on CAC tertile limits. Effect sizes were adjusted for clinical correlates identified in stepwise linear regression. The co-variables included for e’ included age, age,2 Asian or Black race, body height and weight, pulse pressure, use of beta blockers, HbA1C, and triglycerides. For E/e’, these included age,2 female sex, Asian or Black race, body height and weight, pulse pressure, use of diuretics, HbA1C and total cholesterol. For E/A ratio, the co-variables included age, age,2 Asian race, body height and weight, use of ACE inhibitors, cardiovascular disease, HbA1C, LDL-C and triglycerides. For LAVI, the co-variables included age,2 Black race, pulse pressure, heart rate, use of beta blockers, use of diuretics, cardiovascular disease, COPD, HbA1C, and eGFR. CAC, coronary artery calcium; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; LAVI, left atrial volume index; SE, standard error.
3.4. Multivariable analysis of LVDD with traditional risk factors and CAC
3.4.1. Analysis of LVDD using PBHS population-based thresholds
Prior to the analysis of LVDD and CAC score, we defined thresholds based on diastolic parameters in the low-risk subgroup. LV diastolic parameters, including E/A, e’, and E/e’, changed non-linearly with age. Age-specific percentiles for LV diastolic parameters are presented in Fig. 3 and S2. To establish limits of reference, we used the 2.5th percentile for E/A ratio and e’ and the 97.5th percentile for E/e’.26,27 The threshold for E/e’ in the entire group was 9.95 (includes 10 in its confidence interval [CI]). The threshold for LAVI in the entire population was 41.7 mL/m2 (approximated to 42 mL/m2) while the threshold for LA volume scaled to stroke volume was 1.0.
Fig. 3.
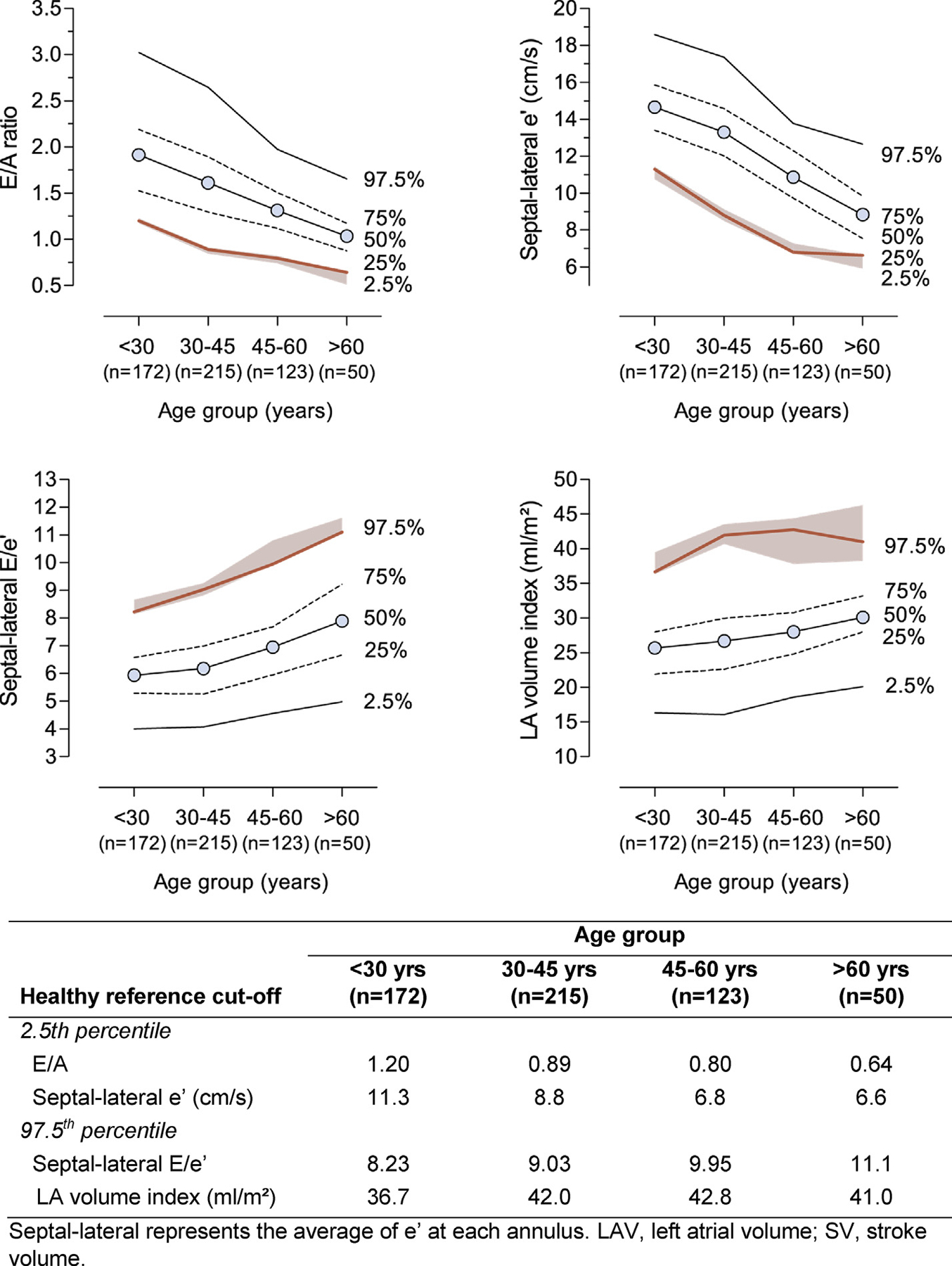
Age-specific percentiles of left ventricular diastolic function indexes from 560 low-risk reference group participants. The shaded area represents 95% confidence intervals of the 2.5% or 97.5% thresholds (red line) as derived from their Bootstrap distributions. LAV, left atrial volume; SV, stroke volume. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
LVDD (PBHS criteria) was observed in 554 individuals (26.6%) with 233 (11.2%) having grade 1 LVDD and 321 (15.4%) having grade 2 LVDD. Isolated E/e’ was observed in 78 participants (3.7%) (Table S3). Participants with LVDD were significantly older, had a higher body mass index, higher systolic and diastolic blood pressure, a higher prevalence of hypertension and diabetes mellitus, higher HbA1C, lower HDL cholesterol, higher triglycerides, lower eGFR, and a higher CAC score (Table S4).
The multivariable analysis with traditional risk scores is summarized in Fig. 4. Grade 2 LVDD was independently associated with older age, female sex, Black race, lower height, higher body weight, higher pulse pressure, higher HbA1C, lower HDL-C, and prevalent CAD (P < 0.05 for all). Unadjusted, age- and sex-adjusted, and fully adjusted models between LVDD and CAC tertiles are presented in Table S5. In the fully adjusted model, the odds for LVDD were significantly higher in the low and high CAC tertiles (P ≤ 0.006) but not in the intermediate CAC tertile (P ≥ 0.25) compared with individuals who had CAC = 0. While the intermediate CAC tertile group had a higher prevalence of LVDD, the older age and higher risk factor profile likely captured the increased risk (Table 4). Similar findings were observed when LVDD was categorized according to CAC score (0, 1–100, 101–300, and ≥300) as shown in Table S6.
Fig. 4.
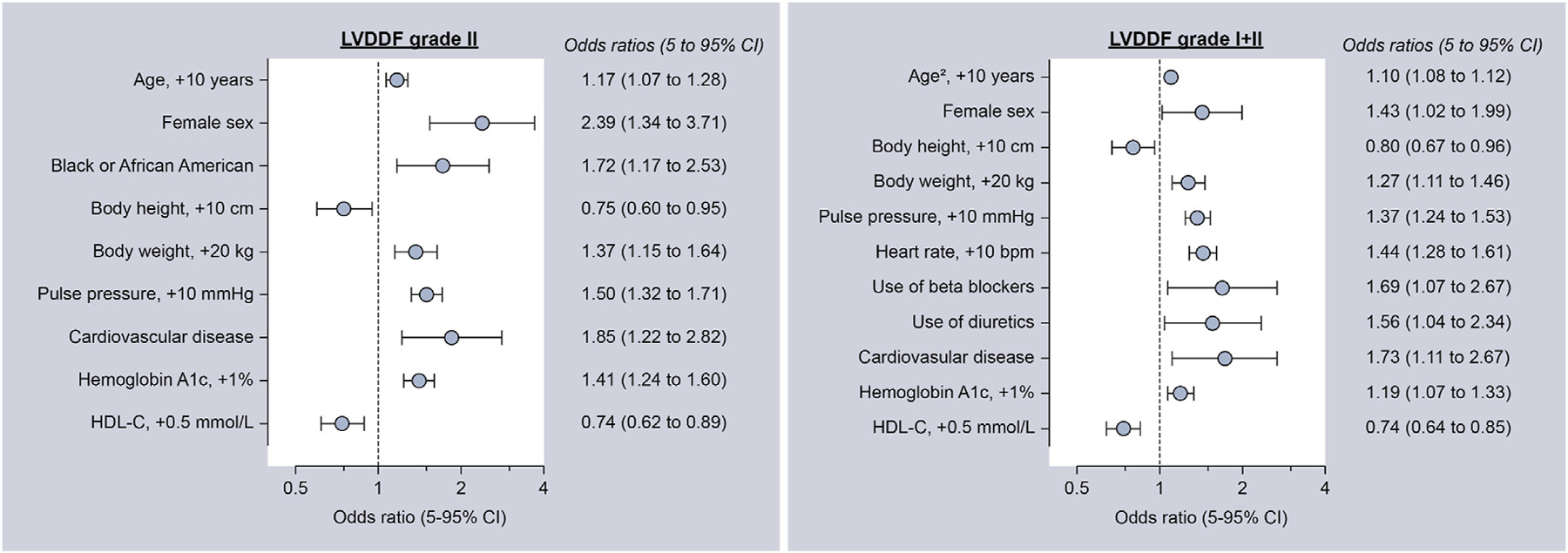
Clinical correlates of left ventricular diastolic dysfunction. Variables considered included age, age,2 sex, race, smoking status (never smoker, past smoker, and current smoker), body height and weight, pulse pressure, heart rate, diabetes, chronic kidney disease, COPD, eGFR, cardiovascular disease, type of antihypertensive medication (beta-blockers, diuretics, calcium channel blockers, ACE inhibitors, angiotensin receptor blocker, and alpha antagonists), lipid-lowering drugs, total cholesterol, HDL cholesterol, LDL cholesterol, HbA1c and triglycerides. ACE, angiotensin-converting-enzyme; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LVDDF, left ventricular diastolic dysfunction.
Table 4.
General characteristics of the study population by CAC score.
| Characteristics | CAC = 0 (n = 1251) | CAC 0–17.5 (n = 277) | CAC 17.5–176 (n = 277) | CAC >176 (n = 277) |
|---|---|---|---|---|
| General clinical data | ||||
| Age, y | 43.5 ± 14.7 | 51.7 ± 15.0 | 63.0 ± 11.1 | 69.3 ± 10.1 |
| Female, n (%) | 788 (63.0) | 162 (58.5) | 131 (47.3) | 97 (35.0) |
| Body mass index, kg/m2 | 27.4 ± 6.4 | 30.8 ± 7.6 | 28.9 ± 6.0 | 29.8 ± 6.5 |
| Systolic BP, mm Hg | 119.0 ± 14.7 | 126.8 ± 14.0 | 130.5 ± 16.3 | 132.0 ± 17.4 |
| Diastolic BP, mm Hg | 74.4 ± 9.8 | 79.2 ± 9.9 | 78.5 ± 9.8 | 75.6 ± 9.7 |
| Heart rate, bpm | 67.4 ± 11.2 | 68.7 ± 11.8 | 65.9 ± 12.0 | 65.8 ± 11.8 |
| Race | ||||
| White, n (%) | 705 (56.4) | 179 (64.6) | 196 (70.8) | 223 (80.5) |
| Black or African American, n (%) | 221 (17.7) | 52 (18.8) | 50 (18.1) | 31 (11.2) |
| Asian, n (%) | 159 (12.7) | 18 (6.5) | 23 (8.3) | 14 (5.1) |
| Other, n (%) | 166 (13.3) | 28 (10.1) | 8 (2.9) | 9 (3.2) |
| Questionnaire and medical history | ||||
| Current smoker, n (%) | 158 (12.6) | 37 (13.4) | 50 (18.1) | 48 (17.3) |
| Hypertension, n (%) | 303 (24.2) | 116 (41.9) | 169 (61.0) | 211 (76.2) |
| Antihypertensive treatment, n (%) | 136 (10.9) | 66 (23.8) | 96 (34.7) | 143 (51.6) |
| Diabetes mellitus type 2, n (%) | 96 (7.7) | 39 (14.1) | 67 (24.2) | 74 (26.7) |
| History of CHD, n (%) | 4 (0.32) | 2 (0.72) | 11 (4.0) | 43 (15.5) |
| Biochemistry | ||||
| HbA1c, % | 5.50 ± 0.86 | 5.75 ± 0.92 | 5.95 ± 1.11 | 6.07 ± 1.16 |
| Total cholesterol, mmol/L | 4.81 ± 0.95 | 4.95 ± 1.03 | 4.82 ± 1.05 | 4.40 ± 1.09 |
| HDL cholesterol, mmol/L | 1.57 ± 0.48 | 1.47 ± 0.48 | 1.51 ± 0.51 | 1.39 ± 0.47 |
| Triglycerides, mmol/L | 1.14 (0.62–2.74) | 1.52 (0.72–3.11) | 1.34 (0.73–4.16) | 1.38 (0.74–3.30) |
| eGFR, ml/min/1.73 m2 | 91.0 ± 19.4 | 89.8 ± 19.8 | 81.2 ± 17.5 | 78.1 ± 17.6 |
| LV diastolic function | ||||
| E/A ratio | 1.42 ± 0.50 | 1.20 ± 0.42 | 1.05 ± 0.41 | 0.98 ± 0.42 |
| e’ septal-lateral, cm/s | 11.5 ± 3.0 | 9.7 ± 2.8 | 8.2 ± 1.8 | 7.4 ± 1.8 |
| E/e’ ratio | 7.2 ± 2.1 | 8.2 ± 2.5 | 9.3 ± 2.6 | 10.3 ± 3.5 |
| LA volume index, mL/m2 | 27.3 ± 6.8 | 27.1 ± 6.7 | 29.2 ± 9.1 | 30.7 ± 8.6 |
| Grade I LVDD, n (%) | 85 (6.8) | 58 (20.9) | 65 (23.5) | 113 (40.8) |
| Grade I and 2 LVDD, n (%) | 202 (16.1) | 92 (33.2) | 103 (37.2) | 157 (56.7) |
Values are mean ± SD, number of subjects (%) or median (10–90 percentile). BP, blood pressure; CAC, coronary artery calcification; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; LA, left atrial; LV, left ventricular; LVDD, left ventricular diastolic dysfunction.
3.4.2. Analysis of LVDD based on ASE classification of diastolic dysfunction
LVDD according to ASE criteria was present in 252 individuals (12.1%) with a majority of patients having grade I LVDD (76.6%) while indeterminate LVDD was present in 147 individuals (7.1%). As expected, LVDD was more common using PBHS criteria, which uses age-specific E/A and e’ (Table S7). Diastolic dysfunction according to both ASE and PBHS criteria was observed in participants with isolated LVH and young individuals with LVH and high E/A ratios.
A strong association was observed between CAC score (classified according to tertile or literature-based CAC thresholds) and LVDD according to ASE criteria when all grades of dysfunction were considered (Tables S8 and S9). In the unadjusted and age- and sex-adjusted models, the odds ratio increased with a higher CAC score.
3.5. Age-stratified analysis
To further account for the potential confounding effect of age, we performed an age-stratified analysis according to the median age of the cohort (50 years). An association between e’, E/e’, and LVDD (PBHS criteria) with CAC tertile was only observed in individuals >50 years of age (Tables S10 and S11). We used CAC tertiles to account for the skewed distribution of CAC scores and in view of the low prevalence of a CAC score >100 in younger individuals.
3.6. Association between ASCVD risk, CAC, and LVDD
The 10-year ASCVD median risk using the pooled cohort equations was 2.69% [95% CI: 0.66–9.36]. As shown in Fig. 5, the prevalence of grade 2 LVDD was higher across increasing CAC score and increasing 10-year ASCVD risk (P < 0.001 for the frequency trends in the groups with LVDD grade 2 and increasing CAC score and/or ASCVD risk). In individuals with CAC >0 and a 10-year ASCVD risk above the median, the prevalence of grade 1 and 2 LVDD was similar to that in individuals with a previous history of cardiovascular disease.
Fig. 5.
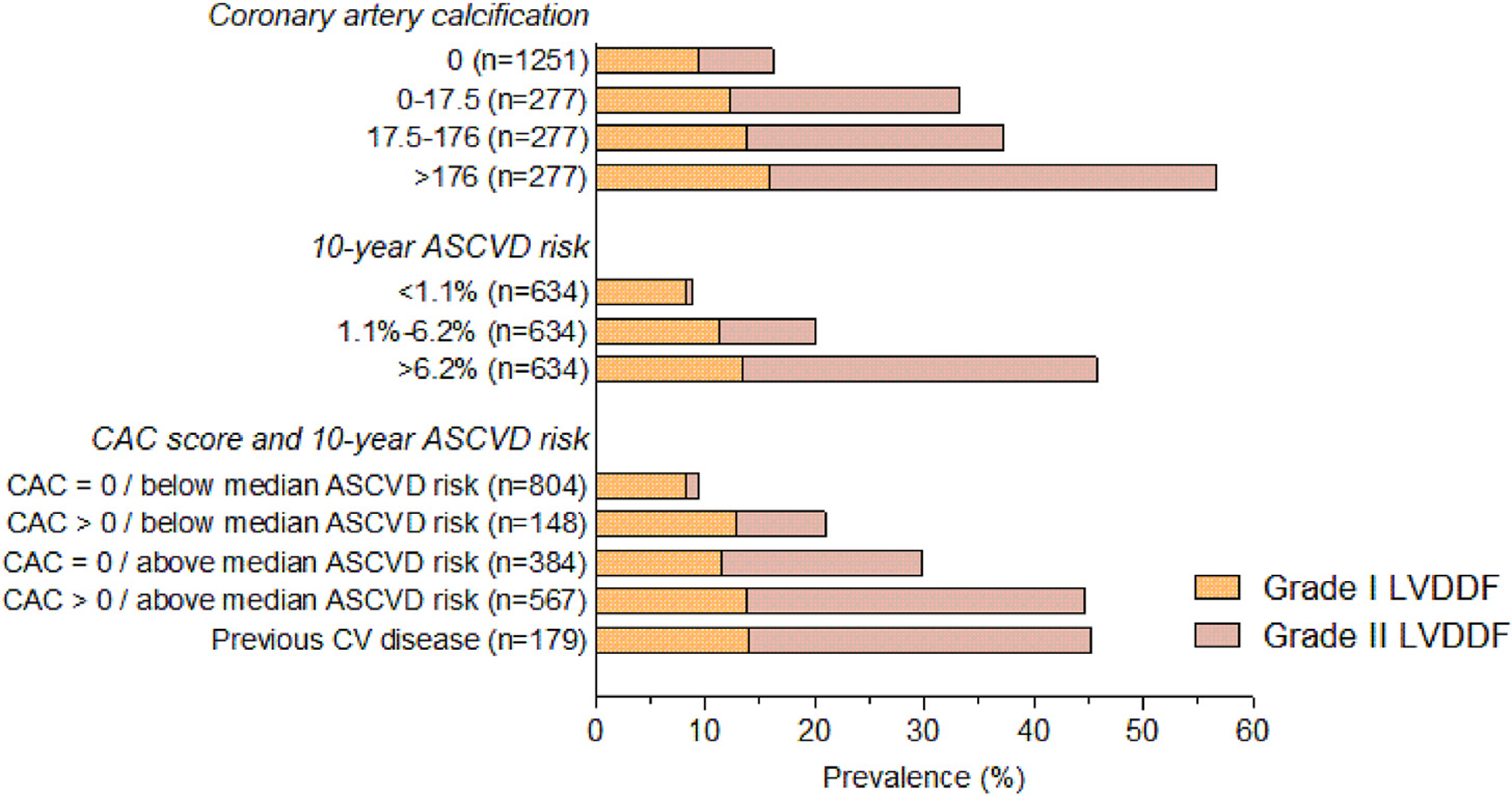
Prevalence of left ventricular diastolic dysfunction (LVDDF) by CAC and 10-year ASCVD risk score. P < 0.001 for frequencies by CAC and/or 10-year ASCVD risk in Grade II LVDDF (P < 0.05 for all frequency trends in grade I LVDDF). ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcium; CV, cardiovascular disease.
4. Discussion
Leveraging a diverse cohort with coronary CT and echocardiographic imaging, we found that LV diastolic function was associated with CAC independently of age, female sex, race, and traditional cardiometabolic risk factors. Furthermore, in the PBHS study, individuals with evidence of CAC and higher ASCVD risk score had a prevalence of LV diastolic dysfunction comparable to that in individuals with established cardiovascular disease.
4.1. LV diastolic dysfunction and CAC
Previous studies have found an association between diastolic function and CAC. In the CARDIA study, Gardin et al. showed that CAC was independently associated with LA dimension, LV mass and geometry (n = 2724).11 In a more recent analysis, Yared et al. confirmed independent associations between CAC and E/e’, LAVI, LVMI, and LV longitudinal strain at 25-year follow-up.10,11 In addition, they found that change in CAC from year 15–25 was associated with higher LV mass and volumes and worse LV diastolic function.10 In contrast, in the Framingham Offspring Heart Study, Castro-Diehl et al. did not observe an independent relationship between CAC and LV diastolic function although an independent association was found with LVMI and aortic root diameter.12 Our findings are consistent with the observations of the CARDIA study, with a stronger effect size noted for e’ velocity, E/e’ ratio, and LVMI. Since CAC and LVDD share several risk factors, finding an independent association will depend on the uncoupling of CAC and ASCVD risk, ie, finding individuals with lower ASCVD risk but elevated CAC score or individuals with higher ASCVD risk but “disproportionally” higher CAC score.8 It is therefore not surprising that an independent association between LV diastolic function and CAC is not observed in all community-based studies.8 Several factors may explain the association between LV diastolic function and CAC. First, as Wang et al. showed in the Multi-Ethnic Study of Atherosclerosis (MESA), coronary vasodilatory response was inversely associated with the presence and severity of CAC in asymptomatic adults.28 This impairment in coronary flow reserve could in turn potentially slow ventricular relaxation, impair ventricular distensibility and thus trigger diastolic dysfunction.29 CAC may also be indirectly associated with LVDD as an indirect marker of increased central arterial stiffness, which may in turn contribute to LVDD.30,31 Increased arterial stiffness may also contribute to aortic root enlargement, which was observed in both our study and the Framingham Heart Study.12 Finally, shared pathophysiological pathways, eg, inflammatory, growth factor, or oxidative stress may modulate both LVDD and subclinical coronary artery disease.32,33
The presence of CAC in individuals with LVDD represents a substrate for future cardiovascular events. In both FLEMENGHO and the Framingham Heart Study, incident CV events in individuals with LVDD were driven by atherosclerotic events, such as symptomatic coronary disease, acute coronary syndrome, stroke, and peripheral arterial disease.1,2
4.2. LV diastolic dysfunction and traditional risk factors
In our study, LVDD was common, occurring in close to 27% of participants when using population-based age-specific criteria for E/A and e’. Notwithstanding the different definitions of LVDD in epidemiological studies and different baseline characteristics, a similar prevalence was reported in the FLEMENGHO study (25% when using age-specific thresholds for E/A but a lower E/e’ threshold) and the Framingham Heart Study (25%–32% across age ranges when using age- and sex-adjusted thresholds).1, 2Consistent with other community-based studies, we found a strong association between LVDD and traditional risk factors, including age, female sex, blood pressure, height (inverse), weight, HbA1C, and HDL cholesterol (inverse).1–4 In addition, our multiracial/ethnic study design allowed us to identify Black race as a correlate of LV diastolic function in both the continuous and categorical analyses (grade 2 LVDD). Based on the CARDIA study, Rasmussen-Torvik also found that Black/African American race was associated with a higher prevalence of LVDD.34 This is also consistent with the recent multi-cohort study of Lewis et al. who found that Black race was more often associated with malignant LVH.35
4.3. Defining LVDD in community-based studies
While not the main objective of our analysis, the criteria identified for LVDD were consistent with several epidemiological-based studies. Similar to studies by Kuznetsova et al. and Nayor et al. we used an age-adjusted threshold for E/A and e’ velocities as it provides a greater sensitivity for detecting LVDD in younger age groups and a greater specificity in older age groups.1,4 A single threshold of 10 for E/e’ is supported by many epidemiologic studies and hemodynamic stress studies.1,4,17,33,36 In the study by Reddy et al. an E/e’ >9 was included in the H2FPEF score and helped discriminate heart failure with preserved ejection fraction from noncardiac causes of dyspnea.36 Adding supportive criteria as advocated by the American Society of Echocardiography/European Association of Cardiovascular Imaging (ASE/EACVI) recommendations on diastolic dysfunction allows for the differentiation of isolated E/e’ abnormalities from grade 2 diastolic dysfunction and minimizes overdiagnosis of LVDD.8 Finally, consistent with the World Alliance of Societies of Echocardiography Normal Values Study (WASE), we also identified a higher threshold for LAVI (42 mL/m2), which could influence future recommendations on the evaluation of diastolic dysfunction if further validated.27 Not surprisingly, the prevalence of LVDD according to ASE criteria is lower and the overlap may vary depending on the definition of cardiac substrate.
4.4. Clinical implications
Our study provides several clinical insights or implications. First, the fact that subclinical atherosclerosis is very common among patients with LVDD may explain the association between LVDD and incident atherosclerotic events.1,2 Second, the presence of LVDD in individuals with a 10-year risk of an atherosclerotic event <7.5% could prompt further evaluation for high CAC as this could influence preventive strategies.18 Future studies are however needed to determine whether age- and sex-specific thresholds for diastolic parameters (epidemiologically based criteria) will better identify individuals at risk of high CAC scores. Third, our study provides insights for outcome models. In fact, since traditional risk factors, CAC score, and diastolic function are closely intertwined, larger samples will be needed to demonstrate independent associations with outcome. Critical to this analysis will be the proportion of resilient (high ASCVD risk profile without LVDD and low CAC score) or vulnerable (low ASCVD risk with LVDD or high CAC score) phenotypes.
4.5. Limitations
Our analysis has several limitations, including its cross-sectional nature, the relatively small low risk reference group, and the absence of an outcome analysis (as insufficient events have occurred to date). Moreover, B-type natriuretic peptides, LV or atrial myocardial strain or LV diastolic stress measures were not available. The PBHS cohort has however several strengths, including its enrichment for metabolic risk, its multiracial and multiethnic representation, and core laboratory analysis.
5. Conclusion
Our study demonstrates an independent association between CAC score and diastolic function. The presence of LVDD in individuals with a 10-year risk of an atherosclerotic event <7.5% could prompt further evaluation for high CAC as this could influence preventive strategies.
Summary
The study highlights the multidimensional associations between diastolic function, coronary artery calcium score, and cardiometabolic risk factors in a multiethnic community-based study. Coronary artery calcium score emerged as interdependently associated with left ventricular diastolic parameters (mainly e’ and E/e’ velocity), left ventricular mass index, and aortic root dimension. Left ventricular diastolic dysfunction remains common in a contemporary cohort of individuals with cardiometabolic risk factors. Consistent with recent studies, we found higher reference limits for body surface area indexed left atrial volume in low-risk individuals.
Supplementary Material
Acknowledgements
We would like to thank the participants of the Project Baseline Health Study (PBHS), the research staff, and the support of Verily. We also would like to thank the Research Foundation Flanders, Belgium (grant numbers G.0880.13; 1225021N, 1S07421N, and G0C5319N). We wish to thank Brooke Walker, MS, of the Duke Clinical Research Institute, Durham, NC, for editorial assistance in preparing this manuscript.
Sources of funding
The Project Baseline Health Study was funded by Verily Life Sciences, San Francisco, California. Stanford University and Duke University partialled funded the individual researchers. NC and TK were supported by the Research Foundation Flanders, Belgium (grant numbers 1225021N, 1S07421N, and G0C5319N).
Role of the funding source
The funding source had no role in the study design; collection, analysis, and interpretation of data; writing of the report; and decision to submit the article for publication.
Abbreviations
- ASCVD
Atherosclerotic cardiovascular disease
- ASE
American Society of Echocardiography
- CAC
Coronary artery calcium
- CAD
Coronary artery disease
- COPD
Chronic pulmonary obstructive disease
- CV
Cardiovascular
- DM
Diabetes mellitus
- e’
Early diastolic mitral annular velocity
- E
Mitral inflow velocity
- E/A
ratio Early and late diastolic peak velocities of mitral inflow
- E/e’
Mitral inflow to annular velocity ratio
- eGFR
Estimated glomerular filtration rate
- HDL-C
High-density lipoprotein-C
- LAVI
Left atrial volume index
- LDL-C
Low-density lipoprotein-C
- LVDD
Left ventricular diastolic dysfunction
- LVMI
Left ventricular mass index
- NCCT
Non-contrast computed tomography
- RWT
Relative wall thickness
- TRVmax
Maximal tricuspid regurgitation velocity
Footnotes
Clinicaltrials.gov identifier:
Data statement
The Baseline Study data will be available to qualified researchers for exploratory analysis after the data are adequately curated and initial planned primary manuscripts are written. Qualified external researchers will be able to apply through applications reviewed by the Proposal Review and Publications Committee and Scientific Executive Committee.
Declaration of competing interest
All authors acknowledge institutional research grants from Verily Life Sciences. FH received an institutional research grant from Actelion Ltd. Within the last 2 years and an institutional research grant from Precordior Ltd. KM reports grants from Verily, Afferent, the American Heart Association (AHA), Cardiva Medical Inc, Gilead, Luitpold, Medtronic, Merck, Eidos, Ferring, Apple Inc, Sanifit, and St. Jude; grants and personal fees from Amgen, AstraZeneca, Bayer, CSL Behring, Johnson & Johnson, Novartis, and Sanofi; and personal fees from Anthos, Applied Therapeutics, Elsevier, Inova, Intermountain Health, Medscape, Mount Sinai, Mundi Pharma, Myokardia, Novo Nordisk, Otsuka, Portola, SmartMedics, and Theravance outside the submitted work. AH reports grants from Verily; grants and personal fees from AstraZeneca, Amgen, Bayer, Merck, and Novartis; and personal fees from Boston Scientific outside the submitted work. RC reports grants from Verily Life Sciences and Google Health, and personal fees from Cytokinetics Inc. And Centessa Inc. NC reports grants from the Research Foundation Flanders. FR reports equity from HealthPals and Carta, and advisory board and consulting fees from NovoNordisk, HealthPals, and Novartis. JB reports grants from the National Institutes of Health (U01-HL146382-03, R01-MD013493-03, D43TW009337, U01-HL123336-06, U01-HL142099-03, and D43 TW011625–01) and royalties or licensing fees from UpTo-Date. The other authors have no conflicts of interest to disclose.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcct.2022.06.003.
References
- 1.Kuznetsova T, Thijs L, Knez J, Herbots L, Zhang Z, Staessen JA. Prognostic value of left ventricular diastolic dysfunction in a general population. J Am Heart Assoc 2014; 3, e000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nayor M, Cooper LL, Enserro DM, et al. Left ventricular diastolic dysfunction in the community: impact of diagnostic criteria on the burden, correlates, and prognosis. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chetrit M, Cremer PC, Klein AL. Imaging of diastolic dysfunction in community-based epidemiological studies and randomized controlled trials of HFpEF. JACC Cardiovasc Imaging 2020;13:310–326. [DOI] [PubMed] [Google Scholar]
- 4.Kuznetsova T, Herbots L, Lopez B, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail 2009;2:105–112. [DOI] [PubMed] [Google Scholar]
- 5.Perak AM, Khan SS, Colangelo LA, et al. Age-related development of cardiac remodeling and dysfunction in young Black and white adults: the coronary artery risk development in young adults study. J Am Soc Echocardiogr 2021;34:388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grossman W, Barry WH. Diastolic pressure-volume relations in the diseased heart. Fed Proc 1980;39:148–155. [PubMed] [Google Scholar]
- 7.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure–abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 2004;350:1953–1959. [DOI] [PubMed] [Google Scholar]
- 8.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 9.Tisdale RL, Haddad F, Kohsaka S, Heidenreich PA. Trends in left ventricular ejection fraction for patients with a new diagnosis of heart failure. Circ Heart Fail 2020;13, e006743. [DOI] [PubMed] [Google Scholar]
- 10.Yared GS, Moreira HT, Ambale-Venkatesh B, et al. Coronary artery calcium from early adulthood to middle age and left ventricular structure and function. Circ Cardiovasc Imaging 2019;12, e009228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardin JM, Iribarren C, Detrano RC, et al. Relation of echocardiographic left ventricular mass, geometry and wall stress, and left atrial dimension to coronary calcium in young adults (the CARDIA study). Am J Cardiol 2005;95:626–629. [DOI] [PubMed] [Google Scholar]
- 12.Castro-Diehl C, Song RJ, Mitchell GF, et al. Association of subclinical atherosclerosis with echocardiographic indices of cardiac remodeling: the Framingham Study. PLoS One 2020;15, e0233321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddad F, Contrepois K, Amsallem M, et al. The right heart network and risk stratification in pulmonary arterial hypertension. Chest 2022;161:1347–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fruchterman TMJ, Reingold EM. Graph drawing by force-directed placement. Software Pract Ex 1991;21:1129–1164. [Google Scholar]
- 15.Reshef DN, Reshef YA, Finucane HK, et al. Detecting novel associations in large data sets. Science 2011;334:1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arges K, Assimes T, Bajaj V, et al. The Project Baseline Health Study: a step towards a broader mission to map human health. NPJ Digit Med 2020;3:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 18.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. Tomaselli GF and American college of cardiology/American heart association task force on practice G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39. e14. [DOI] [PubMed] [Google Scholar]
- 20.Douglas PS, Waugh RA, Bloomfield G, et al. Implementation of echocardiography core laboratory best practices: a case study of the PARTNER I trial. J Am Soc Echocardiogr 2013;26:348–358. e3. [DOI] [PubMed] [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 22.Strange G, Stewart S, Celermajer DS, et al. Threshold of pulmonary hypertension associated with increased mortality. J Am Coll Cardiol 2019;73:2660–2672. [DOI] [PubMed] [Google Scholar]
- 23.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenland P, Bonow RO, Brundage BH, et al. American college of cardiology foundation clinical expert consensus task F, society of atherosclerosis I, prevention and society of cardiovascular computed T. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American college of cardiology foundation clinical expert consensus task force (ACCF/AHA writing committee to update the 2000 expert consensus document on electron beam computed tomography) developed in collaboration with the society of atherosclerosis imaging and prevention and the society of cardiovascular computed tomography. J Am Coll Cardiol 2007;49:378–402. [DOI] [PubMed] [Google Scholar]
- 25.Albanese D, Filosi M, Visintainer R, Riccadonna S, Jurman G, Furlanello C. Minerva and minepy: a C engine for the MINE suite and its R, Python and MATLAB wrappers. Bioinformatics 2013;29:407–408. [DOI] [PubMed] [Google Scholar]
- 26.Solberg HE. The IFCC recommendation on estimation of reference intervals. The RefVal program. Clin Chem Lab Med 2004;42:710–714. [DOI] [PubMed] [Google Scholar]
- 27.Miyoshi T, Addetia K, Citro R, et al. Left ventricular diastolic function in healthy adult individuals: results of the World alliance societies of echocardiography normal values study. J Am Soc Echocardiogr 2020;33:1223–1233. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Jerosch-Herold M, Jacobs DR Jr, et al. Coronary artery calcification and myocardial perfusion in asymptomatic adults: the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2006;48:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohara T, Little WC. Evolving focus on diastolic dysfunction in patients with coronary artery disease. Curr Opin Cardiol 2010;25:613–621. [DOI] [PubMed] [Google Scholar]
- 30.Torngren K, Rylance R, Bjork J, et al. Association of coronary calcium score with endothelial dysfunction and arterial stiffness. Atherosclerosis 2020;313:70–75. [DOI] [PubMed] [Google Scholar]
- 31.Haydar AA, Covic A, Colhoun H, Rubens M, Goldsmith DJ. Coronary artery calcification and aortic pulse wave velocity in chronic kidney disease patients. Kidney Int 2004;65:1790–1794. [DOI] [PubMed] [Google Scholar]
- 32.Sanders-van Wijk S, Tromp J, Beussink-Nelson L, et al. Proteomic evaluation of the comorbidity-inflammation paradigm in heart failure with preserved ejection fraction: results from the PROMIS-HFpEF study. Circulation 2020;142:2029–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuznetsova T, Haddad F, Knez J, et al. Cytokines profile in hypertensive patients with left ventricular remodeling and dysfunction. J Am Soc Hypertens 2015;9: 975–984. e3. [DOI] [PubMed] [Google Scholar]
- 34.Rasmussen-Torvik LJ, Colangelo LA, Lima JAC, et al. Prevalence and predictors of diastolic dysfunction according to different classification criteria: the coronary artery risk development in young in adults study. Am J Epidemiol 2017;185:1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis AA, Ayers CR, Selvin E, et al. Racial differences in malignant left ventricular hypertrophy and incidence of heart failure: a multicohort study. Circulation 2020; 141:957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018;138:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


