Abstract
Background
This document updates previously published Clinical Practice Guidelines for the management of patients with acute respiratory distress syndrome (ARDS), incorporating new evidence addressing the use of corticosteroids, venovenous extracorporeal membrane oxygenation, neuromuscular blocking agents, and positive end-expiratory pressure (PEEP).
Methods
We summarized evidence addressing four “PICO questions” (patient, intervention, comparison, and outcome). A multidisciplinary panel with expertise in ARDS used the Grading of Recommendations, Assessment, Development, and Evaluation framework to develop clinical recommendations.
Results
We suggest the use of: 1) corticosteroids for patients with ARDS (conditional recommendation, moderate certainty of evidence), 2) venovenous extracorporeal membrane oxygenation in selected patients with severe ARDS (conditional recommendation, low certainty of evidence), 3) neuromuscular blockers in patients with early severe ARDS (conditional recommendation, low certainty of evidence), and 4) higher PEEP without lung recruitment maneuvers as opposed to lower PEEP in patients with moderate to severe ARDS (conditional recommendation, low to moderate certainty), and 5) we recommend against using prolonged lung recruitment maneuvers in patients with moderate to severe ARDS (strong recommendation, moderate certainty).
Conclusions
We provide updated evidence-based recommendations for the management of ARDS. Individual patient and illness characteristics should be factored into clinical decision making and implementation of these recommendations while additional evidence is generated from much-needed clinical trials.
Keywords: acute respiratory distress syndrome, corticosteroids, extracorporeal membrane oxygenation, neuromuscular blockade, positive end-expiratory pressure
Contents
Summary of Recommendations
Introduction
-
Methods
Committee Composition
Conflict of Interest Policy
Formulating Clinical Questions
Literature Search
Evidence Review and Appraisal
Development of Clinical Recommendations
Manuscript Preparation
-
Recommendations for Specific Treatment Questions
Question 1: Should Patients with ARDS Receive Systemic Corticosteroids?
Question 2: Should Patients with ARDS Receive Venovenous Extracorporeal Membrane Oxygenation?
Question 3: Should Patients with ARDS Receive Neuromuscular Blockade?
Question 4: Should Patients with ARDS Receive Higher Compared with Lower PEEP, with or without LRMs?
Discussion
Conclusions
Overview
This guideline updates and adds to recommendations for the management of patients with acute respiratory distress syndrome (ARDS) (Figure 1). New recommendations in this guideline include:
-
•
We suggest using corticosteroids for patients with ARDS (conditional recommendation, moderate certainty of evidence).
-
•
We suggest using venovenous extracorporeal membrane oxygenation (VV-ECMO) in selected patients with severe ARDS (conditional recommendation, low certainty of evidence).
-
•
We suggest using neuromuscular blockers in patients with early severe ARDS (conditional recommendation, low certainty of evidence).
-
•With regard to positive end-expiratory pressure (PEEP):
-
○We suggest using higher PEEP without lung recruitment maneuvers (LRMs) as opposed to lower PEEP in patients with moderate to severe ARDS (conditional recommendation, low to moderate certainty).
-
○We recommend against using prolonged LRMs in patients with moderate to severe ARDS (strong recommendation, moderate certainty).
-
○
Figure 1.
Current American Thoracic Society guidelines for the management of acute respiratory distress syndrome. *New or updated recommendations in current guideline. †Recommendations addressed in 2017 guideline. ARDS = acute respiratory distress syndrome; FiO2 = fraction of inspired oxygen; PaO2 = partial pressure of oxygen; PBW = predicted body weight; PEEP = positive end-expiratory pressure; Pplat = plateau pressure; VT = tidal volume; VV-ECMO = venovenous extracorporeal membrane oxygenation.
Recommendations from the 2017 guideline that remain in place include:
-
•
We recommend using mechanical ventilation strategies that limit tidal volume (4–8 mL/kg predicted body weight) and inspiratory pressures (plateau pressure <30 cm H2O) in patients with ARDS (strong recommendation, moderate certainty of evidence).
-
•
We recommend prone positioning for >12 hours per day in patients with severe ARDS (strong recommendation, moderate certainty of evidence).
-
•
We recommend against the routine use of high-frequency oscillatory ventilation in patients with moderate or severe ARDS (strong recommendation, high certainty of evidence).
Introduction
ARDS is a life-threatening form of respiratory failure characterized by acute hypoxemia and bilateral radiographic infiltrates (1–4). More than 50 years have passed since its initial recognition, and its definition has evolved over time, with a recent suggestion that it be expanded to include intubated and nonintubated patients (5, see pp. 37–47 of this issue). ARDS management remains largely supportive, focusing on strategies intended to limit further lung injury, and high mortality rates persist, with those who survive often facing long-term impairments (6). In 2017, the American Thoracic Society (ATS), in conjunction with the European Society of Intensive Care Medicine (ESICM) and Society of Critical Care Medicine, published a Clinical Practice Guideline summarizing the evidence supporting ventilatory and adjunctive measures in ARDS and providing recommendations on their use (7). Since that time, new data have emerged addressing multiple ARDS therapies and supportive care interventions, including corticosteroids, VV-ECMO, neuromuscular blocking agents (NMBAs), and PEEP, prompting an update to the guidelines.
Methods
Committee Composition
The update was proposed by the chairs (E.F. and A.W.) and co-chairs (L.M., N.Q., S.S., and C.S.) to the ATS Critical Care Assembly and was approved by the ATS Board of Directors. The chairs and co-chairs identified a diverse group of panelists with expertise in ARDS epidemiology, clinical trials, methodology, pharmacology, and physiology. We formed four groups to address individual interventions, each led by a co-chair with an assigned methodologist with expertise in Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology (8). We assigned panel members to groups based on their expressed interest and expertise. All guideline meetings were held via video conference.
Conflict of Interest Policy
All committee members disclosed potential conflicts of interest and financial relationships in accordance with ATS policy (9). New or updated conflicts of interest were solicited annually by the chair (E.F.).
Formulating Clinical Questions
The panel co-chairs developed an initial set of four PICO (patient, intervention, comparison, and outcome) questions centered around ARDS management that were not addressed in the initial guideline (corticosteroids, NMBAs) or for which substantial and potentially practice-changing new evidence had emerged since the last iteration (VV-ECMO, PEEP, LRMs). We assigned each question to a subcommittee. Subcommittee members finalized the specific elements of the four questions after detailed discussion and consideration of importance, availability of evidence, and perceived patient preferences. The panel a priori identified outcomes of interest for each question and ranked them in relative importance from the perspective of a patient with ARDS (10, 11) The top five ranked outcomes of interest (rating score ⩾8.0), in order of prioritization, included long-term mortality (at 90 d or 6 mo), health-related quality of life at 6 months or later, long-term cognitive impairment, short-term mortality (28 d; ICU or in-hospital), and cardiac arrest. Delirium and post-ICU weakness were identified as additional important patient-centered outcomes based on prior evidence (11) and feedback from a patient representative.
Literature Search
We planned to conduct a systematic review for each PICO question. Because all of the PICO questions had recent high-quality systematic reviews that had been conducted by coauthors of this guideline, we proceeded to update each of these systematic reviews, ensuring that we captured any recently published trials. We searched the following databases for randomized controlled trials (RCTs) published in any language from the date of the last systematic review to October 27, 2022: MEDLINE, Embase, CDC Library of Coronavirus Disease (COVID) Research, the Cumulative Index to Nursing and Allied Health Literature, and the Cochrane Central Register of Controlled Trials. Using the Covidence tool, a team of reviewers (D.C., B.R., and S.P.) screened titles and abstracts and then full-text manuscripts independently and in duplicate. We performed data extraction and risk-of-bias assessment independently and in duplicate for each included trial per standard systematic review methodology (see online supplement).
Evidence Review and Appraisal
To generate an evidence summary for each PICO question, we used RevMan v5.3 to generate pooled effect estimates using inverse variance weighting and a random effects model. We presented the results of the analyses using relative risks (RRs) for binary outcomes and mean differences for continuous outcomes, both with 95% CIs. We assessed the certainty in effect estimates and generated evidence profiles using GRADE methodology (see online supplement) (12); certainty of evidence for each comparison and outcome was categorized as high, moderate, low, or very low (Table 1).
Table 1.
Implications of Certainty of Evidence Categories
| Certainty | Meaning |
|---|---|
| High | There is a high level of confidence that the true effect is close to the estimated effect |
| Moderate | There is a moderate level of confidence in the effect estimate; true effect is probably close to the estimated effect |
| Low | The confidence in the effect estimate is limited; true effect may be substantially different from the estimated effect |
| Very low | There is very little confidence in the effect estimate; true effect is probably substantially different from estimated effect |
Development of Clinical Recommendations
Each group convened to develop initial recommendations for the individual PICO questions. The co-chairs and methodologists led the groups through a discussion of the evidence profiles and GRADE Evidence to Decision framework (13) to determine the direction and strength of the recommendations (see online supplement). As part of the GRADE Evidence to Decision process, we considered the certainty of evidence, balance of desirable and undesirable consequences of an intervention, patient preferences and values, resource use, implications for health equity, acceptability of the intervention to stakeholders, and clinical feasibility. Evidence across the full spectrum of ARDS severity was unavailable for some interventions. For those interventions, recommendations were limited to the specific severity subgroups (i.e., mild, moderate, or severe) for which evidence was sufficient, and no recommendation was made for the subgroups for which it was not. Each recommendation was designated as “strong” or “conditional” (Table 2) (14, 15). After the individual groups generated draft recommendations, these were presented to the full panel for detailed discussion, input, and approval. Final recommendations were determined by consensus of the full panel. Consistent with the GRADE approach, we had planned to use voting for recommendations that could not achieve consensus through discussion, but this was not required.
Table 2.
Implications of Strong versus Conditional Recommendations
| Strength of Recommendation | ||
|---|---|---|
| Stakeholder | Strong | Conditional |
| Patients | Nearly all individuals in this situation would want the recommended course of action; only a small proportion would not | The majority of individuals in this situation would want the suggested course of action, but many would not |
| Clinicians | Most patients should receive the recommended course of action; adherence to this recommendation could be used as a quality criterion or performance indicator | Different choices will be appropriate for different patients; the clinician must help patients arrive at management decisions consistent with their preferences and values; clinicians should expect to spend more time with patients when working toward a decision |
| Policy makers | The recommendation can be adapted as policy in most situations; quality-improvement initiatives could use adherence to this recommendation as a performance indicator | Policy making will require substantial debate and involvement of many stakeholders; policies may also vary between regions and health systems |
Manuscript Preparation
The writing committee composed of the chairs and co-chairs drafted the guideline document for subsequent review by the panel. We summarized the rationale and supporting evidence for each recommendation, as well as issues raised during the GRADE Evidence to Decision process. A patient representative reviewed the draft guidelines and provided feedback regarding the recommendations and selected patient-centered outcomes, which were incorporated into the document. We then integrated feedback from all panel members into the manuscript. The entire panel approved the final wording of the recommendations and justifications, which was then submitted to ATS for review and approval.
Recommendations for Specific Treatment Questions
Question 1: Should Patients with ARDS Receive Systemic Corticosteroids?
Recommendation
We suggest using corticosteroids for patients with ARDS (conditional recommendation, moderate certainty of evidence).
Background
Corticosteroids are anti-inflammatory medications that inhibit the synthesis of proinflammatory mediators present in ARDS. They are widely administered to patients with ARDS for the management of ARDS specifically and for concurrent conditions such as septic shock or pneumonia (16). More recently, corticosteroids have been found to reduce mortality in COVID-19–related acute hypoxemic respiratory failure (17) and severe community-acquired pneumonia (18). Corticosteroids were not addressed in the 2017 guidelines. Since that time, several multicenter RCTs evaluating the effect of corticosteroids on patients with ARDS have been published (19), prompting a recommendation for this intervention.
Evidence summary
Corticosteroids were evaluated in 19 RCTs including 2,790 patients (20–35). Pooled analysis demonstrated that corticosteroids probably decrease mortality (n = 17 studies; RR, 0.84; 95% CI, 0.73–0.96; moderate certainty) (20–33) and may reduce the duration of mechanical ventilation (n = 9 studies; mean difference (MD), 4 d less; 95% CI, −5.5 to −2.5; low certainty) (22, 24–27, 30, 34, 35) and the length of hospital stay (n = 4 studies; MD, 8 d shorter; 95% CI, −13 to −3; low certainty) (22, 25, 35), although the effect on the length of ICU stay is uncertain (n = 4 studies; MD, 0.8 d shorter; 95% CI, −4.1 to +5.7; very low certainty) (21, 22, 25, 34). With regard to safety outcomes, corticosteroids probably increase the risk of serious hyperglycemia (n = 6 studies; RR, 1.11; 95% CI, 1.01–1.23; moderate certainty) (22, 23, 26, 27, 30), may increase the risk of gastrointestinal bleeding (n = 5 studies; RR, 1.20; 95% CI, 0.43–3.34; low certainty) (20, 23, 26), and have an uncertain effect on neuromuscular weakness (n = 2 studies; RR, 0.85; 95% CI, 0.62–1.18; very low certainty) (22, 25).
Justification and implementation considerations
Although pooled analysis demonstrated a mortality benefit with moderate certainty of evidence, multiple caveats prompted a conditional recommendation. There is substantial heterogeneity in the dosing, timing, and duration of corticosteroids in clinical trials in patients with ARDS, resulting in uncertainty about the optimal course of treatment. Data addressing the short- and long-term adverse effects of corticosteroids are also limited; infectious complications could not be systematically evaluated, and there is low- to very low-certainty evidence for other safety outcomes. Additionally, previous studies assessing the use of corticosteroids for varied indications have demonstrated the potential for harm even when used in short courses (36, 37).
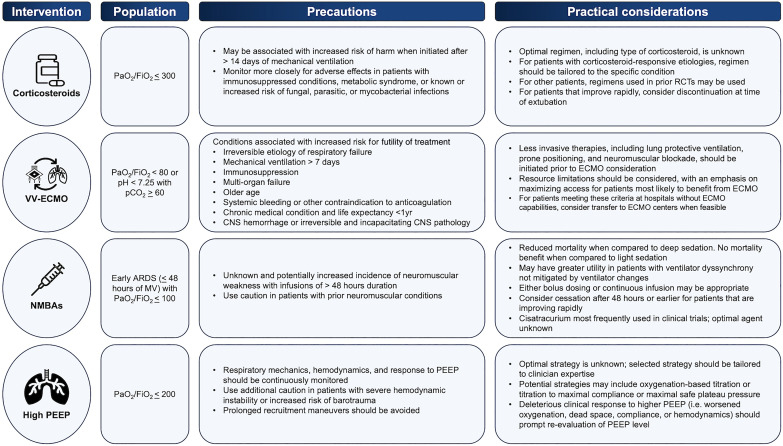
There are several factors to consider for implementation (Figure 2). Corticosteroids are widely available, low in cost, and easy to administer. As such, they have the potential to reach and benefit a substantial number of patients. With regard to corticosteroid dosing and administration, although the panel was not comfortable making recommendations for a specific agent and course of therapy, there are some considerations that may help guide clinicians when selecting a regimen. Some conditions presenting as ARDS (i.e., severe community-acquired pneumonia, Pneumocystis jirovecii pneumonia in patients with HIV infection) are known to benefit from corticosteroids, with regimens that have been defined and evaluated in large RCTs (18, 38). For other ARDS etiologies, any of several regimens used in clinical trials (Table E10 in the online supplement) could reasonably be chosen based on the individual patient’s risk profile for steroid side effects. Although the duration of corticosteroid treatment has varied in clinical trials, corticosteroids were stopped at the time of extubation in a number of the included studies. Additionally, although the optimal timing of therapy is also unclear, it is important to note that the initiation of corticosteroid treatment >2 weeks after the onset of ARDS may be associated with harm (25). Furthermore, the use of corticosteroids should be accompanied by close surveillance for adverse effects, particularly in patient populations that may be at higher risks of harm, such as patients who are immunocompromised, have metabolic syndrome, or live in regions where infections such as tuberculosis and parasitic disease are endemic. Finally, although this recommendation is based on evidence from trials on intubated patients with ARDS and applies specifically to this group, corticosteroids have demonstrated benefit in some groups of nonintubated patients with ARDS. For nonintubated individuals, corticosteroids should be administered for those with ARDS etiologies known to benefit from corticosteroid treatment (i.e., COVID-19, severe community-acquired pneumonia). The role of steroids in nonintubated patients with ARDS of other etiologies remains uncertain.
Figure 2.
Precautions and practical considerations for the use of corticosteroids, venovenous extracorporeal membrane oxygenation, neuromuscular blocking agents, and positive end-expiratory pressure. ARDS = acute respiratory distress syndrome; CNS = central nervous system; FiO2 = fraction of inspired oxygen; MV = mechanical ventilation; NMBA = neuromuscular blocking agent; PaO2 = partial pressure of oxygen; pCO2 = partial pressure of CO2; PEEP = positive end-expiratory pressure; RCT = randomized controlled trial; VV-ECMO = venovenous extracorporeal membrane oxygenation.
Uncertainties and research priorities
Several questions about corticosteroids remain unanswered. The optimal corticosteroid regimen remains unknown; further research is needed to determine the appropriate formulation, dose, timing, and course of therapy to better guide clinical care. Additional longitudinal data are also needed to better understand the adverse consequences of corticosteroids. Finally, there is a possibility that corticosteroids have variable effects on different subpopulations of patients based on ARDS etiology, severity, patient characteristics, or other factors. Understanding the impact of corticosteroids on potentially vulnerable patients, such as those at increased risk for superimposed infections (e.g., immunocompromised patients) and metabolic complications (e.g., those with diabetes mellitus), is of particular importance. Two large, multicenter RCTs assessing the impact of corticosteroids on ARDS outcomes will soon be underway – Glucocorticoids in Adults with Acute Respiratory Distress Syndrome (GuARDS) and Corticosteroid Early and Extended (CORT-E2). These trials may help answer questions about differential treatment effects in ARDS subgroups and strengthen the certainty of evidence surrounding corticosteroid use in ARDS overall.
Question 2: Should Patients with ARDS Receive VV-ECMO?
Recommendation
We suggest the use of VV-ECMO in selected patients with severe ARDS (conditional recommendation, low certainty of evidence)
Background
VV-ECMO facilitates oxygenation and carbon dioxide removal in patients with ARDS by draining blood from the venous system, allowing it to pass through a gas-exchange device, and then returning it to the venous system (39). It is an invasive, resource-intensive technology available at specialized centers that incurs significant cost and requires a considerable amount of human health resources. The use of VV-ECMO has increased substantially during the past several years, with notable increases seen after the 2009 H1N1 pandemic and subsequently during the COVID-19 pandemic (40, 41). The 2017 ATS guidelines addressed VV-ECMO in patients with ARDS but found insufficient evidence to make a recommendation for or against its use (7). Since that time, a multicenter RCT evaluating the effect of early initiation of VV-ECMO on patients with severe ARDS was published (42), prompting an updated recommendation.
Evidence summary
VV-ECMO was evaluated in two RCTs that included 429 patients (42–44). In the first trial, 180 patients were randomized to conventional ARDS management or referral for consideration of VV-ECMO, with follow-up at 6 months; a specific management protocol was not mandated in the control arm (43). In the second trial, 249 patients were randomized to VV-ECMO or conventional management and followed for 60 days. Ventilator management was protocolized in the control arm, and the use of neuromuscular blockade and prone positioning was encouraged (42). Pooled analysis demonstrated that VV-ECMO probably decreased mortality at the latest follow-up (RR, 0.76; 95% CI, 0.60–0.95; moderate certainty) and probably increased ventilator-free days (MD, 8 d more; 95% CI, 2–15; moderate certainty), vasopressor-free days (MD, 8 d more; 95% CI, 3–13; moderate certainty), and renal replacement therapy–free days (MD, 7 d more; 95% CI, 2–13; moderate certainty). With regard to safety outcomes, VV-ECMO probably increased the risk of hemorrhage (RR, 1.64; 95% CI, 1.17–2.31; moderate certainty), but may have little to no effect on the risk of pneumothorax (RR, 1.13; 95% CI, 0.61–2.12; low certainty) and an uncertain effect on the risk of stroke (RR, 0.38; 95% CI, 0.10–1.39; very low certainty).
Justification and implementation considerations
Although pooled analysis demonstrated a benefit from ECMO, with moderate certainty of the evidence of decreased mortality and days of organ support, there were multiple considerations that prompted a conditional recommendation, including the limitations of available data and practical concerns. The CESAR (Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure) trial (43) had several limitations, including the lack of standardized ventilator management in the control arm and a substantial number of patients randomized to VV-ECMO not receiving the intervention. Additionally, the CESAR trial predated the establishment of prone positioning as a guideline-recommended adjunctive therapy, and its use was limited in this trial. For these reasons, the certainty of evidence was downgraded from moderate to low for indirectness. Additionally, there is considerable variability in center experience, pre-ECMO care, and outcomes (16, 45, 46), leading to uncertainty about the real-world generalizability of data obtained from both trials, which were conducted at high-volume, expert ECMO centers.
Because VV-ECMO is a resource-intensive therapy, there are several considerations for implementation (Figure 2). First, less invasive therapies recommended for ARDS, such as lung protective ventilation, higher PEEP, neuromuscular blockade, and prone positioning, should be used before the consideration of VV-ECMO because their use may obviate escalation of treatment. Furthermore, selection criteria for VV-ECMO should be carefully considered and focus on maximizing access for the individuals most likely to benefit from its use, specifically those with reversible etiologies of respiratory failure and very severe hypoxemia (PaO2/FiO2 ratio <80 mm Hg) or hypercapnia (pH <7.25 with PaCO2 ⩾60 mm Hg) despite optimal conventional management, who are early (<7 d) in their ARDS course, and have few risk factors for futility of treatment (42, 47, 48). For patients meeting these criteria who present to facilities without ECMO capabilities, transfer to ECMO centers should be considered when feasible. However, it is important to note that real-world patient selection criteria and access to ECMO centers are variable, and this variability may have serious implications for health equity. Indeed, disparities in patient selection based on insurance status, income, and gender have been reported (49). Finally, there may be considerable variability in feasibility, cost effectiveness, and acceptability for different centers and health systems (43, 50, 51). Because of its resource-intensive nature with regard to staffing, equipment, and costs, VV-ECMO has the potential to divert resources from other institutional needs, a factor that should be considered by established ECMO centers, those considering new ECMO implementation, and policy makers. Additionally, a higher institutional case volume is associated with improved outcomes (45, 46). Accordingly, ECMO should be provided in high-volume, dedicated centers, and efforts should be made to organize ECMO programs on a regional level wherever possible to provide the safest and most efficient care (52).
Uncertainties and research priorities
There are several areas of uncertainty that warrant further research. Little is known about long-term outcomes in ECMO survivors. Pooled data from existing studies suggest that ECMO survivors may have greater decrements in health-related quality of life than patients who were managed with conventional mechanical ventilation, although these findings are limited by small sample sizes and significant heterogeneity in outcome measures and timing of follow-up (53, 54). It is crucial to understand whether increased survival comes with a potential increase in disability because this may have implications for patient preferences, cost effectiveness, and general utility of ECMO. Additionally, there are limited data regarding appropriate supportive measures for patients receiving ECMO, such as early mobilization and ventilator management (55). Further research is needed to understand if approaches to these aspects of care should differ from those used for patients who are treated with conventional mechanical ventilation. Finally, additional studies are needed to address the impact of ECMO on resource allocation in different settings and healthcare systems.
Question 3: Should Patients with ARDS Receive Neuromuscular Blockade?
Recommendation
We suggest using neuromuscular blockade in patients with early severe ARDS (conditional recommendation, low certainty)
Background
NMBAs are a commonly used adjunctive therapy for patients with ARDS (16, 56). The mechanism of benefit is unclear, but likely involves decreasing ventilator-induced lung injury via a reduction in patient–ventilator dyssynchrony in addition to reducing oxygen consumption, inflammation, and alveolar fluid (57–59). NMBAs were not addressed in the 2017 guidelines. Since that time, increasing use and evolving evidence prompted the committee to evaluate NMBAs for the new guidelines (60, 61).
Evidence summary
NMBAs were evaluated in seven RCTs that included 1,598 patients (58–60, 62–66). Pooled analysis demonstrated that NMBAs may decrease mortality for patients with moderate to severe ARDS compared with those who did not receive NMBAs (RR, 0.74; 95% CI, 0.56–0.98; low certainty). However, concerns related to inconsistency and individual study risk of bias led to a low certainty of evidence. Subgroup analyses demonstrated a reduction in mortality for patients receiving NMBAs compared with deep sedation (n = 3 studies, 431 patients; RR, 0.72; 95% CI, 0.58–0.91) (58, 59, 64), an effect not seen in the single RCT that compared NMBAs versus light sedation (RR, 0.99; 95% CI, 0.86–1.15) (60). Additionally, NMBA use was probably associated with a reduced incidence of barotrauma (n = 4 studies, 1,437 patients; RR, 0.55; 95% CI, 0.35–0.85; moderate certainty) and a possible increase in ventilator-free days (n = 5 studies; MD, 0.89 d more; 95% CI, 0.38 fewer to 2.18 more; low certainty), but also probably increased the rates of ICU-acquired weakness (n = 4 studies, 885 patients; RR, 1.16; 95% CI, 0.98–1.37; moderate certainty).
Justification and implementation considerations
Although the largest and most recent RCT comparing NMBAs versus a strategy targeting light sedation did not demonstrate a mortality benefit, pooled data from seven RCTs demonstrated a possible reduction in mortality and an increase in ventilator-free days, prompting the recommendation in favor of NMBA use. Nevertheless, several concerns led to a conditional recommendation, and there are a number of caveats to consider before using NMBAs (Figure 2). First, because of the use of variable sedation strategies in different RCTs, the certainty of evidence was downgraded for a risk of bias and inconsistency. Additionally, a reduction in mortality was seen only when NMBAs were compared with deep sedation, whereas current Clinical Practice Guidelines recommend the use of lighter as opposed to deeper sedation targets (67). The panel identified ongoing uncertainty around the harms of the concomitant sedation required with NMBA and discussed qualifying the recommendation to apply to patients who were already deeply sedated yet were experiencing ventilator dyssynchrony. However, this approach was abandoned because clear thresholds for the degree of dyssynchrony and depth of sedation at which to implement this recommendation could not be identified. Finally, there were concerns related to the potential increased risk for ICU-acquired weakness, as well as the lack of data addressing long-term outcomes.
ARDS severity and the timing of NMBA therapy also factored into the conditional recommendation. Although the included trials enrolled patients with moderate to severe ARDS, the baseline PaO2/FiO2 ratio of enrolled patients was closer to 100 mm Hg. Additionally, the majority of patients included were enrolled within the first 48 hours of mechanical ventilation. Given these considerations, the panel limited this recommendation to early (<48 h since ARDS onset) severe (PaO2/FiO2 ratio ⩽100 mm Hg) ARDS; no recommendation could be made for later initiation or less severe ARDS.
Other considerations for implementation include agent selection and duration of therapy. Although this guideline does not recommend a specific NMBA, cisatracurium was used in the two largest RCTs (60, 64) and may be associated with pleiotropic effects, including a decrease in inflammatory cytokines (68, 69), suggesting that it may be a preferable NMBA for patients with ARDS. Additionally, although the included studies primarily used continuous NMBA infusions, bolus dosing may also be suitable for some patients. With regard to duration, NMBAs were administered for as long as 48 hours in the majority of study patients, with earlier termination in patients whose condition improved rapidly; it is unknown whether a longer duration of use is associated with an increased risk of adverse events. In light of these factors, an appropriate strategy for NMBAs may involve reserving their use for patients with early severe ARDS who are already receiving deep sedation or who, while under light sedation, have evidence of significant ventilator dyssynchrony with associated clinical deterioration that is not mitigated by adjustments to ventilator settings or sedation. In keeping with the included trials, NMBA duration should be limited to a maximum of 48 hours whenever possible.
Uncertainties and research priorities
There are several unanswered questions about NMBAs in ARDS. Although their presumed mechanism of action is through the reduction of ventilator-induced lung injury by decreasing ventilator dyssynchrony, it remains unknown whether NMBAs might also be of benefit in sedated patients who are already fully passively ventilated. It is also unclear if there is a dose–response relationship across the spectrum of passive breathing to strong or dyssynchronous efforts. Some level of spontaneous breathing may be important to prevent diaphragmatic atrophy, whereas too much respiratory effort may cause lung and diaphragm injury (70); accordingly, NMBAs may have a variable impact on patients. Further research efforts should also focus on answering questions about NMBA agent selection, as well as the impact of the timing of initiation (i.e., early vs. late, immediately after meeting criteria vs. after a period of stabilization), dosing (i.e., partial blockade vs. full blockade, intermittent vs. continuous dosing), and duration (71). Finally, longitudinal data are needed to understand the impact of NMBAs on long-term outcomes.
Question 4: Should Patients with ARDS Receive Higher Compared with Lower PEEP, with or without LRMs?
Recommendation
We suggest using higher PEEP without LRMs rather than lower PEEP in patients with moderate to severe ARDS (conditional recommendation, low-moderate certainty). We recommend against using prolonged (PEEP ⩾35 cm H2O for >60 s) LRMs in patients with moderate to severe ARDS (strong recommendation, moderate certainty).
Background
Higher PEEP can facilitate alveolar recruitment and prevent cyclic opening/closing injury, which may in turn improve gas exchange by decreasing intrapulmonary shunting and reduce lung stress (72). However, PEEP can also cause injurious overdistension in aerated lung and hemodynamic compromise via increased right ventricular afterload and decreased venous return. The net balance of benefit to harm is reliant on the proportion of recruitment to overdistension in an individual patient. The 2017 Clinical Practice Guideline previously issued conditional recommendations suggesting higher versus lower PEEP and the use of LRMs in patients with moderate to severe ARDS (7). Since that time, several large RCTs evaluating various PEEP strategies have been published (73, 74). Some have included cointerventions of prolonged LRMs, defined as incremental increases in PEEP to achieve airway pressures ⩾35 cm H2O for ⩾60 seconds. Thus, it was important to incorporate these most recent studies into an updated recommendation.
Evidence summary
This recommendation was based on evidence from two meta-analyses. The first was a recently published network meta-analysis comparing the relative effects of different PEEP strategies using a Bayesian analysis framework; 18 RCTs with 4,646 participants with moderate to severe ARDS were included (75). Compared with lower PEEP, higher PEEP without LRMs probably reduced mortality (n = 4 trials, 1,162 patients; RR, 0.77; 95% credible interval [CrI], 0.60–0.96; high certainty) (76–79), improved oxygenation (MD PaO2/FiO2 ratio 63.7 mm Hg higher; 95% CrI, 51.5–75.9 mm Hg; high certainty), and possibly increased ventilator-free days (MD, 1.3 d more; 95% CI, 2.5 d fewer to 4.3 d more; low certainty). The impact on barotrauma was uncertain (RR, 1.13; 95% Crl, 0.87–1.86; very low certainty). Compared with higher PEEP without LRMs, higher PEEP with prolonged LRMs probably increased mortality (RR, 1.37; 95% CrI, 1.04–1.81; moderate certainty), whereas strategies involving higher PEEP with brief LRMs or esophageal pressure-guided PEEP titration may have no effect on mortality (RR, 1.07; 95% CrI, 0.79–1.48; low certainty; and RR, 1.00; 95% CrI, 0.65–1.54; moderate certainty, respectively). The second meta-analysis was a prior meta-analysis of individual patient data that included three RCTs with 2,299 patients with ARDS and demonstrated that higher PEEP probably improved survival compared with lower PEEP in patients with moderate to severe ARDS (RR, 0.90; 05% CI, 0.81–1.00; P = 0.049), but possibly increased mortality in patients with mild ARDS (adjusted RR, 1.29; 95% CI, 0.91–1.83; P = 0.02) (80).
Justification and implementation considerations
Although higher PEEP was consistently associated with lower mortality in patients with moderate to severe ARDS, the panel issued a conditional recommendation because of a high level of heterogeneity among higher PEEP strategies in the included RCTs. For patients with mild ARDS, there were insufficient data to make a recommendation on PEEP strategy because these patients were excluded from the network meta-analysis, but there appears to be no benefit of high PEEP versus low PEEP, and there is a potential trend toward harm (80). With regard to prolonged LRMs, the panel issued a strong recommendation against their use in combination with high PEEP strategies based on the network meta-analysis demonstrating a high posterior probability of harm, presumably due to serious adverse hemodynamic effects. Although shorter LRMs may be better tolerated, we do not know the safe upper limit for LRM pressure or duration, which may vary between individual patients. Finally, there was a lack of consensus among the panel on brief LRMs and the use of esophageal pressures to set PEEP as a result of high levels of uncertainty of the true effect of these strategies.
A reasonable implementation approach for patients with moderate to severe ARDS would be to use a higher PEEP strategy previously implemented in the RCTs included in the aforementioned meta-analyses (Figure 2). Techniques that have been described included oxygenation-based titration (i.e., using a PEEP/FiO2 table) (76, 81), increasing PEEP to a maximal safe plateau pressure (77), and titration to maximal compliance (78) (Table E11 in the online supplement). The strategy chosen should be tailored to the clinician’s expertise and accompanied by continuous monitoring of respiratory mechanics, hemodynamics, and assessments of the patient’s physiologic response to PEEP.
Uncertainties and research priorities
The optimal strategy for setting PEEP in patients with ARDS remains uncertain. None of the included RCTs incorporated assessments of lung “recruitability” in response to higher PEEP strategies. Validating strategies to assess for lung recruitability at the bedside, such as the use of oxygenation response (82), driving pressure change (83), recruitment/inflation ratio (84), stress index (85), or electrical impedance tomography (86), may help guide individualized PEEP titration. A large multicenter trial evaluating setting PEEP based on respiratory mechanics (recruitability and effort) is ongoing (CAVI-ARDS [Careful Ventilation in Acute Respiratory Distress Syndrome] trial; www.clinicaltrials.gov ID, NCT03963622). There is an essential need for further studies to evaluate the effect of PEEP strategies in specific populations (e.g., obese patients) and specific ARDS phenotypes (e.g., hyper-/hypoinflammatory) and with concomitant interventions (e.g., proning) (87, 88). There is likely no uniform best PEEP strategy for all patients with ARDS, and these future research efforts may help identify patients who are most likely to benefit from each PEEP strategy.
Discussion
Although significant advancements have been made in the management of ARDS, many questions remain. Several recommendations in this guideline are conditional in nature and, as such, require careful evaluation of patient and illness characteristics when considering their use. Future studies may serve to strengthen these recommendations or provide additional caveats to their implementation. Measures with more established evidence of benefit also exist, including lung protective ventilation for all patients with ARDS and prone positioning for those with severe ARDS. Although strong recommendations in favor of these measures have previously been made, translating evidence into practice has been fraught with challenges (89). Considerable practice variation exists in ARDS management, and evidence-based modalities remain underused. This underuse is associated with increased mortality, suggesting that there is significant opportunity to improve ARDS outcomes (16, 90, 91). To maximize these opportunities, future efforts must be made to facilitate access to readily available, granular data about ARDS management practices in real-world settings to allow for benchmarking, auditing, and continuous quality improvement. Additionally, it is crucial to understand the clinician-, systems-, and patient-level barriers to, and facilitators of, the use of evidence-based supportive care in ARDS to inform a comprehensive approach to implementation.
In addition to implementation research, there are several opportunities to address other areas of uncertainty. Much remains unknown about the impact of supportive measures used for ARDS on the long-term outcomes of survivors, an issue of vital importance to patients (92, 93). There is a critical need for future clinical trials to not only consistently collect these data, but also to involve patient and family representatives to help identify and guide the selection of specific outcomes to study (94, 95). There are also other modalities used in a small but significant minority of patients with ARDS, such as pulmonary vasodilators and alternative ventilator modes (16, 56, 96), for which further data are needed before meaningful recommendations can be made. Additionally, although supportive therapies are often used in combination rather than in isolation (16), it remains unknown whether combination treatments are synergistic. Treatment effects can also vary across individuals, a concept known as heterogeneity of treatment effect, which is an issue that may be especially relevant to ARDS (97). There is substantial heterogeneity in ARDS, including patient characteristics, underlying etiologies, mechanisms of injury, and degrees of severity. In light of these issues, there has been growing interest in identifying homogeneous subgroups in ARDS with potential differential responses to treatment (98). Although the methods for subphenotyping patients with ARDS are currently investigational, the identification of distinct subsets of patients may provide an opportunity to improve patient selection for clinical trials in the future and ultimately increase the likelihood of finding effective interventions (99).
Our recommendations are largely consistent with recent guidelines published by the ESICM (100), although differences in methodology and the specific elements of clinical questions addressed account for some areas of divergence. With regard to PEEP, the ESICM guideline makes no recommendation for or against the routine use of higher versus lower PEEP strategies in ARDS, whereas we suggest the use of higher PEEP in select patients. However, it is important to note that our recommendation is narrower with regard to the patient population (moderate to severe ARDS only) and intervention (higher PEEP without accompanying recruitment maneuvers). Recommendations on NMBAs are also notably different: the ESICM guideline recommends against routine NMBA use in moderate to severe ARDS, whereas we suggest its use in early severe ARDS. This contrast reflects differences in studies and outcomes included in the evidence syntheses and, as with PEEP, our recommendations focus on a more limited patient population (severe ARDS only) and more specific intervention (early use of NMBAs).
Conclusions
The evidence base for supportive modalities for ARDS continues to evolve. As part of this guideline, we provide conditional recommendations supporting the use of corticosteroids in ARDS, VV-ECMO in selected patients with severe ARDS, neuromuscular blockers in early severe ARDS, and higher PEEP without LRMs in moderate to severe ARDS. Implementation of these recommendations should take into account individual patient and illness characteristics. These guidelines update and build on those developed in 2017 and will be revisited as new information is available.
The ATS Quality Improvement and Implementation Committee reviewed the guideline and determined that none of the new recommendations are suitable for performance measure development. However, two recommendations that remain in place from the 2017 guidelines are suitable for performance measure development: 1) the use of mechanical ventilation strategies that limit tidal volume (4–8 ml/kg predicted body weight) and inspiratory pressures (plateau pressure <30 cm H2O) in patients with ARDS and 2) prone positioning for >12 hours per day in patients with severe ARDS.
Acknowledgments
Acknowledgment: The authors thank John Harmon, Rachel Kaye, and the ATS Documents and Implementation Committee.
This official Clinical Practice Guideline was prepared by an ad hoc subcommittee of the ATS Assembly on Critical Care.
Members of the subcommittee are as follows:
Eddy Fan, M.D., Ph.D. (Co-Chair)1
Allan J. Walkey, M.D., M.Sc. (Co-Chair)2
Corticosteroids Group:
Charlotte Summers, M.D., Ph.D. (Chair)3
Roy G. Brower, M.D.4
Lisa Burry, Pharm.D., Ph.D.5
Jen-Ting Chen, M.D., M.Sc.6
Catherine L. Hough, M.D., M.Sc.7
Anica Law, M.D., M.S.2
Laurent Papazian, M.D., Ph.D.8
VV-ECMO Group:
Nida Qadir, M.D. (Chair)9
Darryl Abrams, M.D.10
Jen-Ting Chen, M.D., M.Sc.6
Carol Hodgson, Ph.D., B.App.Sc.11
Francois Lamontagne, M.D., M.Sc.12
Matthew Siuba, D.O., M.S.13
Neuromuscular Blockade Group:
Laveena Munshi, M.D. (Chair)1
Darryl Abrams, M.D.10
Jeremy Beitler, M.D., M.P.H.10
Giacomo Bellani, M.D., Ph.D.14,15
Lisa Burry, Pharm.D., Ph.D.5
Catherine L. Hough, M.D., M.Sc.7
Anica Law, M.D., M.S.2
Laurent Papazian, M.D., Ph.D.8
Tai Pham, M.D., Ph.D.16,17
Irene Telias, M.D.1,18,19
PEEP Group:
Sarina Sahetya, M.D., M.H.S. (Chair)4
Jeremy Beitler, M.D., M.P.H.10
Giacomo Bellani, M.D., Ph.D.14,15
Roy G. Brower, M.D.4
Carol Hodgson, Ph.D., B.App.Sc.11
Francois Lamontagne, M.D., M.Sc.12
Tai Pham, M.D., Ph.D.16,17
Matthew Siuba, D.O., M.S.13
Irene Telias, M.D.1,18,19
Methodology Group:
Bram Rochwerg, M.D., M.Sc. (Chair)20,21
Dipayan Chaudhuri, M.D.20,21
Setu Petolia, M.D., M.P.H.22
Patient Representative:
Eileen Rubin, J.D.23
1Interdepartmental Division of Critical Care Medicine, University Health Network and Sinai Health System, University of Toronto, Toronto, Ontario, Canada; 2Division of Pulmonary, Critical Care, Allergy and Sleep Medicine, Department of Medicine, Boston University School of Medicine, Boston, Massachusetts; 3Victor Phillip Dahdaleh Heart and Lung Research Institute, University of Cambridge, Cambridge, United Kingdom; 4Division of Pulmonary & Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland; 5Sinai Health System, University of Toronto Leslie Dan Faculty of Pharmacy, Toronto, Canada; 6Department of Medicine, University of California, San Francisco, California; 7Department of Medicine, Oregon Health and Science University, Portland, Oregon; 8Faculté de Médecine, Centre d’Etudes et de Recherches sur les Services de Santé et Qualité de Vie, Aix-Marseille Université, Marseille, France; 9David Geffen School of Medicine at UCLA, Los Angeles, California; 10Columbia University College of Physicians and Surgeons/New York Presbyterian Hospital, New York, New York; 11Australian and New Zealand Intensive Care Research Centre, Monash University, Melbourne, Victoria, Australia; 12Université Sherbrooke, Centre de Recherche due Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, Quebec, Canada; 13Critical Care Medicine, Respiratory Institute, Cleveland Clinic, Cleveland, Ohio; 14Centre for Medical Sciences CISMed, University of Trento, Trento, Italy; 15Department of Anesthesia and Intensive Care, Santa Chiara Hospital, Azienda Provinciale per i Servizi Sanitari Trento, Trento, Italy; 16Service de Médecine Intensive-Réanimation, Assistance Publique–Hôpitaux de Paris, Hôpital de Bicêtre, DMU CORREVE, FHU SEPSIS, Groupe de Recherche CARMAS, Hôpitaux Universitaires Paris-Saclay, Le Kremlin-Bicêtre, France; 17Université Paris-Saclay, UVSQ, Université Paris-Sud, Institut National de la Santé et de la Recherche Médicale U1018, Equipe d’Epidémiologie Respiratoire Intégrative, CESP, Villejuif, France; 18Keenan Research Centre for Biomedical Science, Division of Respirology, Department of Medicine, University Health Network and Sinai Health System, Toronto, Ontario, Canada; 19Li Ka Shing Knowledge Institute, Unity Health Toronto, Toronto, Ontario, Canada; 20Department of Health Research Methods, Evidence & Impact and 21Department of Medicine, Division of Critical Care, McMaster University, Hamilton, Ontario, Canada; 22SSM Health Saint Louis University Hospital, St. Louis, Missouri; and 23ARDS Foundation, Northbrook, Illinois.
Footnotes
This official Clinical Practice Guideline of the American Thoracic Society was approved September 2023
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Subcommittee disclosures: S.S. received research support from NIH-NHLBI. L.M. served on data safety and monitoring board for AFREE-ECMO; served in leadership or fiduciary role for ICM and TMS; received research support from CIHR, H. Barrie Fairley Award, University of Toronto; received travel support from Blood Diseases in the ICU. C.S. employed by Selwyn College and University of Cambridge; has financial stake in Bottril-Summers Consulting and Exvastat. J.B. served on advisory board for Arrowhead, Biomarck, Global Blood Therapeutics, Hamilton, STIMIT; served as consultant for Biomarck, Global Blood Therapeutics, Sedana; received research support from Hamilton, NIH, Sedana. G.B. served as consultant and received royalties from Flowmeter SpA; received honoraria and research support from Draeger; served as speaker for Chiesi, Draeger, GE Healthcare, Getinge. J.T.C. served on advisory committee for Gilead; served on data safety and monitoring board for CRISP; received honoraria from Baxter; served in leadership or fiduciary role for SCCM-Internal Medicine; received research support from SCCM, Discover Network. C.L.H. served as consultant for NIH; served on data safety and monitoring board for i-SPY COVID, iRehab, Pandora, QuantumLeap, TEAM; received honoraria from Beth Israel and UCLA; received research support from American Lung Association, CDC, NIH. A.L. received research support from NIH; received royalties from McGraw Hill. L.P. served on data safety and monitoring board for Clermont University Hospital; serves as president of the French Intensive Care Society; provided expert testimony for Air Liquide Santé; received honoraria from Gettinge; received research support from Air Liquide Santé and the French Ministry of Health. I.T. served as consultant for MBMed; served on data safety and monitoring board for STIMIT – Drager; received honoraria from Getinge and Medtronic; received research support from Canadian Institutes of Health Research. A.W. received research support from AHRQ, Critical Path Institute, Department of Defense, Gilead, Gordon and Betty Moore Foundation, NIH/NHLBI; received royalties from UpToDate. E.F. served on advisory committee for ALung Technologies, Baxter, Inspira, Vasomune; served as consultant for ALung Technologies, Baxter, GE Healthcare, Getinge, Inspira, MC3 Cardiopulmonary, Vasomune, Zoll Medical; served on executive committee for ECMONet; received research support from Baxter, LivaNova, Mallinckrodt, Medtronic; served as speaker for Aerogen and Getinge. N.Q., D.A., R.G.B., L.B., C.H., F.L., T.P., E.R., M.S., S.P., D.C., B.R. reported no commercial or relevant non-commercial interests from ineligible companies.
References
- 1. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med . 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 3. Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med . 2017;377:1904–1905. doi: 10.1056/NEJMc1711824. [DOI] [PubMed] [Google Scholar]
- 4. Bos LDJ, Ware LB. Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. Lancet . 2022;400:1145–1156. doi: 10.1016/S0140-6736(22)01485-4. [DOI] [PubMed] [Google Scholar]
- 5. Matthay MA, Arabi Y, Arroliga AC, Bernard G, Bersten AD, Brochard LJ, et al. A new global definition of acute respiratory distress syndrome. Am J Respir Crit Care Med . 2024;209:37–47. doi: 10.1164/rccm.202303-0558WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gorman EA, O’Kane CM, McAuley DF. Acute respiratory distress syndrome in adults: diagnosis, outcomes, long-term sequelae, and management. Lancet . 2022;400:1157–1170. doi: 10.1016/S0140-6736(22)01439-8. [DOI] [PubMed] [Google Scholar]
- 7. Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med . 2017;195:1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 8. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol . 2011;64:380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 9.American Thoracic Society. 2015. https://www.thoracic.org/statements/document-development/resources/cpg-specific-coi-policy.pdf
- 10. Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol . 2011;64:395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 11. Dinglas VD, Faraone LN, Needham DM. Understanding patient-important outcomes after critical illness: a synthesis of recent qualitative, empirical, and consensus-related studies. Curr Opin Crit Care . 2018;24:401–409. doi: 10.1097/MCC.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol . 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 13. Li SA, Alexander PE, Reljic T, Cuker A, Nieuwlaat R, Wiercioch W, et al. Evidence to decision framework provides a structured “roadmap” for making GRADE guidelines recommendations. J Clin Epidemiol . 2018;104:103–112. doi: 10.1016/j.jclinepi.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ . 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rochwerg B, Alhazzani W, Jaeschke R. Clinical meaning of the GRADE rules. Intensive Care Med . 2014;40:877–879. doi: 10.1007/s00134-014-3273-0. [DOI] [PubMed] [Google Scholar]
- 16. Qadir N, Bartz RR, Cooter ML, Hough CL, Lanspa MJ, Banner-Goodspeed VM, et al. Severe ARDS: Generating Evidence (SAGE) Study Investigators; Society of Critical Care Medicine’s Discovery Network Variation in early management practices in moderate-to-severe ARDS in the United States: the Severe ARDS: Generating Evidence Study. Chest . 2021;160:1304–1315. doi: 10.1016/j.chest.2021.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamontagne F, Agarwal A, Rochwerg B, Siemieniuk RA, Agoritsas T, Askie L, et al. A living WHO guideline on drugs for covid-19. BMJ . 2020;370:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 18. Dequin PF, Meziani F, Quenot JP, Kamel T, Ricard JD, Badie J, et al. CRICS-TriGGERSep Network Hydrocortisone in severe community-acquired pneumonia. N Engl J Med . 2023;388:1931–1941. doi: 10.1056/NEJMoa2215145. [DOI] [PubMed] [Google Scholar]
- 19. Chaudhuri D, Sasaki K, Karkar A, Sharif S, Lewis K, Mammen MJ, et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med . 2021;47:521–537. doi: 10.1007/s00134-021-06394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Annane D, Sébille V, Bellissant E, Ger-Inf-05 Study Group Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit Care Med . 2006;34:22–30. doi: 10.1097/01.ccm.0000194723.78632.62. [DOI] [PubMed] [Google Scholar]
- 21. Liu L, Li J, Huang YZ, Liu SQ, Yang CS, Guo FM, et al. [The effect of stress dose glucocorticoid on patients with acute respiratory distress syndrome combined with critical illness-related corticosteroid insufficiency] Zhonghua Nei Ke Za Zhi . 2012;51:599–603. [PubMed] [Google Scholar]
- 22. Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest . 2007;131:954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 23. Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA . 1998;280:159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 24. Rezk NA, Ibrahim AM. Effects of methyl prednisolone in early ARDS. Egypt J Chest Dis Tuberc . 2013;62:167–172. [Google Scholar]
- 25. Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med . 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 26. Tongyoo S, Permpikul C, Mongkolpun W, Vattanavanit V, Udompanturak S, Kocak M, et al. Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: results of a randomized controlled trial. Crit Care . 2016;20:329. doi: 10.1186/s13054-016-1511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone in ARDS Network Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med . 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 28. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med . 2020;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, et al. Metcovid Team Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; Metcovid): a randomized, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis . 2021;72:e373–e381. doi: 10.1093/cid/ciaa1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. COALITION COVID-19 Brazil III Investigators Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA . 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. CAPE COVID Trial Group and the CRICS-TriGGERSep Network Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA . 2020;324:1298–1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Writing Committee for the REMAP-CAP Investigators Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA . 2020;324:1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jamaati H, Hashemian SM, Farzanegan B, Malekmohammad M, Tabarsi P, Marjani M, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: a preliminary report of a randomized clinical trial. Eur J Pharmacol . 2021;897:173947. doi: 10.1016/j.ejphar.2021.173947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhi-fang S, Li-jun Y. Effect of glucocorticoid on extravascular lung water in the patients with acute respiratory distress syndrome. Chinese J Crit Care Med . 2016;2016:443–447. [Google Scholar]
- 35. Zhou X, Liu D, Long Y, Zhang Q, Cui N, He H, et al. [The effects of prone position ventilation combined with recruitment maneuvers on outcomes in patients with severe acute respiratory distress syndrome] Zhonghua Nei Ke Za Zhi . 2014;53:437–441. [PubMed] [Google Scholar]
- 36. Waljee AK, Rogers MA, Lin P, Singal AG, Stein JD, Marks RM, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ . 2017;357:j1415. doi: 10.1136/bmj.j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ayodele OA, Cabral HJ, McManus DD, Jick SS. Glucocorticoids and risk of venous thromboembolism in asthma patients aged 20-59 years in the United Kingdom’s CPRD 1995–2015. Clin Epidemiol . 2022;14:83–93. doi: 10.2147/CLEP.S341048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ewald H, Raatz H, Boscacci R, Furrer H, Bucher HC, Briel M. Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV infection. Cochrane Database Syst Rev . 2015;2015:CD006150. doi: 10.1002/14651858.CD006150.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Munshi L, Brodie D, Fan E. Extracorporeal support for acute respiratory distress syndrome in adults. NEJM Evid . 2022;1:10. doi: 10.1056/EVIDra2200128. [DOI] [PubMed] [Google Scholar]
- 40. Brodie D, Slutsky AS, Combes A. Extracorporeal life support for adults with respiratory failure and related indications: a review. JAMA . 2019;322:557–568. doi: 10.1001/jama.2019.9302. [DOI] [PubMed] [Google Scholar]
- 41.Extracorporeal Life Support Organization. 2023. https://www.elso.org/registry/internationalsummaryandreports/internationalsummary.aspx
- 42. Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. EOLIA Trial Group, REVA, and ECMONet Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med . 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 43. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. CESAR Trial Collaboration Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet . 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 44. Combes A, Peek GJ, Hajage D, Hardy P, Abrams D, Schmidt M, et al. ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med . 2020;46:2048–2057. doi: 10.1007/s00134-020-06248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med . 2015;191:894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Verma A, Hadaya J, Williamson C, Kronen E, Sakowitz S, Bakhtiyar SS, et al. A contemporary analysis of the volume-outcome relationship for extracorporeal membrane oxygenation in the United States. Surgery . 2023;173:1405–1410. doi: 10.1016/j.surg.2023.02.004. [DOI] [PubMed] [Google Scholar]
- 47. Tonna JE, Abrams D, Brodie D, Greenwood JC, Rubio Mateo-Sidron JA, Usman A, et al. Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): guideline from the Extracorporeal Life Support Organization (ELSO) ASAIO J . 2021;67:601–610. doi: 10.1097/MAT.0000000000001432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med . 2014;189:1374–1382. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 49. Mehta AB, Taylor JK, Day G, Lane TC, Douglas IS. Disparities in adult patient selection for extracorporeal membrane oxygenation in the United States: a population-level study. Ann Am Thorac Soc . 2023;20:1166–1174. doi: 10.1513/AnnalsATS.202212-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barrett KA, Hawkins N, Fan E. Economic evaluation of venovenous extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Crit Care Med . 2019;47:186–193. doi: 10.1097/CCM.0000000000003465. [DOI] [PubMed] [Google Scholar]
- 51. García A, Giraldo ND. Cost-effectiveness of extracorporeal membrane oxygenation in patients with acute respiratory distress syndrome in Colombia. Biomedica . 2022;42:707–716. doi: 10.7705/biomedica.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Combes A, Brodie D, Bartlett R, Brochard L, Brower R, Conrad S, et al. International ECMO Network (ECMONet) Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med . 2014;190:488–496. doi: 10.1164/rccm.201404-0630CP. [DOI] [PubMed] [Google Scholar]
- 53. Wilcox ME, Jaramillo-Rocha V, Hodgson C, Taglione MS, Ferguson ND, Fan E. Long-term quality of life after extracorporeal membrane oxygenation in ARDS survivors: systematic review and meta-analysis. J Intensive Care Med . 2020;35:233–243. doi: 10.1177/0885066617737035. [DOI] [PubMed] [Google Scholar]
- 54. Hodgson CL, Higgins AM, Bailey MJ, Anderson S, Bernard S, Fulcher BJ, et al. EXCEL Study Investigators on behalf of the International ECMO Network and the Australian and New Zealand Intensive Care Society Clinical Trials Group Incidence of death or disability at 6 months after extracorporeal membrane oxygenation in Australia: a prospective, multicentre, registry-embedded cohort study. Lancet Respir Med . 2022;10:1038–1048. doi: 10.1016/S2213-2600(22)00248-X. [DOI] [PubMed] [Google Scholar]
- 55. Fan E, Gattinoni L, Combes A, Schmidt M, Peek G, Brodie D, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory failure: a clinical review from an international group of experts. Intensive Care Med . 2016;42:712–724. doi: 10.1007/s00134-016-4314-7. [DOI] [PubMed] [Google Scholar]
- 56. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. LUNG SAFE Investigators; ESICM Trials Group Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA . 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 57. Slutsky AS. Neuromuscular blocking agents in ARDS. N Engl J Med . 2010;363:1176–1180. doi: 10.1056/NEJMe1007136. [DOI] [PubMed] [Google Scholar]
- 58. Forel JM, Roch A, Marin V, Michelet P, Demory D, Blache JL, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med . 2006;34:2749–2757. doi: 10.1097/01.CCM.0000239435.87433.0D. [DOI] [PubMed] [Google Scholar]
- 59. Gainnier M, Roch A, Forel JM, Thirion X, Arnal JM, Donati S, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med . 2004;32:113–119. doi: 10.1097/01.CCM.0000104114.72614.BC. [DOI] [PubMed] [Google Scholar]
- 60. Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, et al. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med . 2019;380:1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tatham KC, Ferguson ND, Zhou Q, Hand L, Austin P, Taneja R, et al. Evolution of practice patterns in the management of acute respiratory distress syndrome: a secondary analysis of two successive randomized controlled trials. J Crit Care . 2021;65:274–281. doi: 10.1016/j.jcrc.2021.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guervilly C, Bisbal M, Forel JM, Mechati M, Lehingue S, Bourenne J, et al. Effects of neuromuscular blockers on transpulmonary pressures in moderate to severe acute respiratory distress syndrome. Intensive Care Med . 2017;43:408–418. doi: 10.1007/s00134-016-4653-4. [DOI] [PubMed] [Google Scholar]
- 63. Lyu G, Wang X, Jiang W, Cai T, Zhang Y. [Clinical study of early use of neuromuscular blocking agents in patients with severe sepsis and acute respiratory distress syndrome] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue . 2014;26:325–329. doi: 10.3760/cma.j.issn.2095-4352.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 64. Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. ACURASYS Study Investigators Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med . 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 65. Rao S, Messacar K, Torok MR, Rick AM, Holzberg J, Montano A, et al. Enterovirus D68 in critically ill children: a comparison with pandemic H1N1 influenza. Pediatr Crit Care Med . 2016;17:1023–1031. doi: 10.1097/PCC.0000000000000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tarazan N, Alshehri M, Sharif S, Al Duhailib Z, Møller MH, Belley-Cote E, et al. GUIDE Group Neuromuscular blocking agents in acute respiratory distress syndrome: updated systematic review and meta-analysis of randomized trials. Intensive Care Med Exp . 2020;8:61. doi: 10.1186/s40635-020-00348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med . 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 68. Fanelli V, Morita Y, Cappello P, Ghazarian M, Sugumar B, Delsedime L, et al. Neuromuscular blocking agent cisatracurium attenuates lung injury by inhibition of nicotinic acetylcholine receptor-α1. Anesthesiology . 2016;124:132–140. doi: 10.1097/ALN.0000000000000907. [DOI] [PubMed] [Google Scholar]
- 69. Sottile PD, Albers D, Moss MM. Neuromuscular blockade is associated with the attenuation of biomarkers of epithelial and endothelial injury in patients with moderate-to-severe acute respiratory distress syndrome. Crit Care . 2018;22:63. doi: 10.1186/s13054-018-1974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Goligher EC, Dres M, Patel BK, Sahetya SK, Beitler JR, Telias I, et al. Lung- and diaphragm-protective ventilation. Am J Respir Crit Care Med . 2020;202:950–961. doi: 10.1164/rccm.202003-0655CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Doorduin J, Nollet JL, Roesthuis LH, van Hees HW, Brochard LJ, Sinderby CA, et al. Partial neuromuscular blockade during partial ventilatory support in sedated patients with high tidal volumes. Am J Respir Crit Care Med . 2017;195:1033–1042. doi: 10.1164/rccm.201605-1016OC. [DOI] [PubMed] [Google Scholar]
- 72. Sahetya SK, Mancebo J, Brower RG. Fifty years of research in ARDS. Vt selection in acute respiratory distress syndrome. Am J Respir Crit Care Med . 2017;196:1519–1525. doi: 10.1164/rccm.201708-1629CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cavalcanti AB, Suzumura ÉA, Laranjeira LN, Paisani DM, Damiani LP, Guimarães HP, et al. Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA . 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hodgson CL, Cooper DJ, Arabi Y, King V, Bersten A, Bihari S, et al. Maximal recruitment open lung ventilation in acute respiratory distress syndrome (PHARLAP). A phase II, multicenter randomized controlled clinical trial. Am J Respir Crit Care Med . 2019;200:1363–1372. doi: 10.1164/rccm.201901-0109OC. [DOI] [PubMed] [Google Scholar]
- 75. Dianti J, Tisminetzky M, Ferreyro BL, Englesakis M, Del Sorbo L, Sud S, et al. Association of positive end-expiratory pressure and lung recruitment selection strategies with mortality in acute respiratory distress syndrome: a systematic review and network meta-analysis. Am J Respir Crit Care Med . 2022;205:1300–1310. doi: 10.1164/rccm.202108-1972OC. [DOI] [PubMed] [Google Scholar]
- 76. Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. National Heart, Lung, and Blood Institute ARDS Clinical Trials Network Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med . 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 77. Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. Expiratory Pressure (Express) Study Group Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA . 2008;299:646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 78. Pintado MC, de Pablo R, Trascasa M, Milicua JM, Rogero S, Daguerre M, et al. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care . 2013;58:1416–1423. doi: 10.4187/respcare.02068. [DOI] [PubMed] [Google Scholar]
- 79. Salem MS, Eltatwy HS, Abdelhafez AA, Alsharif SEI. Lung ultrasound- versus FiO2-guided PEEP in ARDS patients. Egypt J Anaesth . 2020;36:31–37. [Google Scholar]
- 80. Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA . 2010;303:865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 81. Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Lung Open Ventilation Study Investigators Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA . 2008;299:637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 82. Yehya N, Hodgson CL, Amato MBP, Richard JC, Brochard LJ, Mercat A, et al. Response to ventilator adjustments for predicting acute respiratory distress syndrome mortality. Driving pressure versus oxygenation. Ann Am Thorac Soc . 2021;18:857–864. doi: 10.1513/AnnalsATS.202007-862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sahetya SK, Fan E. Driving pressure: the road ahead. Respir Care . 2019;64:1017–1020. doi: 10.4187/respcare.07226. [DOI] [PubMed] [Google Scholar]
- 84. Chen L, Del Sorbo L, Grieco DL, Junhasavasdikul D, Rittayamai N, Soliman I, et al. Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome. A clinical trial. Am J Respir Crit Care Med . 2020;201:178–187. doi: 10.1164/rccm.201902-0334OC. [DOI] [PubMed] [Google Scholar]
- 85. Ferrando C, Suárez-Sipmann F, Gutierrez A, Tusman G, Carbonell J, García M, et al. Adjusting tidal volume to stress index in an open lung condition optimizes ventilation and prevents overdistension in an experimental model of lung injury and reduced chest wall compliance. Crit Care . 2015;19:9. doi: 10.1186/s13054-014-0726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Franchineau G, Bréchot N, Lebreton G, Hekimian G, Nieszkowska A, Trouillet JL, et al. Bedside contribution of electrical impedance tomography to setting positive end-expiratory pressure for extracorporeal membrane oxygenation-treated patients with severe acute respiratory distress syndrome. Am J Respir Crit Care Med . 2017;196:447–457. doi: 10.1164/rccm.201605-1055OC. [DOI] [PubMed] [Google Scholar]
- 87. Fumagalli J, Santiago RRS, Teggia Droghi M, Zhang C, Fintelmann FJ, Troschel FM, et al. Lung Rescue Team Investigators Lung recruitment in obese patients with acute respiratory distress syndrome. Anesthesiology . 2019;130:791–803. doi: 10.1097/ALN.0000000000002638. [DOI] [PubMed] [Google Scholar]
- 88. Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, NHLBI ARDS Network Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med . 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Qadir N, Chen JT. Adjunctive therapies in ARDS: the disconnect between clinical trials and clinical practice. Chest . 2020;157:1405–1406. doi: 10.1016/j.chest.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al. STOP-COVID Investigators Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med . 2020;180:1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dam TA, de Grooth HJ, Klausch T, Fleuren LM, de Bruin DP, Entjes R, et al. Some patients are more equal than others: variation in ventilator settings for coronavirus disease 2019 acute respiratory distress syndrome. Crit Care Explor . 2021;3:e0555. doi: 10.1097/CCE.0000000000000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med . 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 93. Herridge MS. Recovery and long-term outcome in acute respiratory distress syndrome. Crit Care Clin . 2011;27:685–704. doi: 10.1016/j.ccc.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 94. Needham DM, Sepulveda KA, Dinglas VD, Chessare CM, Friedman LA, Bingham CO, III, et al. Core outcome measures for clinical research in acute respiratory failure survivors. An international modified Delphi consensus study. Am J Respir Crit Care Med . 2017;196:1122–1130. doi: 10.1164/rccm.201702-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Dinglas VD, Chessare CM, Davis WE, Parker A, Friedman LA, Colantuoni E, et al. Perspectives of survivors, families and researchers on key outcomes for research in acute respiratory failure. Thorax . 2018;73:7–12. doi: 10.1136/thoraxjnl-2017-210234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Duggal A, Rezoagli E, Pham T, McNicholas BA, Fan E, Bellani G, et al. LUNG SAFE Investigators and the ESICM Trials Group Patterns of use of adjunctive therapies in patients with early moderate to severe ARDS: insights from the LUNG SAFE Study. Chest . 2020;157:1497–1505. doi: 10.1016/j.chest.2020.01.041. [DOI] [PubMed] [Google Scholar]
- 97. Khan YA, Fan E, Ferguson ND. Precision medicine and heterogeneity of treatment effect in therapies for ARDS. Chest . 2021;160:1729–1738. doi: 10.1016/j.chest.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care . 2019;25:12–20. doi: 10.1097/MCC.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ware LB, Matthay MA, Mebazaa A. Designing an ARDS trial for 2020 and beyond: focus on enrichment strategies. Intensive Care Med . 2020;46:2153–2156. doi: 10.1007/s00134-020-06232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Grasselli G, Calfee CS, Camporota L, Poole D, Amato MBP, Antonelli M, et al. European Society of Intensive Care Medicine Taskforce on ARDS ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med . 2023;49:727–759. doi: 10.1007/s00134-023-07050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]