Abstract
Background
Fatigue is the most common symptom among cancer survivors. Cancer-related fatigue (CRF) may occur at any point in the cancer care continuum. Multiple factors contribute to CRF development and severity, including cancer type, treatments, presence of other symptoms, comorbidities, and medication side effects. Clinically, increasing physical activity, enhancing sleep quality, and recognizing sleep disorders are integral to managing CRF. Unfortunately, CRF is infrequently recognized, evaluated, or treated in lung cancer survivors despite more frequent and severe symptoms than in other cancers. Therefore, increased awareness and understanding of CRF are needed to improve health-related quality of life in lung cancer survivors.
Objectives
1) To identify and prioritize knowledge and research gaps and 2) to develop and prioritize research questions to evaluate mechanistic, diagnostic, and therapeutic approaches to CRF among lung cancer survivors.
Methods
We convened a multidisciplinary panel to review the available literature on CRF, focusing on the impacts of physical activity, rehabilitation, and sleep disturbances in lung cancer. We used a three-round modified Delphi process to prioritize research questions.
Results
This statement identifies knowledge gaps in the 1) detection and diagnostic evaluation of CRF in lung cancer survivors; 2) timing, goals, and implementation of physical activity and rehabilitation; and 3) evaluation and treatment of sleep disturbances and disorders to reduce CRF. Finally, we present the panel’s initial 32 research questions and seven final prioritized questions.
Conclusions
This statement offers a prioritized research agenda to 1) advance clinical and research efforts and 2) increase awareness of CRF in lung cancer survivors.
Keywords: lung cancer, cancer-related fatigue, physical activity, rehabilitation, exercise training, sleep disturbance, sleep disorder, survivorship
Contents
Overview
Key Points and Recommendations
Introduction
Methods
-
Results
Section I: Overview of CRF in Lung Cancer Survivors
Section II: General Considerations for Research in CRF in Lung Cancer
Section III: Detection and Diagnostic Evaluation of CRF in Lung Cancer
Section IV: Timing, Goals, and Implementation of Physical Activity, Rehabilitation, and Exercise Training
Section V: Evaluation of Sleep Disruption and Underlying Sleep Disorders in Lung Cancer Survivors and CRF
Conclusions
Overview
Cancer-related fatigue (CRF) is common in lung cancer survivors and can occur at any point in the care continuum. CRF encompasses physical, emotional, and/or cognitive tiredness; is related to cancer or cancer treatments; and interferes with multiple dimensions of health-related quality of life (1–3). CRF is poorly understood, underrecognized, and undertreated. Furthermore, CRF often presents with a cluster of symptoms, including pain, mood disorders, and sleep disturbance. Compared with survivors of other cancer types, lung cancer survivors (i.e., anyone living with or beyond a lung cancer diagnosis) may have more severe and difficult-to-manage CRF symptoms. Unfortunately, most CRF-related research has focused on patients with other cancer types. The lack of data on CRF in lung cancer likely arises from the historically poor survival in this cohort. However, as lung cancer survival steadily improves, there is a critical need to prioritize CRF detection and treatment before, during, and after cancer-directed care. This American Thoracic Society (ATS) research statement aims to identify knowledge gaps and prioritize research questions to improve the recognition, evaluation, and treatment of CRF among lung cancer survivors to guide future clinical and research efforts.
Key Points and Recommendations
-
•
Fatigue negatively affects up to 80% of lung cancer survivors.
-
•
CRF is defined by the National Comprehensive Cancer Network as a “distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” (1).
-
•
The epidemiology, pathogenesis, evaluation, and preferred treatment of CRF in lung cancer survivors are poorly understood.
-
•
Symptom clusters in lung cancer may be unique compared with other cancer types; clinical recognition and management of symptom clusters are uncertain.
-
•
It is unclear if specific tumor characteristics (i.e., histology, location, and stage) and cancer treatment modalities influence CRF development, progression, and severity in lung cancer survivors.
-
•
Several screening tools for CRF have been validated in various cancer populations, but the optimal screening tool for lung cancer survivors is unknown.
-
•
Although physical activity appears beneficial in lung cancer survivors, there are significant knowledge gaps regarding the potential for physical activity programs to improve CRF (and the resultant biological changes).
-
•
Given the unique clinical characteristics of lung cancer survivors (i.e., the effects of advanced age, cigarette smoking, comorbid cardiopulmonary disease, and lung cancer and its treatments), there is a need to personalize CRF treatment.
-
•
There is a need for physical activity programs to have effective and sustainable implementation strategies to reduce CRF among lung cancer survivors, including program components, location, setting, and timing along the lung cancer course.
-
•
Sleep disturbances often coexist with CRF and may share common pathophysiologic pathways; however, there are limited data demonstrating whether treatment of sleep disturbances and/or symptom clusters can reduce CRF in lung cancer survivors.
Introduction
CRF is a common, distressing, complex, understudied, and underrecognized symptom during cancer survivorship (defined by the National Cancer Institute and the National Coalition for Cancer Survivorship as any time after cancer diagnosis; Figure 1) (1, 4–6). Although fatigue is reported by up to 40% of patients at cancer diagnosis (7), there is significant variability in CRF prevalence among studies, ranging from 12% to 80% in different cancers (8–11). In patients with metastatic disease, the prevalence of CRF exceeds 75% (12–14). CRF encompasses physical, emotional, and/or cognitive tiredness; development of mood symptoms (i.e., anxiety and depression); impaired sleep; and reduced physical activity (2, 3, 15). CRF overlaps with comorbidities among lung cancer survivors and significantly interferes with health-related quality of life (HRQoL) (3). As higher symptom burden, depression scores, and impairments of HRQoL are associated with worsened lung cancer survival, identification and treatment of CRF are critically important for lung cancer survivors (16–20). Unfortunately, strategies for evaluation, diagnosis, and treatment of CRF are inconsistent (1). As a result, CRF often goes undiagnosed and unmanaged, potentially impairing cancer treatment adherence, disease control, and patient outcomes (3).
Figure 1.
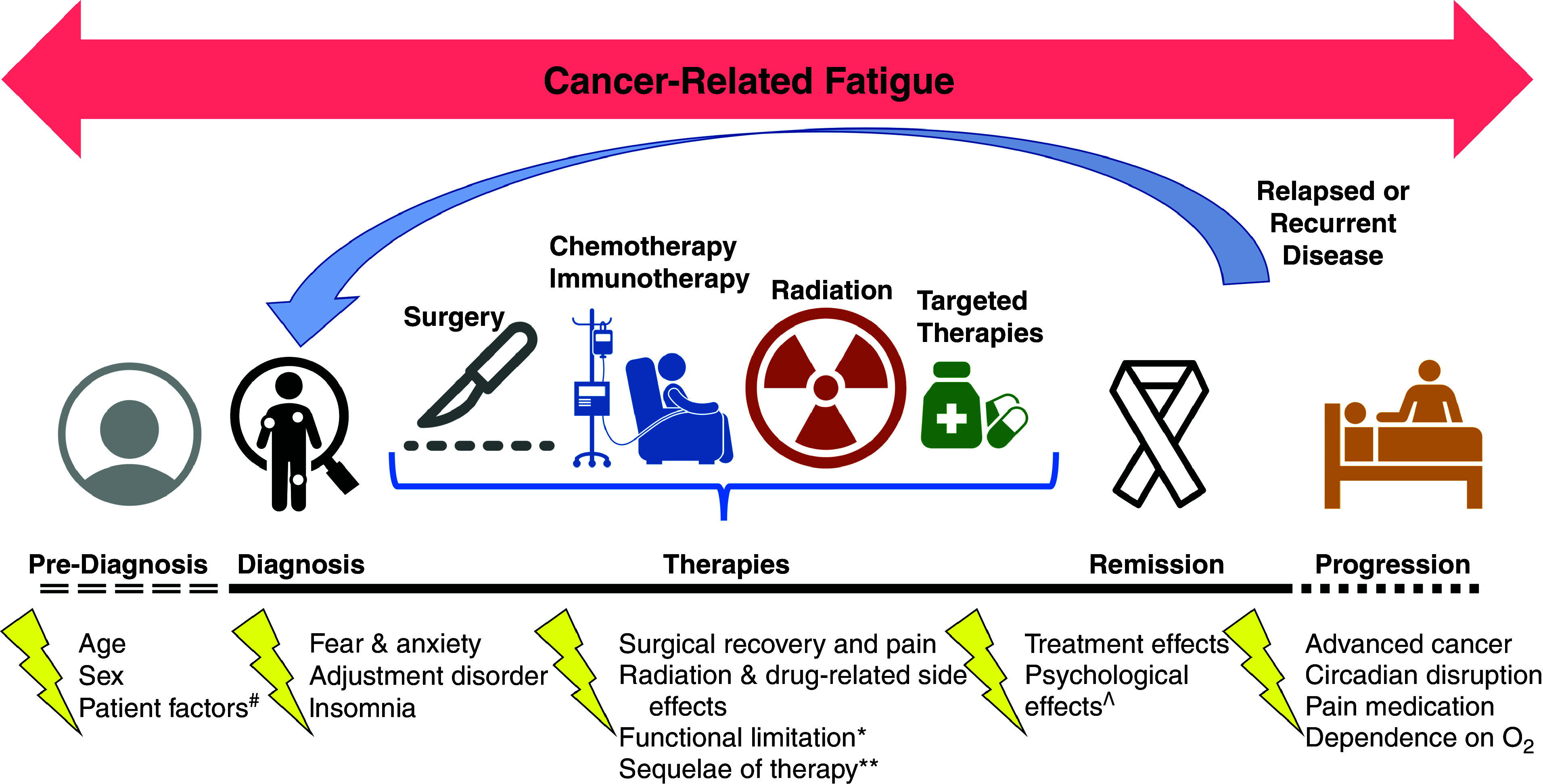
Cancer-related fatigue (CRF) over the continuum of cancer care. CRF may affect lung cancer survivors at any point during their cancer care. The National Comprehensive Cancer Network defines CRF as “Distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” (1). Routine screening for the presence of fatigue is encouraged from the point of diagnosis onward. #Comorbid (medical and psychiatric), habits (physical activity, sleep), and others. *Cardiac, pulmonary, and musculoskeletal. **Infections, hospitalizations, anemia, thyroid dysfunction, and metabolic conditions. ^Anxiety, depression, and post-traumatic stress disorder.
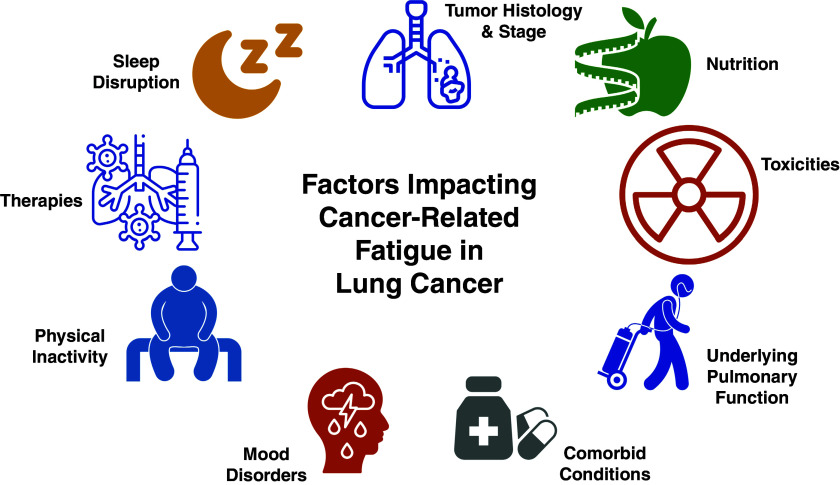
The biological basis and treatment strategies for CRF are unclear. The pathophysiology may be multifactorial and the result of a complex interplay among biological mediators, comorbidities (medical and psychiatric), chronic pain, treatment-related effects, physical activity, deconditioning, skeletal muscle dysfunction, and sleep disruption (Figure 2) (2, 3). Similarly, the optimal treatment for CRF is uncertain. The National Comprehensive Cancer Network (NCCN) guidelines for CRF include nonpharmacologic and pharmacologic strategies (1). Although the guidelines recognize that management of contributory comorbidities may improve CRF, available studies support that nonpharmacologic interventions (including physical activity, sleep, and psychosocial interventions) may be more efficacious than pharmacologic interventions (21–27), and some pharmacologic options (e.g., psychostimulants) remain investigational (1, 28). Therefore, in this work, we focus on nonpharmaceutical treatment options approachable by multiple cancer team members. Although physical activity may be an effective strategy to reduce CRF (21, 23–25, 27), physical activity generally decreases with time and treatment in most lung cancer survivors (29–33). Sleep disruption and sleep disorders are highly prevalent, underrecognized (34), and strongly correlate with CRF (35).
Figure 2.
Factors affecting cancer-related fatigue (CRF) in lung cancer. Multiple factors can affect the development and severity of CRF, including those related to the patient, cancer, or treatment. The etiology of CRF is uncertain, with multiple probable factors: immune/inflammatory, metabolic, neuroendocrine, and genetic.
Cancer survivorship guidelines recommend maintaining a physically active lifestyle with aerobic and resistance exercises to optimize symptoms, physical functioning, and HRQoL (36). A physically active lifestyle may also be an effective strategy to reduce CRF (21, 23–25, 27). Unfortunately, most lung cancer survivors are inactive, with physical activity and functional capacity generally decreasing with time and treatment (29–33). In addition, sleep disruption and sleep disorders are highly prevalent, underrecognized (34), and strongly correlate with CRF (35). The NCCN guideline on CRF recommends evaluating and treating underlying sleep disturbances (1). Both physical activity and sleep may play a central role in developing and/or ameliorating CRF, so an evidence-based framework for clinicians to evaluate and address physical inactivity, sleep disturbances, and CRF is needed.
As advances in cancer care have led to improved survival (37), the number of lung cancer survivors is expected to increase by ∼25% in the next decade (38). Furthermore, as lung cancer screening identifies disease in earlier stages, the number of lung cancer survivors struggling with CRF will continue to increase. An improved understanding of the frequency, pathophysiology, and options for diagnosing and treating CRF in lung cancer survivors is paramount. To this end, the ATS convened a multidisciplinary panel to 1) identify and prioritize knowledge and research gaps and 2) develop and prioritize research strategies to evaluate mechanistic, diagnostic, and treatment priorities in CRF in lung cancer survivors. The panel members agreed to focus on physical activity and sleep, as they may play a central role in developing and/or ameliorating CRF; evidence-based strategies for clinicians to evaluate and address these factors are needed.
Methods
A multidisciplinary and international panel was assembled, including members of the ATS Thoracic Oncology, Sleep and Respiratory Neurobiology, Pulmonary Rehabilitation, and Nursing Assemblies. The panel provided expertise in lung cancer, pulmonology, internal medicine, medical oncology, sleep medicine, thoracic surgery, physical medicine and rehabilitation, palliative care, psychiatry, physiotherapy, nursing, and social work and included a lung cancer survivor. We recognized that not all patients prefer the term “cancer survivor,” but we use this term throughout the document for clarity and consistency. Table 1 provides a summary of definitions of terms used throughout the statement.
Table 1.
Definitions of Terms
| Definition | Advantages (A) and Disadvantages (D) of Individual Terminology | |
|---|---|---|
| General | ||
| Cancer survivor | Anyone with a diagnosis of cancer, starting from the time of diagnosis to the end of life (National Coalition of Cancer Survivorship; National Cancer Institute) (5, 6) | A: Inclusive of all phases of the cancer experience D: Can be confused with “long-term” cancer survivor (typically ⩾5 yr after diagnosis) |
| Cancer-related fatigue | “A distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” (3) | A: Focuses on one symptom common among cancer survivors D: May be too focused or narrow and may miss other aspects patients experience related to fatigue, including concurrent symptoms and/or underlying mechanisms |
| Symptom cluster | Two or more concurrent symptoms characterized by stability over time, independence of other clusters, possibly shared underlying mechanism(s), shared outcomes(s), and a temporal relationship between symptoms (190) | A: Contains multiple symptoms that may better capture patients’ experiences D: No well-defined or validated symptom cluster, particularly in lung cancer |
| Physical activity | ||
| Physical activity | “Any bodily movement produced by skeletal muscles that require energy expenditure” (World Health Organization) (92) | A: Broadest definition of a physical behavior D: Nonspecific |
| Exercise | “Planned, structured, repetitive, and purposive in the sense that improvement or maintenance of one or more components of physical fitness is an objective” (93) | A: More specific than physical activity D: Lacks individual aspects of specific programs; omits activities that are occupationally related |
| Rehabilitation | “A set of interventions designed to optimize functioning and reduce disability in individuals with health conditions in interaction with their environment” (World Health Organization) (94) | A: Prioritizes interventions focusing on function D: “Rehab” may have negative connotation among some patients |
| Telerehabilitation | “The delivery of therapeutic rehabilitation at a distance or offsite using telecommunication technologies” (145); may be delivered to the patient’s home, to a remote healthcare facility, or in the community and characteristically contains some degree of two-way interaction between the patient and healthcare professional (95) | A: Includes a broad array of telecommunication technologies including video, text-based, and/or telephone interactions D: May be confused with other models of rehabilitation, including home-based and/or web-based rehabilitation |
| Pulmonary rehabilitation | “A comprehensive intervention based on a thorough patient assessment followed by patient tailored therapies that include, but are not limited to, exercise training, education, and behavior change, designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence to health-enhancing behaviors” (217) | A: 1) A multidisciplinary program focusing on optimizing function in patients with chronic lung disease; 2) individualized regimens incorporate interventions on multiple aspects of care: physical activity, education, psychologic, relaxation D: Might not be designed for patients with lung cancer without concomitant lung disease |
| Physical activity programs | In this document, the term “physical activity programs” encompasses • Physical activity • Exercise Rehabilitation (all types) |
A: Focuses on an important patient-centered outcome during or after rehabilitation D: Omits other components of rehabilitation, including psychological and/or social support |
| Sleep | ||
| Sleep disruption or disturbance | Entity that prevents or interrupts consolidated sleep, thus resulting in daytime symptoms including fatigue and sleepiness | A: Inclusive of various etiologies that may disturb sleep D: Nonspecific factor disrupting sleep that includes comorbid medical or psychiatric condition, pain, noise, potentially underlying sleep disorder |
| Sleep disorder | Refers to sleep disorder according to criteria defined by the International Classification of Sleep Disorders from one the following categories: insomnia, sleep-related breathing disorders, central disorders of hypersomnolence (narcolepsy, idiopathic hypersomnia), circadian rhythm sleep–wake disorders, parasomnias, sleep-related movement disorders (restless leg syndrome, periodic limb movement disorder) (218) | A: Sleep disorders meet specific criteria and subsequent treatment interventions well defined D: Term may be used to imply symptom and may be used without meeting specified criteria |
| Sleep-related breathing disorders | Disorder characterized by abnormal respiration during sleep, which may include obstructive sleep apnea, central sleep apnea, or sleep-related hypoventilation | A: Specific term based on risk factors or other characteristics D: Can be used without meeting specified criteria |
| Insomnia | Difficulty falling asleep or staying asleep; can also include early morning awakenings Specific criteria exist to qualify it as a sleep disorder and includes three distinct subtypes: short-term insomnia disorder, chronic insomnia disorders, and other insomnia disorder (has symptom but does not meet criteria) |
A: Versatile and may be used as a symptom or imply sleep disorder D: Often used to describe symptom and not sleep disorder with specific criteria unless specified |
| Circadian rhythm | Physical, mental, and behavior changes that follow a 24-h cycle Circadian rhythm sleep–wake disorders reflect a misalignment between the environment and an individual’s sleep–wake cycle and result in a chronic or recurrent sleep disturbance |
A: Identifies a pattern to target for evaluation and intervention D: Simplified term for a complex process |
Conflicts of interest were disclosed and managed according to ATS policies and procedures. The co-chairs (B.C.B., S.A.F., D.M.H., M.T., and M.P.R.) developed an overview of current knowledge gaps of CRF in lung cancer survivors. These themes were further defined during premeeting conference calls with select panel members. Two virtual meetings were held with the entire panel during the ATS International Conference (May 14, 2021, and May 18, 2021) and consisted of six presentations, three breakout sessions, and a conclusion session to expand discussions related to three overarching themes in CRF: 1) detection and diagnostic evaluation in patients with lung cancer; 2) timing, goals, and implementation of physical activity and rehabilitation; and 3) evaluation of sleep disturbances and disorders. The breakout sessions were moderated by one co-chair and included the entire panel. The panel discussed research gaps for each theme in the breakout sessions. The panel summarized the identified gaps and potential research topics during the concluding session. After the meetings, the co-chairs compiled a comprehensive summary of the virtual meetings’ discussions and research topics. On the basis of the synopsis and knowledge gaps, the chairs drafted 32 research questions on the overarching themes. Writing committee members helped refine the key research questions. A modified Delphi process prioritized each question to achieve consensus through subsequent online surveys (Table 2) (39, 40). All panel members (n = 18) participated in each round (i.e., 100% response rate) (Table 3). In round 1, participants were asked to indicate the priority on an eight-point Likert-type scale (0 = not at all important, 7 = extremely important) of the 32 research questions; 1 question inadvertently included a five-point (rather than an eight-point) Likert-type scale, with comparable labels. After round 1, questions were maintained if ⩾80% of participants ranked the question as “extremely important,” “important,” or “somewhat important.” Consensus of ⩾80% using a modified Delphi approach agreeing or strongly agreeing with the research question has been used elsewhere (39–41). In round 2, a six-point Likert-type scale (0 = not at all important, 5 = extremely important) was used, and questions were selected for round 3 if ⩾80% of participants ranked themes as extremely important or important. In round 3, participants were shown the seven selected questions and asked to rank them from “most important” to “least important.” Consensus of ⩾80% was not obtained for prioritization of these final seven questions (i.e., there was significant heterogeneity in what were considered the top questions). We present the prioritized research questions according to the most votes (Table 2). A limitation of this ranking process, due to the lack of consensus, was that the order of ranks depended on where we started (i.e., if we started with ranking the least important of the seven questions, we would have a different ranking order). However, we elected to rank the questions as “most” to “least” important because this is likely to be more interpretable and helpful to readers. Key points and recommendations were derived via panel member consensus.
Table 2.
Prioritization of Research Questions Using Delphi Process
| All Research Questions Identified from ATS Workshop | Round 1 Delphi | Round 2 Delphi | Round 3 Delphi | |
|---|---|---|---|---|
| Number of questions | 32 | 31 | 7 | Rank |
| Section II: General considerations for research in CRF in lung cancer | ||||
| 1. How does CRF in lung cancer differ from other cancer types regarding prevalence, severity, and course? | ✓ | ✓ | ✓ | 1 |
| 2. How do tumoral factors (i.e., location, histology, and stage) and treatment modalities (i.e., surgery, radiation, chemotherapy, and immunotherapy) affect the development, severity, and progression of CRF? | ✓ | ✓ | ✓ | 2 |
| 3. Beyond individual patient data and biomarkers, can other available metrics help clarify mechanisms and diagnosis of CRF: population-based studies, machine learning, imaging (CT, PET, MRI), or tissue samples? | ✓ | ✓ | ||
| 4. Does the pathophysiology of CRF in patients with lung cancer elucidate potential therapeutic targets? | ✓ | ✓ | ||
| 5. Which symptom clusters are associated with the greatest degree of CRF severity? | ✓ | ✓ | ||
| Section III: detection and diagnostic evaluation of CRF in lung cancer | ||||
| 1. What are the roles of multidisciplinary teams in a patient-driven care model for recognizing and treating CRF in patients with lung cancer? | ✓ | ✓ | ||
| 2. What is the optimal screening tool and score cutoff for CRF in patients with lung cancer? | ✓ | ✓ | ||
| 3. What assessments can be used for CRF identification and surveillance: symptom scores, biomarkers, circadian disruption, sleep fragmentation? | ✓ | ✓ | ✓ | 3 |
| 4. How do patient factors (socioeconomic status, race, sex, functional status, comorbidities, medications, nutrition) affect the diagnosis and treatment of CRF in patients with lung cancer? | ✓ | ✓ | ✓ | 5 |
| Section IV: timing, goals, and implementation of physical activity, rehabilitation, and exercise training | ||||
| 1. How do multidisciplinary teams optimize delivery of physical activity programs (dose, timing, location) to improve CRF for patients with lung cancer? | ✓ | ✓ | ✓ | 6 |
| 2. Do comorbid conditions (e.g., COPD, ILD, CAD, heart failure) predict benefit from physical activity programs for patients with lung cancer? | ✓ | ✓ | ||
| 3. What is the effect of physical activity programs, including frequency, intensity, duration, and type, on CRF in patients with lung cancer, before, during, and after treatment? | ✓ | ✓ | ✓ | 7 |
| 4. What are the associated biological and physiological mechanisms relating to physical activity programs and potential impact of CRF for patients with lung cancer? | ✓ | ✓ | ||
| 5. What is the optimal timing to commence physical activity programs to prevent or treat CRF along the lung cancer treatment continuum (before treatment, during treatment, after treatment, palliative care)? | ✓ | ✓ | ||
| 6. Is there effect modification of physical activity programs along the lung cancer treatment course (e.g., before treatment; within 1, 3, or 6 mo after treatment)? | ✓ | ✓ | ||
| 7. What are the effects of physical activity programs in combination with psychological and/or symptom management on CRF and HRQoL in patients with lung cancer along their life course (i.e., before, during, and after treatment, including specific time intervals after treatment)? | ✓ | ✓ | ||
| 8. Are there predictors of the benefit of multidisciplinary rehabilitation for patients with lung cancer and CRF (e.g., older or younger age, presence or absence of comorbid cardiopulmonary disease, early or advanced stage, curative-intent or non–curative-intent therapy)? | ✓ | ✓ | ||
| 9. How are physical activity programs being used clinically for patients with lung cancer, and what are the cost implications for these services? | ✓ | ✓ | ||
| 10. What factors (e.g., patient, socioenvironmental, health care organization) are associated with referrals and uptake of physical activity programs among patients with lung cancer that may identify subgroups of patients with high needs and/or low uptake? | ✓ | ✓ | ||
| 11. What is the optimal strategy to increase access to and uptake of physical activity programs to reduce CRF and improve HRQoL among patients with lung cancer? | ✓ | ✓ | ||
| 12. How can multidisciplinary teams disseminate the importance of physical activity programs for patients with lung cancer? | ✓ | ✓ | ||
| 13. How can multidisciplinary teams incorporate patient-relevant goals and integrate physical activity services to manage patients with lung cancer? | ✓ | ✓ | ||
| 14. What is the relationship between nutritional health and the ability to undertake physical activity and reduce fatigue in lung cancer? | ✓ | ✓ | ||
| 15. How do multidisciplinary teams facilitate participation and adherence in appropriate patient-centered physical activity programs to alleviate symptoms and improve HRQoL among patients with lung cancer? | ✓ | ✓ | ||
| 16. Does e-health enhance strategies for adherence to maintenance of physical activity? | ✓ | |||
| Section V: evaluation of sleep disruption and underlying sleep disorders in lung cancer survivors and CRF | ||||
| 1. What factors contribute to sleep disturbance: biomarkers, genetics, nocturnal hypoxemia, sleep-disordered breathing? | ✓ | ✓ | ||
| 2. What is the prevalence of different sleep disorders (sleep-disordered breathing, insomnia, circadian dysregulation, movement disorders)? | ✓ | ✓ | ✓ | 4 |
| 3. How do symptom clusters affect sleep disruption in patients with lung cancer affect CRF? | ✓ | ✓ | ||
| 4. Which diagnostic modalities (nocturnal oximetry, home sleep testing, polysomnography, actigraphy, wearables) can uncover underlying sleep disorders? | ✓ | ✓ | ||
| 5. How do sleep disorders and their treatments (chronotherapy, positive airway pressure therapy, cognitive-behavioral therapy, oxygen) affect quality of life and response to therapies in patients with lung cancer and CRF? | ✓ | ✓ | ||
| 6. What strategies can multidisciplinary groups use to raise awareness of sleep health, reduce sleep disruption, and emphasize sleep hygiene? | ✓ | ✓ | ||
| 7. What is the role of integrative or complementary therapies (yoga, acupuncture, herbal medication) to address sleep disruption in patients with lung cancer and CRF? | ✓ | ✓ | ||
Definition of abbreviations: ATS = American Thoracic Society; CAD = coronary artery disease; CRF = cancer-related fatigue; COPD = chronic obstructive pulmonary disease; CT = computed tomography; HRQoL = health-related quality of life; ILD = interstitial lung disease; MRI = magnetic resonance imaging; PET = positron emission tomography.
Questions in boldface type represent the seven questions ranked “extremely important” or “important” presented in round 3 of the survey.
Table 3.
American Thoracic Society Lung Cancer and Cancer-related Fatigue Panel
| Participant | Institution, Country | Discipline | Project Role |
|---|---|---|---|
| Brett C. Bade, M.D. | Yale University, United States | Pulmonology, rehabilitation | Co-chair, speaker |
| Duc M. Ha, M.D. | Rocky Mountain Regional Veterans Affairs Medical Center, United States | Pulmonology, rehabilitation | Co-chair, speaker |
| Saadia A. Faiz, M.D. | The University of Texas MD Anderson Cancer Center, United States | Pulmonology, sleep medicine | Co-chair |
| Miranda Tan, D.O. | Memorial Sloan Kettering Cancer Center, United States | Pulmonology, sleep medicine | Co-chair |
| M. Patricia Rivera, M.D. | University of Rochester Medical Center, United States | Pulmonology, thoracic oncology | Co-chair, mentor |
| Margaret Barton-Burke, Ph.D., R.N. | Memorial Sloan Kettering Cancer Center, United States | Nursing research, sleep medicine | Speaker |
| Andrea L. Cheville, M.D., M.S.C.E. | Mayo Clinic, United States | Physical medicine and rehabilitation | Speaker |
| Carmen P. Escalante, M.D. | The University of Texas MD Anderson Cancer Center, United States | Internal medicine | Speaker |
| David Gozal, M.D., M.B.A., Ph.D. | University of Missouri, United States | Pulmonology, sleep medicine | Speaker |
| Catherine L. Granger, Ph.D., P.T., G.C.U.T. | University of Melbourne, Australia | Physiotherapy | Speaker |
| Dawn M. Chamberlaine, M.A. | New Haven, United States | Patient experience | Panel member |
| Jason M. Long, M.D., M.P.H. | University of North Carolina at Chapel Hill, United States | Thoracic surgery | Panel member |
| Daniel J. Malone, P.T., Ph.D. | University of Colorado, United States | Physical therapy | Panel member |
| William F. Pirl, M.D., M.P.H. | Dana-Farber Cancer Institute, United States | Psychiatry | Panel member |
| Carolyn J. Presley, M.D., M.H.S | The Ohio State University, United States | Oncology, thoracic oncology | Panel member |
| Sheree M. Smith, B.N., M.S.P.D., G.C.H.E., Ph.D. | Western Sydney University, Australia | Nursing, sleep medicine | Panel member |
| Halley L. Robinson, L.C.S.W. | Yale New Haven Hospital, United States | Social work | Panel member |
| Kazuhiro Yasufuku, M.D., Ph.D. | University of Toronto, Canada | Thoracic surgery | Panel member |
The co-chairs drafted the initial version of the manuscript with the assistance of the writing committee (M.B.-B., A.L.C., C.P.E., C.L.G., D.G., C.J.P., and S.M.S.). The manuscript was then disseminated to the entire panel and iteratively revised. The final manuscript underwent peer review and was approved by the ATS Board of Directors.
Results
The results are organized into five sections to assist scientists and funding agencies in conducting and supporting CRF research. The first section provides an overview of CRF among lung cancer survivors. Sections II–V provide an overview of general considerations for research in CRF in lung cancer (etiology, evaluation, pharmacologic and nonpharmacologic treatment); detection and diagnostic evaluation of CRF in lung cancer; timing, goals, and implementation of physical activity, rehabilitation, and exercise training in lung cancer survivors and CRF; and evaluation of sleep disruption and underlying sleep disorders in CRF. Finally, the panel’s prioritized research questions for sections II–V are listed in Table 2.
Section I: Overview of CRF in Lung Cancer Survivors
CRF prevalence in lung cancer survivors ranges from 40% to 80% (8, 42–45). Compared with other common malignancies (e.g., breast, prostate, colon), several studies suggest a greater likelihood of fatigue in patients with lung cancer (8, 46). Studies evaluating CRF report higher prevalence in mixed cancer cohorts (56%), earlier cohorts (i.e., 64% in studies from 1996 to 2000 vs. 43% in studies from 2016 to 2020), and advanced cancer (61%) and during cancer treatment (62%) (4). In a study among early-stage lung cancer survivors after surgical treatment, fatigue was present in 57%, but as most lung cancer survivors receive diagnoses of advanced-stage disease, prevalence is likely underestimated (43).
Comorbidities aggravate fatigue and are present in 53% of lung cancer survivors compared with 32%, 31%, and 41% among survivors of breast, prostate, and colon cancers, respectively (47). In lung cancer survivors, tobacco-related comorbidities, including chronic obstructive pulmonary disease (COPD), coronary artery disease, and mood disorders, are frequently encountered. For example, COPD and depression are present in 24–73% and 11–44% of lung cancer survivors, respectively. Women with lung cancer have the highest prevalence of depression (25%) compared with other cancer types (48). Chronic pain also contributes to symptom burden and CRF in lung cancer survivors (Figures 1 and 2), and the NCCN guidelines on CRF recommend evaluation and treatment of pain (1). Thus, comorbidities in lung cancer survivors are particularly challenging in CRF management.
Cancer treatments also affect CRF. Prior studies have suggested even higher rates of CRF in patients actively receiving chemotherapy or radiotherapy (>80%) (8). As immunotherapy becomes increasingly integrated into lung cancer treatment, attention should also be paid to the impact on CRF. Fatigue is a consistent adverse event reported in studies with a single-agent immune checkpoint inhibitor, with an incidence of 16–37% with anti–PD-1 (programmed cell death protein 1) agents and 12–24% with anti–PD-L1 agents. Slightly higher reported rates of fatigue, ranging from 21% to 71%, are noted in those clinical studies that combine anti–PD-1/PD-L1 agents with other immune checkpoint inhibitors, chemotherapy, antiangiogenic agents, and targeted therapies (10).
Treatment guidelines are available for CRF and include pharmacologic and nonpharmacologic approaches (1). Pharmacologic treatments address comorbidity management (e.g., antidepressants, anxiolytics, or pain medications) and directed therapies (e.g., psychostimulants or corticosteroids). Because there is more supportive evidence for nonpharmacologic interventions, our discussion focused on these topics.
CRF in lung cancer is strongly related to decreased physical activity, exercise, and sleep, but established treatments lack validation in lung cancer survivors. Physical activity and cardiopulmonary fitness predict lung cancer–related survival (49–53) and correlate with symptom burden, depressive symptoms, and HRQoL, with the least active patients having the most severe impairments (54, 55). Although aerobic exercise is beneficial in reducing CRF, few studies have included lung cancer survivors (27). Of 113 randomized controlled trials (RCTs) including approximately 11,500 participants evaluating CRF treatment, only 1 study focused solely on lung cancer (21). As lung cancer survivors are at heightened risk for CRF, identifying strategies to reduce CRF effectively is of utmost importance to improve HRQoL.
Patients with baseline symptoms, inactivity, or functional impairment may benefit from physical activity interventions (56–58). A walking intervention in lung cancer survivors improved sleep quality in patients with poor baseline rest–activity rhythms (56). Pulmonary rehabilitation interventions have shown a similar trend. A study showed no difference between “conventional” and “comprehensive” pulmonary rehabilitation, but a subgroup analysis suggested a benefit in patients with higher comorbidity and preoperative risk scores (58). Tarumi and colleagues used pulmonary rehabilitation during chemoradiation and reported greater spirometric improvement in patients with baseline respiratory impairment (57). However, the clinical significance of slight improvements in lung function after rehabilitation remains uncertain. In summary, available data suggest that 1) lung cancer survivors are at risk for CRF associated with functional impairment, and 2) alleviation of symptoms, improvement in HRQoL, and potentially improvement in spirometry may be modified by baseline impairment. Therefore, improved evaluation, diagnosis, and treatment of CRF in lung cancer survivors are paramount to improving HRQoL.
Section II: General Considerations for Research in CRF in Lung Cancer
Summary of evidence and knowledge gaps
The extant literature highlights a significant difference between CRF in lung cancer and other types of cancer. Despite the pervasiveness of CRF and its effect on HRQoL, there is a dearth of evidence on the prevalence, etiology, epidemiology, pathogenesis, and clinical sequelae of CRF in lung cancer survivors. In a systematic review of CRF, including 126 studies between 1993 and 2020, only 3 focused on lung cancer survivors (4). Furthermore, studies are limited by small sample sizes and heterogeneous mixes of different cancers or therapies (i.e., postresection, patients undergoing radiation, or those with advanced-stage disease) (59–62). It is uncertain if specific tumor characteristics (i.e., location, histology, and stage) influence CRF’s development, progression, and severity in lung cancer survivors.
CRF may behave differently in lung cancer survivors as opposed to other types of cancers, in part because of the differences in demographics, smoking history, and comorbid symptoms commonly described in patients with lung cancer. Smoking is a known risk factor for lung cancer (63), and after tobacco cessation, patients with lung cancer experience decreased fatigue and dyspnea and increased performance status (64, 65). Ethnic and racial minorities, however, are less likely to receive and use tobacco cessation interventions than White individuals (66), which may increase risk for tobacco-related disease (e.g., COPD, cardiovascular disease). Symptom burden has been higher in lung cancer survivors than in other cancer survivors (67). Further studies are thus necessary to understand and distinguish the differences between CRF in lung cancer and different types of cancer.
A complex interplay of mechanisms is presumed to influence the development and persistence of CRF in cancer survivors. CRF may be central and/or peripheral in origin. Hypotheses behind central fatigue include cytokine dysregulation, hypothalamic–pituitary–adrenal axis disruption, circadian rhythm disruption, serotonin dysregulation, and vagal afferent nerve dysfunction (68). Energy dysregulation, muscle metabolism, and muscle contractile properties are potential mechanisms of peripheral fatigue (68). Because the exact pathogenesis for CRF and the relative contributions of central and peripheral fatigue mechanisms remain unclear, targeted pharmacologic therapies have not been studied.
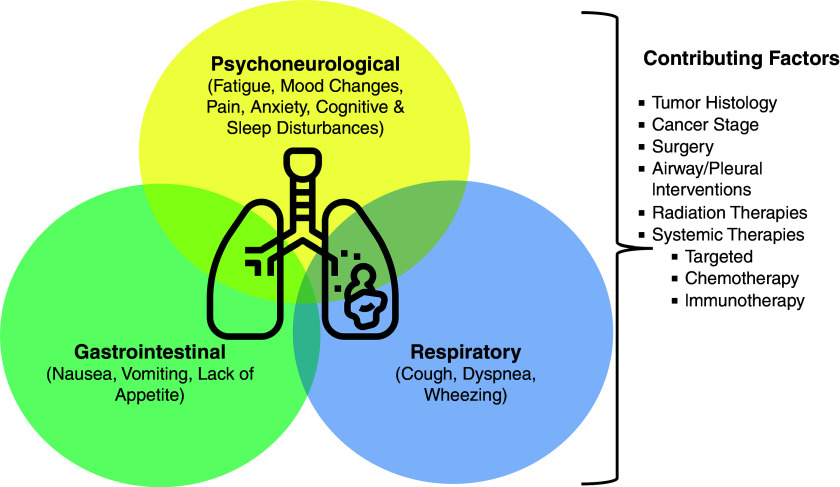
CRF often occurs as part of a symptom cluster and coexists with pain, sleep disturbance, and depression (Figure 3) (1). Several studies describe the occurrence of these factors together. In a cross-sectional study of elderly patients with cancer, patients reported the coexistence of any two (20%) or three (29.2%) symptoms of either pain, fatigue, sleep, or mood disturbance (69). Among Chinese patients with lung cancer undergoing surgery, fatigue, sleep disturbance, and distress were the most common and severe symptoms; 76.6% of patients reported cooccurrence of all four symptoms (70). Moreover, the presence of sleep disturbance exacerbated fatigue, pain, and depression symptoms in one small study of patients with lung cancer (71). Dyspnea and cough have also been identified as possible components of a symptom cluster in patients with lung cancer (72, 73). The cooccurrence of depression, fatigue, and pain is not limited only to those with active cancer; it has also been described in lung, breast, and prostate cancer survivors (74–76). Although subjects experience these symptoms simultaneously, the optimal modality to identify and treat these symptom clusters is currently unknown.
Figure 3.
Symptom clusters in lung cancer. Concurrent physical and/or psychosocial symptoms in lung cancer may vary on the basis of the cancer stage and disease trajectory. Symptom burden is often particularly high in advanced cancer. Various contributing factors may further exacerbate the patient’s symptom burden, including the side effects of multiple therapies and interventions.
CRF as part of a symptom cluster may be consequent to cause other symptoms or may share a common etiology with them. Proinflammatory cytokines produced in response to the tumor microenvironment, tissue damage from treatment, and cell stress/death can activate CNS pathways, perpetuating pain, disrupting sleep cycles, and eliciting fatigue (77). Cytokines may also induce behavioral changes via serotonin, norepinephrine, and dopamine metabolism alterations (78). Although these shared pathways suggest common biological alterations leading to various symptom clusters, the association of symptoms and their predilection for exacerbating CRF remains uncertain; uncovering these relationships could help focus future interventions.
Prioritized research questions
Of the five questions developed for section II (Table 2), voting results indicated that committee members prioritized questions 1 and 2 as first and second, respectively, with 80% consensus not reached among the final seven questions.
Section III: Detection and Diagnostic Evaluation of CRF in Lung Cancer
Summary of evidence and knowledge gaps
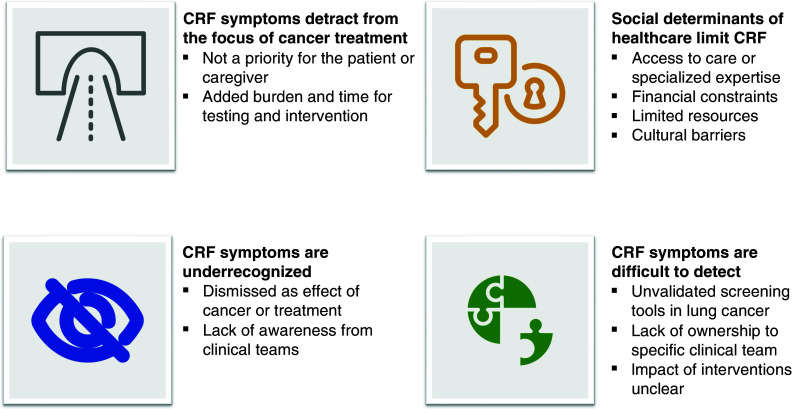
Clinical practice guidelines for CRF developed by the NCCN (1), European Society for Medical Oncology (7), Canadian Partnership against Cancer, Canadian Association of Psychosocial Oncology, and Oncology Nursing Society (79) recommend that all cancer survivors be screened and assessed for fatigue from diagnosis to follow-up; if identified, evaluation and treatment are recommended. However, barriers to identifying CRF may conceivably stem from the patient and health care provider. Fatigue is a subjective symptom perceived by the patient; it may not be spontaneously disclosed because it is considered “expected,” unimportant, or a potential barrier to further cancer treatment if reported. Without clearly defined screening tools, adequate time to obtain a thorough symptom history, and access to an interdisciplinary cancer management team, CRF diagnosis and management may be difficult for healthcare providers.
Several screening tools have been validated in mixed cancer populations, including the Brief Fatigue Inventory (BFI), European Organisation for Research and Treatment of Cancer quality-of-life questionnaire (80, 81), and Daily Fatigue Cancer Scale (Table 4) (82). The BFI is a psychometrically vetted questionnaire that incorporates numerical rating scales to assess fatigue severity and interference (83); is also widely used in the cancer population, including lung cancer survivors (84). As with previously developed measures of similar conceptual content (i.e., “legacy” measures), the BFI is derived through classical test theory, which discriminates well at the mean but may perform suboptimally at the ends of the trait range, thereby impeding the detection of severe CRF. Item response theory (IRT)–based tools have been widely adopted as an alternative to legacy tools because of their flexibility and robust psychometric properties (85). Currently, three IRT-modeled item banks are available to assess fatigue. These include the Patient-reported Outcome Measurement Information System (PROMIS) Bank version 1.0, the PROMIS Cancer Bank version 1.0, and the Neuro-QOL Bank version 1.0 (86). The PROMIS Bank version 1.0 has 95 items. In contrast, the PROMIS Cancer Bank version 1.0 has 54 items, affording the former greater discriminative capacity in cancer and noncancer populations. As IRT was initially validated in a mixed cancer population (only 21.3% of participants had lung cancer [87]), it is unknown if IRT-based tools would perform better at identifying CRF in lung cancer survivors compared with legacy tools or if use of the IRT would be hindered because of its extensive items. The utility and ability of these tools to detect meaningful changes in CRF over time are limited without adequate implementation efforts to ensure clinical responses to actionable scores.
Table 4.
Selected Tools Used to Identify and Quantify Cancer-related Fatigue
| Tools | Description | Strengths | Limitations |
|---|---|---|---|
| NCCN DT and PL | The DT is a 1-item, 0–10 Likert-type scale. A score of >4 triggers the PL. The PL is 40 items covering practical/family/emotional/spiritual physical problems. (Fatigue is 1 item in the physical problems domain.) |
Simple and quick to use (<5 min) | Free for personal, nonpromotional, and educational use; permission required for publication or commercial purpose Primarily a screening tool Dichotomous (yes/no response) Positive findings should trigger a more comprehensive assessment Better at identifying severe fatigue Limited psychometrics |
| One Item Fatigue Screen | 0–10 numerical or verbal scale | Simple and quick to use (<1 min) Semiquantitative assessment |
Unidimensional tool Primarily a screening tool Positive findings should trigger a more comprehensive assessment Limited psychometrics |
| MD Anderson Symptom Inventory | 13 items total (3 related to fatigue) | Simple and quick to use (<5 min) Available in multiple forms (paper, web-based) Translated into multiple languages |
Requires permission and fee for use Positive findings should trigger a more comprehensive assessment |
| Daily Fatigue Cancer Scale | Visual analogue scale (10 cm) 3 items addressing general/physical/emotional fatigue |
Simple and quick to use (<2–5 min) | Primarily a screening tool Limited psychometrics |
| Functional Assessment of Chronic Illness Treatment–Fatigue | 40 items total (13 fatigue specific) | Multidimensional tool (e.g., addresses fatigue impact on physical, social; emotional, and functional well-being over time) Free to use after registering (FACIT.org) |
May take 15 min to complete Translated version may have fee |
| Brief Fatigue Inventory | 9 items total (3 fatigue severity, 6 fatigue impact on daily activities) | Short and easy to use (5 min) Multidimensional tool (e.g., assesses fatigue directly and fatigue impact on general activity; walking; mood; work; relationships; life enjoyment) Translated into multiple languages |
Possible processing fee |
| European Organisation for Research and Treatment of Cancer quality-of-life questionnaire measuring cancer-related fatigue | 12 items total | Short and easy to use (<5 min) Multidimension tool (e.g., distinguishes among physical, emotional, and cognitive fatigue and assesses fatigue impact on daily activities and social life) Free for academic use |
|
| Piper Fatigue Scale–Revised | 22 items total | Short and easy to use (<10 min) Multidimensional (e.g., addresses fatigue impact on behavioral, affective, sensory, cognitive/mood) Translated into multiple languages Free to use |
Multiple versions of the Piper Fatigue Scale have been developed |
| Fatigue Severity Scale | 9 items total, 0–10 Likert-type scale | Short and easy to use (5 min) Multidimensional tool (e.g., evaluates fatigue impact on motivation, activity, physical function, work, family, and social life) |
Frequently used for chronic conditions (less frequently in cancer survivors) Free to use for nonprofit research, permission required for pharmaceutical studies |
| Cancer Related Fatigue Distress Scale | 20 items total, 0–10 Likert-type scale | Free to use | |
| Multidimensional Fatigue Symptom Inventory (short form) | 30 items total, five-point Likert-type scale | Easy to use (5-10 min) Multidimensional tool (e.g., evaluates general, physical, emotional, vigor, and mental fatigue over the past 7 d) Free to use |
Majority of population used in validation study were women, and almost 90% identified as White (219) |
Screening tools should be readily available, be easy to administer and complete, include salient items to clinicians and patients, discriminate across the relevant trait range, ensure appropriate subdomain coverage, and generate interpretable and clinically actionable data. For example, studies that use the BFI among cancer survivors have revealed impaired physical functioning associated with BFI scores ⩾7 (83).
However, given CRF’s common coexistence with other symptoms (including symptom clusters), the optimal tool to assess fatigue in lung cancer survivors (as well as the threshold scores for intervention) is not well defined in lung cancer. The performance of screening tools for CRF varies across mixed cancer populations and throughout the cancer care continuum (i.e., active treatment, immediate post-treatment, and longer term survivorship) (88–90), and one is not currently endorsed over any other (Table 4) (1). A delineated and validated screening tool for lung cancer survivors is needed.
CRF can affect and be affected by diverse clinical and psychosocial domains. Therefore, an integral part of its diagnostic assessment should include evaluating potential contributors, including pain, emotional distress, sleep disturbance, sleep hygiene, degree of physical activity, anemia, nutrition, medications and their side effects, alcohol/substance abuse, comorbidities/cancer treatment sequelae, and the absence of a good support network (1). Currently, there are limited data on the frequency and distribution of the aforementioned patient factors in CRF. A systematic review on exercise and nutrition interventions in advanced lung cancer (n = 5 studies) suggested that exercise and nutrition interventions are not harmful, and potential benefits could include weight maintenance, strengthening, and functional performance (91). Standardized objective clinical and psychosocial assessments to identify CRF beyond patient-reported questionnaires currently do not exist, and whether or to what extent their implementation may reduce CRF in lung cancer survivors remains unclear.
Prioritized research questions
Of the four questions developed for section III (Table 2), voting results indicated that committee members prioritized questions 3 and 4 as third and fifth, respectively, with 80% consensus not reached among the final seven research questions.
Section IV: Timing, Goals, and Implementation of Physical Activity, Rehabilitation, and Exercise Training
Definitions
In clinical practice, the terms “physical activity,” “rehabilitation,” and “exercise training” are often used interchangeably. The World Health Organization defines physical activity as “any bodily movement produced by skeletal muscles that require energy expenditure” (92). Exercise is defined as a type of physical activity that is “planned, structured, repetitive, and purposive in the sense that improvement or maintenance of one or more components of physical fitness is an objective” (93). The World Health Organization defines rehabilitation as “a set of interventions designed to optimize functioning and reduce disability in individuals with health conditions in interaction with their environment” (94). To make terms even more confusing, rehabilitation services are provided by several different disciplines, including physiotherapists, occupational therapists, respiratory therapists, registered nurses, pulmonologists (i.e., pulmonary rehabilitation), and cardiologists (i.e., cardiac rehabilitation). Furthermore, rehabilitation programs can also provide interventions on nonphysical aspects of health (e.g., medication adherence and inhaler techniques, mindfulness training, spiritual support, psychosocial support). Therefore, this document uses the collective term “physical activity programs” when referring to physical activity, rehabilitation, and/or exercise interventions (Table 1). We recognize the limitation of this term, as increasing physical activity alone is not sufficient to meet the definition of “pulmonary rehabilitation” (95). However, because of the absence of a well-accepted term or adequately evaluated program, we believed that this term emphasizes the physical aspects of rehabilitation, which is widely considered an essential component.
Physical activity programs among lung cancer survivors
Summary of evidence and knowledge gaps
Most lung cancer survivors are physically inactive and do not meet physical activity recommendations at diagnosis and treatment initiation (29, 96). On average, lung cancer survivors walk approximately 3,000–6,000 steps/d (29, 54, 97) and 350–450 m on the 6-minute-walk test (29, 49, 84, 98), depending on the stage of disease and functional status. Within six months after diagnosis, patients often experience declines in physical activity and functional exercise capacity associated with treatment (29, 30, 84). Symptom burden and self-reported function can also worsen, including after treatments with curative intent (16, 84, 99–102). High symptom burden, functional impairments, and low physical activity can contribute to diminished HRQoL during and after lung cancer treatment (44, 54, 103, 104), including several years after surgery (105–108).
A 2019 American College of Sports Medicine international multidisciplinary roundtable identified strong evidence to recommend that specific doses of aerobic training, combined aerobic and resistance training, and/or resistance training alone could improve cancer-related health outcomes, including CRF and HRQoL (36). To reduce CRF, cancer survivors should engage in three or more sessions per week of moderate-intensity aerobic exercise (i.e., exercise at 65% of maximum heart rate, 45% o2max, or a rating of perceived exertion of 12), for 30-minute sessions, totaling 90 minutes of moderate aerobic exercise per week. Patients could also engage in two or more sessions per week of moderate-intensity resistance training (i.e., exercise at 60% of 1-repetition maximum or a rating of perceived exertion of 12) of two sets of 12–15 repetitions for major muscle groups. These aerobic and resistance exercises can be performed alone or combined for at least 12 weeks. In addition, there may be a higher benefit on CRF with longer aerobic exercise program duration and length (36). However, a dose–response relationship has not been observed in programs that use combined aerobic and resistance or only resistance exercise (36). Critically, the 2019 roundtable noted that most of the evidence was derived from breast and prostate cancer survivors, with insufficient data to further detail exercise prescriptions according to cancer type, including lung cancer (36).
Among lung cancer survivors, several systematic reviews (109, 110), including a Cochrane review (with moderate-quality evidence from up to 10 RCTs involving 566 participants), identified that preoperative exercise training reduces postoperative pulmonary complications and length of hospital stay. However, no study assessed CRF as an outcome. After pulmonary resection for lung cancer, a single-blind RCT of 61 patients demonstrated that a 20-week high-intensity aerobic and resistance training program starting 5–7 weeks postoperatively, compared with usual care, resulted in clinically meaningful and statistically significant improvements in the primary outcome of maximal exercise capacity and secondary outcomes of physical and mental HRQoL (111). Again, CRF was not assessed in this study. A 2019 Cochrane systematic review concluded that exercise training for patients within 12 months of pulmonary resection for lung cancer improves functional and maximal exercise capacity (112). However, only three RCTs involving 68 patients assessed CRF as an outcome, with results showing no statistically significant difference in the effect of exercise training on CRF (ungraded-certainty evidence). Another Cochrane review showed that exercise training improves functional capacity in patients with inoperable lung cancer (113). However, three RCTs involving 90 patients showed no statistically significant difference in the effect of exercise training on CRF (ungraded-certainty evidence).
Given that exercise training improves CRF in many survivors of other cancers (36), there is a strong rationale that exercise training could also improve CRF in lung cancer survivors undergoing operative and nonoperative therapies. However, there is limited evidence on the effects of exercise training on CRF in lung cancer survivors, with a low number and small sample size of studies that assessed CRF as an outcome. In addition, given that a dose–response relationship between exercise and CRF has not been established in cancer survivors, inference for a causal effect is limited. Identifying biological and/or physiological mechanisms can better delineate the (potentially causal) link between exercise and CRF among lung cancer survivors.
Intervention strategies to meet the complex needs of lung cancer survivors
Summary of evidence and knowledge gaps
Lung cancer survivors are the most advanced in age, with a median age at diagnosis of 71 years, compared with those with breast (62 yr), prostate (66 yr), and colorectal (67 yr) cancer. In addition, lung cancer survivors have the highest comorbidity burden, prevalence of cardiopulmonary disease (i.e., COPD, heart failure, cerebrovascular disease, and peripheral vascular disease) (47), and activity-limiting emotional/psychological distress (114). Across eight different types of cancer, lung cancer survivors have the highest or second highest odds of unmet care needs in physical, emotional, and family/social functioning within approximately one year after diagnosis (115). Importantly, a 2019 Global Burden of Disease Cancer Collaboration systematic analysis showed that of the 29 types of cancer across 195 countries, involving 24.5 million incident cases, lung cancer was the leading and second leading cause of disability-adjusted life-years among men and women, respectively (80). Although most of the disability-adjusted life-years came from years of life lost, with strong interdependence between socioeconomic status and health in general, this analysis and existing literature highlight that many lung cancer survivors live with significant functional impairment and/or disability, likely more so than survivors of other cancer types.
As approximately 50% of lung cancer survivors have COPD, many lung cancer survivors stand to benefit from pulmonary rehabilitation (116–118). However, to comprehensively meet the needs of lung cancer survivors, rehabilitation strategies may also need to address the unique aspects of CRF and HRQoL in lung cancer, including post-traumatic distress and fear of recurrence or progression after cancer diagnosis and treatment (119–122). About 50% of patients with advanced cancer, including lung cancer, perceive their disease as curable (123, 124), with those who highly value disease cure having increased risk of psychological maladjustment (125). Acceptance and commitment to therapy provided by psychologists or psychiatrists may have an important role. For example, in a study of 222 patients after curative-intent therapy for breast/colorectal cancer/melanoma who had high fear of recurrence, those who received a 10-week intervention consisting of metacognition and attention training, acceptance/mindfulness therapy, and monitoring of behaviors and value-based goals (intervention arm) experienced clinically meaningful and statistically significantly greater reductions in fear of cancer recurrence than those who received relaxation therapy only (attention control arm) (126). However, CRF was not assessed in this study. Notably, a meta-analysis of 11,525 patients with cancer identified that exercise and psychological interventions, alone or combined, effectively reduced CRF during and after cancer treatment. These were better than the available pharmaceutical options, including antidepressants and stimulants (21). Psychological interventions included eclectic; cognitive-behavioral therapy; behavioral, cognitive, and psychoeducational support; motivational interviewing; and cognitive-behavioral stress management, with the most effective cognitive-behavioral treatment (21). Yet, of the 113 RCTs included in this meta-analysis, only 1 RCT focused on lung cancer survivors and evaluated a stimulant (127); no study evaluated psychological (nonpharmaceutical) interventions.
Symptom management support from nursing may also be necessary. An RCT of 92 patients with lung cancer (predominately advanced stage) showed that although an eight-week multidisciplinary home-based rehabilitation program, compared with usual care, did not improve functional capacity, patients experienced improvements in secondary exploratory outcomes of symptom severity and HRQoL at six months (128). A multidisciplinary supportive program initiated by nursing assessments described improved psychological distress and HRQoL 12 months after lung cancer resection surgery (129). Finally, the timing after treatment may modify the effects of interventions. In a study of 235 patients with operable lung cancer, a 12-week rehabilitation program did not affect maximal exercise capacity at six months; however, early initiation of this program (two weeks after surgery), compared with late (14 weeks after surgery), may prevent worsening of CRF (130).
The available evidence suggests that lung cancer is associated with significant disability. Physical activity programs may be beneficial and can address the complex needs of lung cancer survivors, but data are scarce. Furthermore, the potential additive or synergistic benefits of program components and how their effect may be modified by the timing along the lung cancer continuum are unknown. Collaborative care (including palliative care services) may also reduce CRF burden and/or associated symptom clusters in lung cancer, particularly for symptom management and/or psychosocial support (131–134). The opportunity to test multimodal programs to reduce CRF could include newer study designs such as SMART (Sequential Multiple Randomized Trials), the MOST (Multiphase Optimization Strategy), and hybrid (e.g., effectiveness–implementation) designs (135–138). For instance, since physical activity programs often provide multifaceted interventions, studies may use the SMART or MOST design to evaluate the impact of different program components and at different times along the lung cancer continuum (139, 140). Such studies could pay attention to patient and clinician goals, as well as timing along the disease course, such as immediately after diagnosis (and before treatment), immediately or shortly after completion of curative-intent therapy, during systemic therapy, or at the time of disease recurrence or progression. Hybrid implementation–effectiveness studies could evaluate program implementation strategies, such as in-person or remote delivery, and associated outcomes (141). Strategies to reduce biases, such as participant or personnel performance bias, may also be needed (142). Moreover, although exercise training is generally safe in lung cancer survivors after surgical treatment and those with advanced/inoperable disease, data among those with bone metastases are limited (112, 113). To address this uncertainty, best practices for cancer survivors with bone metastases may include risk assessment of skeletal complications, medical consultations when appropriate, and involvement of a physical therapist or exercise physiologist with cancer exercise training (143).
Barriers to physical activity programs among lung cancer survivors
Summary of evidence and knowledge gaps
Real-world challenges exist across multiple levels related to the access, uptake, and completion of center-based rehabilitation. Chief among these challenges is limited program accessibility, including referrals and patient-related barriers, including transportation, competing work, and other life demands (144). Access is even more limited for patients residing in remote and rural areas. Remotely delivered programs that allow patients to participate at home may effectively increase access to and uptake of rehabilitation services. Telerehabilitation—the delivery of therapeutic rehabilitation at a distance or offsite using telecommunication technologies—is an alternative strategy to center-based rehabilitation. Telerehabilitation in patients with chronic lung disease is associated with higher patient uptake and completion, with possibly similar benefits and safety outcomes compared with center-based rehabilitation (145–147). In contrast to center-based rehabilitation, a significantly higher proportion (approximately 70%) of eligible patients with COPD (148, 149) and advanced cancer (150) enroll in remotely delivered or home-based rehabilitation interventions. Also, a systematic review showed that, compared with center-based rehabilitation, telerehabilitation is associated with higher patient adherence among patients with cardiopulmonary disease (147). Therefore, there is a critical need for remotely delivered or home-based rehabilitation for lung cancer survivors (151).
Moreover, clinicians caring for lung cancer survivors identify barriers in the lack of integration of physical activity programs in managing lung cancer, limited care referral pathways, and inadequate staffing (152). In addition, studies among patients with COPD suggest that press and media describing stories of failed attempts among popular personalities can galvanize negative views of rehabilitation; patients have voiced concern over the term “rehab,” believing there is an attached stigma. Stigma detrimentally affects lung cancer survivors’ communication, psychosocial, and behavioral outcomes across multiple levels (153). Although lung cancer diagnosis may represent a “teachable moment” for patients to adopt health-enhancing behaviors (154, 155), many lung cancer survivors also require repeated contact/communication to become receptive to exercise or rehabilitation services (156). Among older adults in postacute care, a standardized approach based on motivation and behavioral change models to engage patients in physical and occupational therapy may result in better outcomes than standard rehabilitation strategies (157). Also, although CRF and HRQoL are important health outcomes, lung cancer survivors may have unique and individualized goals, including life goals separate from cancer treatment goals (125). Concordance in treatment goals among survivors of advanced lung cancer and their oncologists can be as low as 20% (158), with little known regarding the individual goals of lung cancer survivors after curative-intent therapy.
Furthermore, in the United States, the Centers for Medicare and Medicaid Services introduced in 2010 the Bundled Payments for Care Improvement Initiative, under which healthcare systems are accountable for the entire episode of care (e.g., associated with a surgical procedure or hospitalization), including postacute care. As a result, healthcare policy makers and hospital administrators are increasingly focused on minimizing cost while balancing the optimal quality of care. Concerns about inadequate reimbursement for pulmonary rehabilitation services have been raised (159). Therefore, early integration and repeated introduction of rehabilitation concepts, particularly in alignment with patients’ goals and with attention to the stigma associated with lung cancer and rehabilitation, may be necessary to engage lung cancer survivors in rehabilitation services effectively. Economic analyses of rehabilitation services may also be needed to inform decisions at the healthcare system and policy levels.
Promoting behavior change to enhance adherence to physical activity programs among lung cancer survivors
Summary of evidence and knowledge gaps
An important goal of physical activity programs is to promote behavior change and adherence to health-enhancing behaviors. Promoting long-term and sustained participation in daily physical activity is essential for pulmonary rehabilitation (160). In patients with COPD, a Cochrane systematic review reported that, compared with no intervention, the mean difference in time spent performing moderate- to vigorous-intensity physical activity after pulmonary rehabilitation was 4 min/d (low-certainty evidence). There was possibly greater improvement, to 6 min/d, after high-intensity interval exercise training (moderate-certainty evidence) (161). There were mixed results on the effects of adding physical activity counseling to pulmonary rehabilitation, with scant evidence for a sustained/lasting impact after completion of interventions (161). Newer strategies to enhance physical activity and improve psychosocial health, such as social prescription for community-based patients, may increase adherence to health-promoting behaviors (162). A recent review of social prescribing highlights poor quality studies with limited time frames for measuring outcomes (163).
Identifying barriers and facilitators to physical activity programs among lung cancer survivors may guide interventions to promote behavior change. A systematic synthesis of quantitative and qualitative studies identified barriers to physical activity and exercise training among lung cancer survivors: older age, male sex, high comorbidity burden including COPD, low physical capacity, high symptom burden, psychological influences, perceived irrelevance, and previous sedentary and inactive exercise behaviors (33). Facilitators of higher engagement in physical activity or exercise training include anticipated and/or experienced benefits in improved fitness, strength, daily functioning, fatigue control, well-being, and influence from clinicians and caregivers (33). There is also high heterogeneity in the preferences of lung cancer survivors on physical activity programming. For instance, although many patients prefer moderate-intensity exercise without supervision, those older and with a high comorbidity burden prefer light-intensity exercise with supervision (33). Quist and colleagues demonstrated that supervised physical activity programs improved psychological symptoms and physical performance among survivors with advanced lung cancer (164). They measured physical functional capacity using the 6-minute-walk test but did not include patient-reported functional status or CRF as outcomes. Temel and colleagues attempted a structured group exercise program twice weekly, but the program was closed early because of slow accrual (165). Program challenges may have been due to the additional weekly visits required for up to two hours and the high rigor of the exercise program. Although the barriers, facilitators, and heterogeneous preferences for exercise programming are not unique compared with survivors of other cancers (166, 167), these systematic syntheses highlight a need for individualized and tailored approaches to promote active change in exercise behavior, including lung cancer survivors. Another Cochrane systematic review of interventions to promote habitual exercise among cancer survivors, including lung cancer, identified essential behavior change techniques that included goal setting, graded tasks, and providing instructions on performing exercise (low-quality evidence) (168). However, as in patients with COPD, long-term follow-up data are also limited (168). The opportunity to test multimodal strategies to reduce CRF could include newer study designs such as SMART, MOST, and hybrid (e.g., effectiveness–implementation) designs (135–138). For instance, as physical activity programs often provide multifaceted interventions, studies may use the SMART or MOST design to evaluate the impact of different program components and at different times along the lung cancer continuum (139, 140). Such studies could pay attention to patient and clinician goals, as well as timing along the disease course, such as immediately after diagnosis (and before treatment), immediately or shortly after completion of curative-intent therapy, during systemic therapy, or at the time of disease recurrence or progression. Hybrid implementation–effectiveness studies could evaluate program implementation strategies, such as in-person or remote delivery (including self-help or live interaction), and associated outcomes (141). Strategies may also be needed to reduce biases, such as participant or personnel performance bias (142). Moreover, although exercise training is generally safe in lung cancer survivors after surgical treatment and those with advanced/inoperable disease, data are scarce among those with bone metastases (112, 113). To address this uncertainty, best practices for cancer survivors with bone metastases may include risk assessment of skeletal complications, medical consultations when appropriate, and involvement of a physical therapist or exercise physiologist with cancer exercise training (143).
Prioritized research questions
Of the 16 questions developed for section IV (Table 2), voting results indicated that committee members prioritized questions 1 and 3 as 6th and 7th, respectively, with 80% consensus not reached among the final 7 research questions.
Section V: Evaluation of Sleep Disruption and Underlying Sleep Disorders in Lung Cancer Survivors and CRF
Summary of evidence and knowledge gaps
As for CRF, data on sleep disruption and underlying sleep disorders in lung cancer survivors are limited. In a study evaluating 982 ambulatory patients, lung cancer survivors constituted 12% of the cohort and had the highest prevalence of sleep disturbances, including fatigue (56%), use of sleeping pills (40%), excessive daytime sleepiness (40%), and insomnia (37%) (169). Palesh and colleagues prospectively evaluated 823 cancer survivors undergoing chemotherapy, of whom 120 had lung cancer, and most (80%) had insomnia symptoms, with half qualifying for clinical insomnia diagnosis (170). Similarly, among a smaller cohort of 75 lung cancer survivors after curative-intent therapy, 73% reported sleep difficulties, and 56% had abnormally high insomnia (171). In a polysomnography study, lung cancer survivors underreported sleep difficulties and had sleep patterns similar to those of otherwise healthy subjects with insomnia (172). Sleep questionnaires targeting only lung cancer survivors, albeit involving smaller cohorts, corroborate symptoms of sleep disruption, insomnia, and excessive daytime sleepiness (Table 5) (71, 173–180). Only a few small studies have suggested an association between sleep-disordered breathing in newly diagnosed and advanced patients with lung cancer with CRF (181–183). However, although preliminary data imply significant sleep disruption and sleep disorders in lung cancer survivors, there are scant data on this population.
Table 5.
Overview of Self-administered Questionnaires and Objective Measures to Evaluate Sleep Disturbance and Disorders
| Tool | Type | Description |
|---|---|---|
| Pittsburgh Sleep Quality Index | Survey | Assesses sleep quality and habits during the past month; 19 items with varying response categories Global score <5 indicates good sleep and >5 indicates poor sleep; maximum score 21 |
| Insomnia Severity Index | Survey | Evaluates for insomnia and severity; 7 items on five-point Likert-type scale. Scores of 22–28 indicate severe clinical insomnia |
| STOP-BANG Questionnaire | Survey | Screening questionnaire for OSA; 8 dichotomous yes/no questions. Scores of 5–8 indicate high risk for receiving a diagnosis of OSA |
| International Restless Legs Syndrome Scale | Survey | Evaluates the severity of restless leg syndrome and the impact on sleep quality, daily affairs, and mood; 10 items on a five-point Likert-type scale (0 = no restless leg symptoms, 4 = very severe restless leg symptoms), scored as a sum (maximum score 40) |
| Epworth Sleepiness Scale | Survey | Estimates likelihood of falling asleep in eight situations on a four-point Likert-type scale. Score >10 indicates daytime sleepiness; maximum score 24 |
| Functional Outcomes of Sleep Questionnaire | Survey | Assesses impact of sleepiness on conducting daily activities and QoL; 30 items (long version) or 10 items (short versions) with a four-point Likert-type scale |
| FACT-L | Survey | Thirty-six items on a four-point Likert-type scale. Higher scores indicated better QoL. Composed of 27-item FACT-general and 7-item lung cancer subscale; maximum score 136 |
| Actigraphy | Diagnostic tool | Wrist-worn monitoring of activity used in assessment of sleep/wake patterns. Software used to analyze data contains several sleep algorithms with correlations to high concordance to PSG measures for estimations of sleep quality, quantity, and circadian phasing on the basis of movement |
| Polysomnogram | Diagnostic tool | In-laboratory sleep study monitored and recorded on a multichannel system used to diagnose various sleep disorders, including sleep-disordered breathing and parasomnias |
| Home Sleep Test | Diagnostic tool | Unattended sleep study with portable monitor sensors to diagnose OSA in the home environment; reliable alternative to PSG in patients with high pretest probability of OSA |
| Sleep Diaries | Patient-generated log | Self-reported daily record of sleep patterns, sleep efficiency, and sleep quality/satisfaction. Useful to diagnose circadian rhythm disorders and characterize insomnia symptoms over time |
Definition of abbreviations: FACT-L = Functional Assessment of Cancer Therapy–Lung; OSA = obstructive sleep apnea; PSG = polysomnography; QoL = quality of life; STOP-BANG = snoring, tiredness, observed sleep apnea, high blood pressure, body mass index > 35 kg/m2, age > 50 years, neck circumference > 40 cm, male gender.
Sleep and fatigue are often reported as a symptom cluster (35) that may include cough, dyspnea, anxiety, depression, and pain in lung cancer survivors (15, 184, 185). The frequent clustering of symptoms with sleep disturbance suggests a shared etiology or bidirectional relationship (70, 73, 186, 187). Although these symptoms are believed to have common pathways, interventions may alleviate some symptoms and simultaneously exacerbate other issues; management options have not been explicitly addressed in lung cancer. For example, opiates may relieve pain but may also worsen sleep-disordered breathing, exacerbate excessive daytime sleepiness, and potentiate gastrointestinal sequelae (nausea, vomiting, constipation) (188). Similarly, bronchodilator treatments can improve respiratory symptoms, with sympathetic activation potentially exacerbating insomnia. Moreover, CRF, sleep disturbance, and mood disorders (such as depression and anxiety) often coexist, sharing common pathophysiologic mechanisms such as dysregulation of proinflammatory cytokines, specifically IL-1, tumor necrosis factor-α, and IL-6 (189, 190). Potential manifestations of depressed mood include insomnia, early morning awakenings, and excessive daytime sleepiness; sleep disturbances may even precede the diagnosis of depression. Risk for depression may also be exacerbated in those with shorter or longer sleep duration. Indeed, estimates of mood disturbances in lung cancer survivors are high, ranging from 15% to 40% for anxiety and 21% to 44% for depressive symptoms (181, 187, 191). Interventions that may have efficacy for symptom clusters likely consist of several behavioral or biobehavioral components. Cognitive-behavioral therapy, complementary therapy, and exercise interventions have demonstrated positive outcomes in several cancer types with various symptom clusters (190, 192). Further research in homogeneous samples with specific symptom clusters is needed and will allow a better understanding of cooccurring symptoms and underlying mechanisms and eventually provide reproducible evidence to develop guidelines for management.
Symptom clusters can also vary on the basis of the disease trajectory and range from dyspnea, fatigue, and cough in early lung cancer to fatigue, pain, dyspnea, depressed appetite, and persistent cough in long-term lung cancer survivors (72, 193). Longitudinal studies reveal that fatigue and poor sleep quality may adversely affect HRQoL and persist beyond completion of treatment (193, 194). Circadian dysregulation is potentiated by high symptom burden in advanced lung cancer, disrupted sleep–wake cycles, behavioral influences, and effects of the tumor or underlying comorbidities on host clock regulation (195, 196). In a study with 62 advanced lung cancer survivors, Sephton and colleagues reported that flattening the diurnal cortisol rhythm portended limited survival (197). Modulating circadian rhythms to alleviate symptoms and outcomes in lung cancer is understudied and underused. Integrative strategies, including cognitive-behavioral therapy for insomnia, light therapy, exercise, yoga, and tai chi, have been used in several studies to ameliorate sleep symptoms (198). Although several small studies have evaluated yoga in patients with lung cancer, most integrative and circadian-based interventions have not been assessed in lung cancer survivors (199–201).
It is also unclear if combinations of comorbidities in lung cancer may differentially influence sleep disturbance and/or CRF. COPD is associated with frequent awakenings, difficulty initiating or maintaining sleep, daytime sleepiness, reduced total sleep efficiency, and REM sleep (202). Respiratory disturbances during sleep in lung cancer survivors, particularly those with COPD, may include sleep-related oxygen desaturation, impaired pulmonary function, and hypoventilation, particularly during REM sleep. During sleep, oxygen desaturation in COPD may exceed the degree of desaturation, further contributing to nocturnal hypoxemia and hypoventilation. Dynamic abnormalities during sleep are well described in COPD (202, 203) but remain unexplored in lung cancer survivors with and without COPD. Patients with concomitant COPD and obstructive sleep apnea (OSA) (i.e., overlap syndrome) have more frequent nocturnal desaturations, hypoxemia, hypercapnia, and dysrhythmias (204). Overlap syndrome is associated with higher morbidity and hospital readmissions, and positive airway pressure therapy can improve survival (205, 206). Lung cancer engenders additional pulmonary dysfunction with tumor and treatment-related changes, and the combination of these processes can further negatively affect airway and respiratory mechanics and gas exchange. The impact of lung cancer with concomitant overlap syndrome has not been explored regarding symptoms, prevalence, diagnosis, interventions, or outcomes.
Aside from other potential cardiovascular sequelae, the role of hypoxia and sleep-disordered breathing has unclear implications in lung cancer. Clinical and epidemiological series have also shown that 1) the degree of nocturnal hypoxia in OSA increases the risk for cancer incidence and mortality, and 2) patients with untreated OSA have worse outcomes (207–209). The hallmarks of OSA include intermittent hypoxia and sleep fragmentation. Both chronic intermittent hypoxia and sleep fragmentation can trigger oxidative stress and systemic inflammation in animal and in vitro cancer models and promote the activation of oncogenic pathways (209–211). The lack of data on lung cancer is of utmost concern given the increased incidence of underlying respiratory disease in this cohort. On a larger scale, population-based independent cohorts have reported an increased incidence of and mortality from cancer in those with sleep-disordered breathing (207, 208, 212, 213). The underlying etiology centers around chronic intermittent hypoxia as the presumptive common link between systemic inflammation and tumor progression and proliferation in the setting of sleep-disordered breathing (207, 208). Interestingly, studies also suggested that treatment for OSA with continuous positive airway pressure may be protective; the association between cancer mortality and OSA severity was less in patients adherent to therapy (207, 212).
Two large retrospective studies using cancer registries demonstrated severe OSA antecedent to a lung cancer diagnosis (214, 215). The SAILS (Sleep Apnea in Lung Cancer Screening) study evaluated OSA prevalence and nocturnal hypoxemia using home sleep apnea testing. This study revealed that 76% of individuals at high risk for lung cancer also had OSA and nocturnal hypoxemia, with nocturnal hypoxemia being associated with an increased risk of positive screen-detected nodules (216). Of note, all the aforementioned studies targeted patients without active cancer undergoing cancer screening, so the prevalence, modality for detection, and impact on CRF, HRQoL, and tumor behavior remain uncertain in lung cancer survivors and should be further investigated.
Prioritized research questions
Of the seven questions developed for section V (Table 2), voting results indicated that committee members prioritized question 2 as fourth, with 80% consensus not reached among the final seven research questions.
Conclusions
The urgency of CRF recognition and treatment in lung cancer survivors is demonstrated by this panel’s patient representative: “If I had to continue living my life this way, I wouldn’t want it.” CRF is an underrecognized entity in lung cancer survivors, and additional research is needed for evaluation and treatment. Our multidisciplinary workshop highlighted the challenges and barriers associated with the diagnosis, evaluation, and treatment of CRF in lung cancer survivors and the impact of physical activity and sleep (Figure 4). This statement offers a prioritized research agenda to 1) advance evaluation, treatment, and research efforts and 2) increase patient and clinician awareness of CRF in lung cancer.
Figure 4.
Challenges in the study and management of cancer-related fatigue in lung cancer. Cancer-related fatigue (CRF) in lung cancer survivors is underrecognized, and both challenges and barriers to the evaluation and treatment of CRF exist.
Acknowledgments
Acknowledgment
The authors would like to thank Kimberly Lawrence and John Harmon of the American Thoracic Society for their help in supporting the meetings and survey administration to develop this statement.
This official research statement was prepared by an ad hoc subcommittee of ATS members from the Thoracic Oncology, Sleep and Respiratory Neurobiology, Pulmonary Rehabilitation, and Nursing Assemblies.
Members of the subcommittee are as follows:
Brett C. Bade, M.D. (Co-Chair)1,2
Saadia A. Faiz, M.D. (Co-Chair)3
Duc M. Ha, M.D. (Co-Chair)4
Miranda Tan, D.O. (Co-Chair)5
M. Patricia Rivera, M.D. (Co-Chair)6
Margaret Barton-Burke, Ph.D., R.N.5
Dawn M. Chamberlaine, M.A.7*
Andrea L. Cheville, M.D., M.S.C.E.8
Carmen P. Escalante, M.D.3
David Gozal, M.D., M.B.A., Ph.D.9
Catherine L. Granger, Ph.D., P.T., G.C.U.T.9,10
Jason M. Long, M.D., M.P.H.11
Daniel J. Malone, P.T., Ph.D.12
William F. Pirl, M.D., M.P.H.13
Carolyn J. Presley, M.D., M.H.S.14
Halley L. Robinson, L.C.S.W.7
Sheree M.S. Smith, B.N., M.S.P.D., G.C.H.E., Ph.D.15
Kazuhiro Yasufuku, M.D., Ph.D.16
1School of Medicine, Yale University, New Haven, Connecticut; 2Veterans Affairs Connecticut Health Care System, West Haven, Connecticut; 3The University of Texas MD Anderson Cancer Center, Houston, Texas; 4Rocky Mountain Regional Veterans Affairs Medical Center, Aurora, Colorado; 5Memorial Sloan Kettering Cancer Center, New York, New York; 6University of Rochester Medical Center, Rochester, New York; 7Smilow Cancer Hospital, New Haven, Connecticut; 8Mayo Clinic, Rochester, Minnesota; 9School of Medicine, University of Missouri, Columbia, Missouri; 10The University of Melbourne and The Royal Melbourne Hospital, Melbourne, Australia; 11School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina; 12Anschutz Medical Campus, University of Colorado, Denver, Colorado; 13Dana-Farber Cancer Institute, Boston, Massachusetts; 14Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio; 15Western Sydney University, Sydney, New South Wales, Australia; and 16University of Toronto, Toronto, Ontario, Canada
*Patient representative.
Footnotes
This official Research Statement of the American Thoracic Society was approved October 2022
Supported by the American Thoracic Society.
An Executive Summary of this document is available at https://www.atsjournals.org/doi/suppl/10.1164/rccm.202210-1963ST.
Subcommittee disclosures: B.C.B. received research support from American Cancer Society, Nucleix Ltd, Small Award Initiative for Impact (SWIFT; VA HSR&D), VACO Contract (Women’s Veteran’s Strategic Health Group), Yale SPORE in Lung Cancer; served as a consultant for the American College of Chest Physicians. M.B.B received research support from Pfizer; received royalties from Jones and Bartlett Learning. C.P.E served as a consultant for IQVIA; served on an advisory committee for Emmi Solutions, LLC. and Jazz Pharmaceuticals. C.J.P received research support from the NIH. S.M.S received research support from GlaxoSmithKline; served on an advisory committee for the Lung Foundation of Australia; served as a speaker for the Thoracic Society of Australia and New Zealand. K.Y served as a consultant at Auris Health, Medtronic, Johnson & Johnson, Olympus America; received research support from Zidan Medical and Olympus Corporation. M.P.R served as a consultant at ABIM; received research support from NIH-NCI. S.A.F., D.M.H., M.T., A.L.C., D.G., C.L.G., D.M.C., J.M.L., D.J.M., W.F.P., H.L.R. reported no commercial or relevant non-commercial interests from ineligible companies.
References
- 1.National Comprehensive Cancer Network. Cancer-related fatigue. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2021. [accessed 2021 Jun 7]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf [Google Scholar]
- 2. Bower JE. Cancer-related fatigue—mechanisms, risk factors, and treatments. Nat Rev Clin Oncol . 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saligan LN, Olson K, Filler K, Larkin D, Cramp F, Yennurajalingam S, et al. Multinational Association of Supportive Care in Cancer Fatigue Study Group-Biomarker Working Group The biology of cancer-related fatigue: a review of the literature. Support Care Cancer . 2015;23:2461–2478. doi: 10.1007/s00520-015-2763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Maqbali M, Al Sinani M, Al Naamani Z, Al Badi K, Tanash MI. Prevalence of fatigue in patients with cancer: a systematic review and meta-analysis. J Pain Symptom Manage. 2021;61:167–189.e14. doi: 10.1016/j.jpainsymman.2020.07.037. [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Institute, Office of Cancer Survivorship. Home page. Bethesda, MD: National Cancer Institute; 2021. [accessed 2022 Jan 1]. Available from: https://cancercontrol.cancer.gov/ocs [Google Scholar]
- 6.National Coalition for Cancer Survivorship. Our mission. Silver Spring, MD: National Coalition for Cancer Survivors; 2021. [accessed 2022 Jan 16]. Available from: https://canceradvocacy.org/about/our-mission/ [Google Scholar]
- 7. Fabi A, Bhargava R, Fatigoni S, Guglielmo M, Horneber M, Roila F, et al. ESMO Guidelines Committee Cancer-related fatigue: ESMO clinical practice guidelines for diagnosis and treatment. Ann Oncol . 2020;31:713–723. doi: 10.1016/j.annonc.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 8. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist . 2007;12:4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 9. Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: results from a cross-sectional national survey in the U.S. Support Care Cancer . 2008;16:791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- 10. Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti–PD-1 and anti–PD-L1 immune checkpoint antibodies. Ann Oncol . 2015;26:2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oxnard GR, Yang JC, Yu H, Kim SW, Saka H, Horn L, et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol . 2020;31:507–516. doi: 10.1016/j.annonc.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 12. Portenoy RK, Kornblith AB, Wong G, Vlamis V, Lepore JM, Loseth DB, et al. Pain in ovarian cancer patients: prevalence, characteristics, and associated symptoms. Cancer . 1994;74:907–915. doi: 10.1002/1097-0142(19940801)74:3<907::aid-cncr2820740318>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 13. Curtis EB, Krech R, Walsh TD. Common symptoms in patients with advanced cancer. J Palliat Care . 1991;7:25–29. [PubMed] [Google Scholar]
- 14. Ventafridda V, De Conno F, Ripamonti C, Gamba A, Tamburini M. Quality-of-life assessment during a palliative care programme. Ann Oncol . 1990;1:415–420. doi: 10.1093/oxfordjournals.annonc.a057794. [DOI] [PubMed] [Google Scholar]
- 15. Charalambous A, Berger AM, Matthews E, Balachandran DD, Papastavrou E, Palesh O. Cancer-related fatigue and sleep deficiency in cancer care continuum: concepts, assessment, clusters, and management. Support Care Cancer . 2019;27:2747–2753. doi: 10.1007/s00520-019-04746-9. [DOI] [PubMed] [Google Scholar]
- 16. Sullivan DR, Forsberg CW, Ganzini L, Au DH, Gould MK, Provenzale D, et al. Longitudinal changes in depression symptoms and survival among patients with lung cancer: a national cohort assessment. J Clin Oncol . 2016;34:3984–3991. doi: 10.1200/JCO.2016.66.8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Movsas B, Moughan J, Sarna L, Langer C, Werner-Wasik M, Nicolaou N, et al. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: an analysis of RTOG 9801. J Clin Oncol . 2009;27:5816–5822. doi: 10.1200/JCO.2009.23.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sloan JA, Zhao X, Novotny PJ, Wampfler J, Garces Y, Clark MM, et al. Relationship between deficits in overall quality of life and non-small-cell lung cancer survival. J Clin Oncol . 2012;30:1498–1504. doi: 10.1200/JCO.2010.33.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med . 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 20. Cheung WY, Barmala N, Zarinehbaf S, Rodin G, Le LW, Zimmermann C. The association of physical and psychological symptom burden with time to death among palliative cancer outpatients. J Pain Symptom Manage . 2009;37:297–304. doi: 10.1016/j.jpainsymman.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 21. Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol . 2017;3:961–968. doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tomlinson D, Diorio C, Beyene J, Sung L. Effect of exercise on cancer-related fatigue: a meta-analysis. Am J Phys Med Rehabil . 2014;93:675–686. doi: 10.1097/PHM.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 23. Tian L, Lu HJ, Lin L, Hu Y. Effects of aerobic exercise on cancer-related fatigue: a meta-analysis of randomized controlled trials. Support Care Cancer . 2016;24:969–983. doi: 10.1007/s00520-015-2953-9. [DOI] [PubMed] [Google Scholar]
- 24. Hilfiker R, Meichtry A, Eicher M, Nilsson Balfe L, Knols RH, Verra ML, et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med . 2018;52:651–658. doi: 10.1136/bjsports-2016-096422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc . 2013;45:2080–2090. doi: 10.1249/MSS.0b013e31829a3b63. [DOI] [PubMed] [Google Scholar]
- 26. Paramanandam VS, Dunn V. Exercise for the management of cancer-related fatigue in lung cancer: a systematic review. Eur J Cancer Care (Engl) . 2015;24:4–14. doi: 10.1111/ecc.12198. [DOI] [PubMed] [Google Scholar]
- 27. Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev . 2012;11:CD006145. doi: 10.1002/14651858.CD006145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yennurajalingam S, Bruera E. Review of clinical trials of pharmacologic interventions for cancer-related fatigue: focus on psychostimulants and steroids. Cancer J . 2014;20:319–324. doi: 10.1097/PPO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 29. Granger CL, McDonald CF, Irving L, Clark RA, Gough K, Murnane A, et al. Low physical activity levels and functional decline in individuals with lung cancer. Lung Cancer . 2014;83:292–299. doi: 10.1016/j.lungcan.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 30. Granger CL, Parry SM, Edbrooke L, Denehy L. Deterioration in physical activity and function differs according to treatment type in non-small cell lung cancer—future directions for physiotherapy management. Physiotherapy . 2016;102:256–263. doi: 10.1016/j.physio.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 31. Avancini A, Sartori G, Gkountakos A, Casali M, Trestini I, Tregnago D, et al. Physical activity and exercise in lung cancer care: will promises be fulfilled? Oncologist . 2020;25:e555–e569. doi: 10.1634/theoncologist.2019-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bade BC, Thomas DD, Scott JB, Silvestri GA. Increasing physical activity and exercise in lung cancer: reviewing safety, benefits, and application. J Thorac Oncol . 2015;10:861–871. doi: 10.1097/JTO.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 33. Granger CL, Connolly B, Denehy L, Hart N, Antippa P, Lin KY, et al. Understanding factors influencing physical activity and exercise in lung cancer: a systematic review. Support Care Cancer . 2017;25:983–999. doi: 10.1007/s00520-016-3484-8. [DOI] [PubMed] [Google Scholar]
- 34. Ancoli-Israel S. Sleep disturbances in cancer: a review. Sleep Med Res . 2015;6:45–49. [Google Scholar]
- 35. Roscoe JA, Kaufman ME, Matteson-Rusby SE, Palesh OG, Ryan JL, Kohli S, et al. Cancer-related fatigue and sleep disorders. Oncologist . 2007;12:35–42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- 36. Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc . 2019;51:2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin . 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 38. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin . 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 39. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs . 2000;32:1008–1015. [PubMed] [Google Scholar]
- 40. Hsu C-C, Sandford BA. The Delphi technique: making sense of consensus. Pract Assess, Res Eval . 2007;12:Article 10. [Google Scholar]
- 41. Oh AL, Mularski RA, Barjaktarevic I, Barr RG, Bowler RP, Comellas AP, et al. SPIROMICS Smoking Resilience Group Defining resilience to smoking-related lung disease: a modified Delphi approach from SPIROMICS. Ann Am Thorac Soc . 2021;18:1822–1831. doi: 10.1513/AnnalsATS.202006-757OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sung MR, Patel MV, Djalalov S, Le LW, Shepherd FA, Burkes RL, et al. Evolution of symptom burden of advanced lung cancer over a decade. Clin Lung Cancer . 2017;18:274–280.e6. doi: 10.1016/j.cllc.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 43. Hung R, Krebs P, Coups EJ, Feinstein MB, Park BJ, Burkhalter J, et al. Fatigue and functional impairment in early-stage non-small cell lung cancer survivors. J Pain Symptom Manage . 2011;41:426–435. doi: 10.1016/j.jpainsymman.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sloan JA, Cheville AL, Liu H, Novotny PJ, Wampfler JA, Garces YI, et al. Impact of self-reported physical activity and health promotion behaviors on lung cancer survivorship. Health Qual Life Outcomes . 2016;14:66. doi: 10.1186/s12955-016-0461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr . 2004;(32):40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 46. Wang XS, Zhao F, Fisch MJ, O’Mara AM, Cella D, Mendoza TR, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer . 2014;120:425–432. doi: 10.1002/cncr.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, et al. Annual report to the nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer . 2014;120:1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Linden W, Vodermaier A, Mackenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord . 2012;141:343–351. doi: 10.1016/j.jad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 49. Kasymjanova G, Correa JA, Kreisman H, Dajczman E, Pepe C, Dobson S, et al. Prognostic value of the six-minute walk in advanced non-small cell lung cancer. J Thorac Oncol . 2009;4:602–607. doi: 10.1097/JTO.0b013e31819e77e8. [DOI] [PubMed] [Google Scholar]
- 50. Jones LW, Watson D, Herndon JE, II, Eves ND, Haithcock BE, Loewen G, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer . 2010;116:4825–4832. doi: 10.1002/cncr.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jones LW, Hornsby WE, Goetzinger A, Forbes LM, Sherrard EL, Quist M, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer . 2012;76:248–252. doi: 10.1016/j.lungcan.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arem H, Moore SC, Park Y, Ballard-Barbash R, Hollenbeck A, Leitzmann M, et al. Physical activity and cancer-specific mortality in the NIH-AARP Diet and Health Study cohort. Int J Cancer . 2014;135:423–431. doi: 10.1002/ijc.28659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ha D, Mazzone PJ, Ries AL, Malhotra A, Fuster M. The utility of exercise testing in patients with lung cancer. J Thorac Oncol . 2016;11:1397–1410. doi: 10.1016/j.jtho.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bade BC, Brooks MC, Nietert SB, Ulmer A, Thomas DD, Nietert PJ, et al. Assessing the correlation between physical activity and quality of life in advanced lung cancer. Integr Cancer Ther . 2018;17:73–79. doi: 10.1177/1534735416684016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ha DM, Prochazka AV, Bekelman DB, Stevens-Lapsley JE, Chan ED, Keith RL. Association of leisure-time physical activity with health-related quality of life among US lung cancer survivors. JNCI Cancer Spectr . 2021;5:pkaa118. doi: 10.1093/jncics/pkaa118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen HM, Tsai CM, Wu YC, Lin KC, Lin CC. Effect of walking on circadian rhythms and sleep quality of patients with lung cancer: a randomised controlled trial. Br J Cancer . 2016;115:1304–1312. doi: 10.1038/bjc.2016.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tarumi S, Yokomise H, Gotoh M, Kasai Y, Matsuura N, Chang SS, et al. Pulmonary rehabilitation during induction chemoradiotherapy for lung cancer improves pulmonary function. J Thorac Cardiovasc Surg . 2015;149:569–573. doi: 10.1016/j.jtcvs.2014.09.123. [DOI] [PubMed] [Google Scholar]
- 58. Harada H, Yamashita Y, Misumi K, Tsubokawa N, Nakao J, Matsutani J, et al. Multidisciplinary team-based approach for comprehensive preoperative pulmonary rehabilitation including intensive nutritional support for lung cancer patients. PLoS One . 2013;8:e59566. doi: 10.1371/journal.pone.0059566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen HL, Liu K, You QS. Self-efficacy, cancer-related fatigue, and quality of life in patients with resected lung cancer. Eur J Cancer Care (Engl) . 2018;27:e12934. doi: 10.1111/ecc.12934. [DOI] [PubMed] [Google Scholar]
- 60. Brown DJ, McMillan DC, Milroy R. The correlation between fatigue, physical function, the systemic inflammatory response, and psychological distress in patients with advanced lung cancer. Cancer . 2005;103:377–382. doi: 10.1002/cncr.20777. [DOI] [PubMed] [Google Scholar]
- 61. Okuyama T, Tanaka K, Akechi T, Kugaya A, Okamura H, Nishiwaki Y, et al. Fatigue in ambulatory patients with advanced lung cancer: prevalence, correlated factors, and screening. J Pain Symptom Manage . 2001;22:554–564. doi: 10.1016/s0885-3924(01)00305-0. [DOI] [PubMed] [Google Scholar]
- 62. Beach P, Siebeneck B, Buderer NF, Ferner T. Relationship between fatigue and nutritional status in patients receiving radiation therapy to treat lung cancer. Oncol Nurs Forum . 2001;28:1027–1031. [PubMed] [Google Scholar]
- 63. Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J . 2016;48:889–902. doi: 10.1183/13993003.00359-2016. [DOI] [PubMed] [Google Scholar]
- 64. Garces YI, Yang P, Parkinson J, Zhao X, Wampfler JA, Ebbert JO, et al. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest . 2004;126:1733–1741. doi: 10.1378/chest.126.6.1733. [DOI] [PubMed] [Google Scholar]
- 65. Baser S, Shannon VR, Eapen GA, Jimenez CA, Onn A, Lin E, et al. Smoking cessation after diagnosis of lung cancer is associated with a beneficial effect on performance status. Chest . 2006;130:1784–1790. doi: 10.1378/chest.130.6.1784. [DOI] [PubMed] [Google Scholar]
- 66. Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000–2015. MMWR Morb Mortal Wkly Rep . 2017;65:1457–1464. doi: 10.15585/mmwr.mm6552a1. [DOI] [PubMed] [Google Scholar]
- 67. Sugimura H, Yang P. Long-term survivorship in lung cancer: a review. Chest . 2006;129:1088–1097. doi: 10.1378/chest.129.4.1088. [DOI] [PubMed] [Google Scholar]
- 68. O’Higgins CM, Brady B, O’Connor B, Walsh D, Reilly RB. The pathophysiology of cancer-related fatigue: current controversies. Support Care Cancer . 2018;26:3353–3364. doi: 10.1007/s00520-018-4318-7. [DOI] [PubMed] [Google Scholar]
- 69. Cheng KK, Lee DT. Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit Rev Oncol Hematol . 2011;78:127–137. doi: 10.1016/j.critrevonc.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 70. Lin S, Chen Y, Yang L, Zhou J. Pain, fatigue, disturbed sleep and distress comprised a symptom cluster that related to quality of life and functional status of lung cancer surgery patients. J Clin Nurs . 2013;22:1281–1290. doi: 10.1111/jocn.12228. [DOI] [PubMed] [Google Scholar]
- 71. Nishiura M, Tamura A, Nagai H, Matsushima E. Assessment of sleep disturbance in lung cancer patients: relationship between sleep disturbance and pain, fatigue, quality of life, and psychological distress. Palliat Support Care . 2015;13:575–581. doi: 10.1017/S1478951513001119. [DOI] [PubMed] [Google Scholar]
- 72. Cheville AL, Novotny PJ, Sloan JA, Basford JR, Wampfler JA, Garces YI, et al. Fatigue, dyspnea, and cough comprise a persistent symptom cluster up to five years after diagnosis with lung cancer. J Pain Symptom Manage . 2011;42:202–212. doi: 10.1016/j.jpainsymman.2010.10.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Molassiotis A, Lowe M, Blackhall F, Lorigan P. A qualitative exploration of a respiratory distress symptom cluster in lung cancer: cough, breathlessness and fatigue. Lung Cancer . 2011;71:94–102. doi: 10.1016/j.lungcan.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 74. Baden M, Lu L, Drummond FJ, Gavin A, Sharp L. Pain, fatigue and depression symptom cluster in survivors of prostate cancer. Support Care Cancer . 2020;28:4813–4824. doi: 10.1007/s00520-019-05268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fox SW, Lyon DE. Symptom clusters and quality of life in survivors of lung cancer. Oncol Nurs Forum . 2006;33:931–936. doi: 10.1188/06.ONF.931-936. [DOI] [PubMed] [Google Scholar]
- 76. Bjerkeset E, Röhrl K, Schou-Bredal I. Symptom cluster of pain, fatigue, and psychological distress in breast cancer survivors: prevalence and characteristics. Breast Cancer Res Treat . 2020;180:63–71. doi: 10.1007/s10549-020-05522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kwekkeboom KL, Tostrud L, Costanzo E, Coe CL, Serlin RC, Ward SE, et al. The role of inflammation in the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. J Pain Symptom Manage . 2018;55:1286–1295. doi: 10.1016/j.jpainsymman.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol . 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oncology Nursing Society. Fatigue. Pittsburgh, PA: Oncology Nursing Society; 2021. [accessed 2022 Jan 16]. Available from: https://www.ons.org/pep/fatigue?display=pepnavigator&sort_by=created&items_per_page=50 [Google Scholar]
- 80. Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global Burden of Disease Cancer Collaboration Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol . 2019;5:1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Weis J, Tomaszewski KA, Hammerlid E, Ignacio Arraras J, Conroy T, Lanceley A, et al. EORTC Quality of Life Group International psychometric validation of an EORTC quality of life module measuring cancer related fatigue (EORTC QLQ-FA12) J Natl Cancer Inst . 2017;109 doi: 10.1093/jnci/djw273. [DOI] [PubMed] [Google Scholar]
- 82. Baussard L, Stoebner-Delbarre A, Bonnabel L, Huteau ME, Gastou A, Cousson-Gélie F. Development and validation of the daily fatigue cancer scale (DFCS): single-item questions for clinical practice. Eur J Oncol Nurs . 2017;26:42–48. doi: 10.1016/j.ejon.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 83. Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer . 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 84. Ha D, Ries AL, Lippman SM, Fuster MM. Effects of curative-intent lung cancer therapy on functional exercise capacity and patient-reported outcomes. Support Care Cancer . 2020;28:4707–4720. doi: 10.1007/s00520-020-05294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fries JF, Krishnan E, Rose M, Lingala B, Bruce B. Improved responsiveness and reduced sample size requirements of PROMIS physical function scales with item response theory. Arthritis Res Ther . 2011;13:R147. doi: 10.1186/ar3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lai JS, Cella D, Dineen K, Bode R, Von Roenn J, Gershon RC, et al. An item bank was created to improve the measurement of cancer-related fatigue. J Clin Epidemiol . 2005;58:190–197. doi: 10.1016/j.jclinepi.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 87. Chang YJ, Lee JS, Lee CG, Lee WS, Lee KS, Bang SM, et al. Assessment of clinical relevant fatigue level in cancer. Support Care Cancer . 2007;15:891–896. doi: 10.1007/s00520-007-0219-x. [DOI] [PubMed] [Google Scholar]
- 88. Donovan KA, Jacobsen PB. The Fatigue Symptom Inventory: a systematic review of its psychometric properties. Support Care Cancer . 2010;19:169–185. doi: 10.1007/s00520-010-0989-4. [DOI] [PubMed] [Google Scholar]
- 89. Donovan KA, Stein KD, Lee M, Leach CR, Ilozumba O, Jacobsen PB. Systematic review of the multidimensional fatigue symptom inventory-short form. Support Care Cancer . 2015;23:191–212. doi: 10.1007/s00520-014-2389-7. [DOI] [PubMed] [Google Scholar]
- 90. Jacobsen PB. Assessment of fatigue in cancer patients. J Natl Cancer Inst Monogr . 2004;(32):93–97. doi: 10.1093/jncimonographs/lgh010. [DOI] [PubMed] [Google Scholar]
- 91. Payne C, Larkin PJ, McIlfatrick S, Dunwoody L, Gracey JH. Exercise and nutrition interventions in advanced lung cancer: a systematic review. Curr Oncol . 2013;20:e321–e337. doi: 10.3747/co.20.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.World Health Organization. Physical activity. Geneva: World Health Organization; 2021. [accessed 2021 Oct 10]. Available from: https://www.who.int/news-room/fact-sheets/detail/physical-activity [Google Scholar]
- 93. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep . 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 94.World Health Organization. Rehabilitation. Geneva: World Health Organization; 2021. [accessed 2021 Oct 10]. Available from: https://www.who.int/news-room/fact-sheets/detail/rehabilitation [Google Scholar]
- 95. Holland AE, Cox NS, Houchen-Wolloff L, Rochester CL, Garvey C, ZuWallack R, et al. Defining modern pulmonary rehabilitation: an official American Thoracic Society workshop report. Ann Am Thorac Soc . 2021;18:e12–e29. doi: 10.1513/AnnalsATS.202102-146ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ha DM, Zeng C, Chan ED, Gray M, Mazzone PJ, Samet JM, et al. Association of exercise behavior with overall survival in stage I-IIIA lung cancer. Ann Am Thorac Soc . 2021;18:1034–1042. doi: 10.1513/AnnalsATS.202003-235OC. [DOI] [PubMed] [Google Scholar]
- 97. Edbrooke L, Granger CL, Clark RA, Denehy L. Physical activity levels are low in inoperable lung cancer: exploratory analyses from a randomised controlled trial. J Clin Med . 2019;8:1288. doi: 10.3390/jcm8091288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hamada K, Irie M, Fujino Y, Hyodo M, Hanagiri T. Prognostic value of preoperative exercise capacity in patients undergoing thoracoscopic lobectomy for non-small cell lung cancer. Lung Cancer . 2019;128:47–52. doi: 10.1016/j.lungcan.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 99. Cella D, Kallich J, McDermott A, Xu X. The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: results from five randomized clinical trials. Ann Oncol . 2004;15:979–986. doi: 10.1093/annonc/mdh235. [DOI] [PubMed] [Google Scholar]
- 100. Koczywas M, Williams AC, Cristea M, Reckamp K, Grannis FW, Jr, Tiep BL, et al. Longitudinal changes in function, symptom burden, and quality of life in patients with early-stage lung cancer. Ann Surg Oncol . 2013;20:1788–1797. doi: 10.1245/s10434-012-2741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nugent SM, Golden SE, Hooker ER, Sullivan DR, Thomas CR, Jr, Deffebach ME, et al. Longitudinal health-related quality of life among individuals considering treatment for stage I non-small-cell lung cancer. Ann Am Thorac Soc . 2020;17:988–997. doi: 10.1513/AnnalsATS.202001-029OC. [DOI] [PubMed] [Google Scholar]
- 102. Brown LM, Gosdin MM, Cooke DT, Apesoa-Varano EC, Kratz AL. Health-related quality of life after lobectomy for lung cancer: conceptual framework and measurement. Ann Thorac Surg . 2020;110:1840–1846. doi: 10.1016/j.athoracsur.2020.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hechtner M, Eichler M, Wehler B, Buhl R, Sebastian M, Stratmann J, et al. Quality of life in NSCLC survivors—a multicenter cross-sectional study. J Thorac Oncol . 2019;14:420–435. doi: 10.1016/j.jtho.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 104. Ha D, Ries AL, Mazzone PJ, Lippman SM, Fuster MM. Exercise capacity and cancer-specific quality of life following curative intent treatment of stage I-IIIA lung cancer. Support Care Cancer . 2018;26:2459–2469. doi: 10.1007/s00520-018-4078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Coups EJ, Park BJ, Feinstein MB, Steingart RM, Egleston BL, Wilson DJ, et al. Correlates of physical activity among lung cancer survivors. Psychooncology . 2009;18:395–404. doi: 10.1002/pon.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ostroff JS, Krebs P, Coups EJ, Burkhalter JE, Feinstein MB, Steingart RM, et al. Health-related quality of life among early-stage, non-small cell, lung cancer survivors. Lung Cancer . 2011;71:103–108. doi: 10.1016/j.lungcan.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Solberg Nes L, Liu H, Patten CA, Rausch SM, Sloan JA, Garces YI, et al. Physical activity level and quality of life in long term lung cancer survivors. Lung Cancer . 2012;77:611–616. doi: 10.1016/j.lungcan.2012.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lowery AE, Krebs P, Coups EJ, Feinstein MB, Burkhalter JE, Park BJ, et al. Impact of symptom burden in post-surgical non-small cell lung cancer survivors. Support Care Cancer . 2014;22:173–180. doi: 10.1007/s00520-013-1968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Steffens D, Beckenkamp PR, Hancock M, Solomon M, Young J. Preoperative exercise halves the postoperative complication rate in patients with lung cancer: a systematic review of the effect of exercise on complications, length of stay and quality of life in patients with cancer. Br J Sports Med . 2018;52:344. doi: 10.1136/bjsports-2017-098032. [DOI] [PubMed] [Google Scholar]
- 110. Assouline B, Cools E, Schorer R, Kayser B, Elia N, Licker M. Preoperative exercise training to prevent postoperative pulmonary complications in adults undergoing major surgery: a systematic review and meta-analysis with trial sequential analysis. Ann Am Thorac Soc . 2021;18:678–688. doi: 10.1513/AnnalsATS.202002-183OC. [DOI] [PubMed] [Google Scholar]
- 111. Edvardsen E, Skjønsberg OH, Holme I, Nordsletten L, Borchsenius F, Anderssen SA. High-intensity training following lung cancer surgery: a randomised controlled trial. Thorax . 2015;70:244–250. doi: 10.1136/thoraxjnl-2014-205944. [DOI] [PubMed] [Google Scholar]
- 112. Cavalheri V, Burtin C, Formico VR, Nonoyama ML, Jenkins S, Spruit MA, et al. Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database Syst Rev . 2019;6:CD009955. doi: 10.1002/14651858.CD009955.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Peddle-McIntyre CJ, Singh F, Thomas R, Newton RU, Galvão DA, Cavalheri V. Exercise training for advanced lung cancer. Cochrane Database Syst Rev . 2019;2:CD012685. doi: 10.1002/14651858.CD012685.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Presley CJ, Canavan M, Wang SY, Feder SL, Kapo J, Saphire ML, et al. Severe functional limitation due to pain & emotional distress and subsequent receipt of prescription medications among older adults with cancer. J Geriatr Oncol . 2020;11:960–968. doi: 10.1016/j.jgo.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Holm LV, Hansen DG, Johansen C, Vedsted P, Larsen PV, Kragstrup J, et al. Participation in cancer rehabilitation and unmet needs: a population-based cohort study. Support Care Cancer . 2012;20:2913–2924. doi: 10.1007/s00520-012-1420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gould MK, Munoz-Plaza CE, Hahn EE, Lee JS, Parry C, Shen E. Comorbidity profiles and their effect on treatment selection and survival among patients with lung cancer. Ann Am Thorac Soc . 2017;14:1571–1580. doi: 10.1513/AnnalsATS.201701-030OC. [DOI] [PubMed] [Google Scholar]
- 117. Williams CD, Salama JK, Moghanaki D, Karas TZ, Kelley MJ. Impact of race on treatment and survival among U.S. veterans with early-stage lung cancer. J Thorac Oncol . 2016;11:1672–1681. doi: 10.1016/j.jtho.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 118. Islam KM, Jiang X, Anggondowati T, Lin G, Ganti AK. Comorbidity and survival in lung cancer patients. Cancer Epidemiol Biomarkers Prev . 2015;24:1079–1085. doi: 10.1158/1055-9965.EPI-15-0036. [DOI] [PubMed] [Google Scholar]
- 119. Nipp RD, El-Jawahri A, D’Arpino SM, Chan A, Fuh CX, Johnson PC, et al. Symptoms of posttraumatic stress disorder among hospitalized patients with cancer. Cancer . 2018;124:3445–3453. doi: 10.1002/cncr.31576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ni J, Feng J, Denehy L, Wu Y, Xu L, Granger CL. Symptoms of posttraumatic stress disorder and associated risk factors in patients with lung cancer: a longitudinal observational study. Integr Cancer Ther . 2018;17:1195–1203. doi: 10.1177/1534735418807970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hall DL, Jimenez RB, Perez GK, Rabin J, Quain K, Yeh GY, et al. Fear of cancer recurrence: a model examination of physical symptoms, emotional distress, and health behavior change. J Oncol Pract . 2019;15:e787–e797. doi: 10.1200/JOP.18.00787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nekhlyudov L, Mollica MA, Jacobsen PB, Mayer DK, Shulman LN, Geiger AM. Developing a quality of cancer survivorship care framework: implications for clinical care, research, and policy. J Natl Cancer Inst . 2019;111:1120–1130. doi: 10.1093/jnci/djz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. El-Jawahri A, Traeger L, Park ER, Greer JA, Pirl WF, Lennes IT, et al. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer . 2014;120:278–285. doi: 10.1002/cncr.28369. [DOI] [PubMed] [Google Scholar]
- 124. Nipp RD, Greer JA, El-Jawahri A, Moran SM, Traeger L, Jacobs JM, et al. Coping and prognostic awareness in patients with advanced cancer. J Clin Oncol . 2017;35:2551–2557. doi: 10.1200/JCO.2016.71.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Rand KL, Banno DA, Shea AM, Cripe LD. Life and treatment goals of patients with advanced, incurable cancer. Support Care Cancer . 2016;24:2953–2962. doi: 10.1007/s00520-016-3113-6. [DOI] [PubMed] [Google Scholar]
- 126. Butow PN, Turner J, Gilchrist J, Sharpe L, Smith AB, Fardell JE, et al. Randomized trial of ConquerFear: a novel, theoretically based psychosocial intervention for fear of cancer recurrence. J Clin Oncol . 2017;35:4066–4077. doi: 10.1200/JCO.2017.73.1257. [DOI] [PubMed] [Google Scholar]
- 127. Spathis A, Fife K, Blackhall F, Dutton S, Bahadori R, Wharton R, et al. Modafinil for the treatment of fatigue in lung cancer: results of a placebo-controlled, double-blind, randomized trial. J Clin Oncol . 2014;32:1882–1888. doi: 10.1200/JCO.2013.54.4346. [DOI] [PubMed] [Google Scholar]
- 128. Edbrooke L, Aranda S, Granger CL, McDonald CF, Krishnasamy M, Mileshkin L, et al. Multidisciplinary home-based rehabilitation in inoperable lung cancer: a randomised controlled trial. Thorax . 2019;74:787–796. doi: 10.1136/thoraxjnl-2018-212996. [DOI] [PubMed] [Google Scholar]
- 129. Raz DJ, Sun V, Kim JY, Williams AC, Koczywas M, Cristea M, et al. Long-term effect of an interdisciplinary supportive care intervention for lung cancer survivors after surgical procedures. Ann Thorac Surg . 2016;101:495–502. doi: 10.1016/j.athoracsur.2015.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Quist M, Sommer MS, Vibe-Petersen J, Staerkind MB, Langer SW, Larsen KR, et al. Early initiated postoperative rehabilitation reduces fatigue in patients with operable lung cancer: a randomized trial. Lung Cancer . 2018;126:125–132. doi: 10.1016/j.lungcan.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 131. Chan CW, Richardson A, Richardson J. Managing symptoms in patients with advanced lung cancer during radiotherapy: results of a psychoeducational randomized controlled trial. J Pain Symptom Manage . 2011;41:347–357. doi: 10.1016/j.jpainsymman.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 132. Steel JL, Geller DA, Kim KH, Butterfield LH, Spring M, Grady J, et al. Web-based collaborative care intervention to manage cancer-related symptoms in the palliative care setting. Cancer . 2016;122:1270–1282. doi: 10.1002/cncr.29906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Stegmann ME, Brandenbarg D, Reyners AK, van Geffen WH, Hiltermann TJN, Berendsen AJ. Prioritisation of treatment goals among older patients with non-curable cancer: the Option randomised controlled trial in Dutch primary care. Br J Gen Pract . 2020;70:e450–e456. doi: 10.3399/bjgp20X710405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bekelman DB, Allen LA, McBryde CF, Hattler B, Fairclough DL, Havranek EP, et al. Effect of a collaborative care intervention vs usual care on health status of patients with chronic heart failure: the CASA randomized clinical trial. JAMA Intern Med . 2018;178:511–519. doi: 10.1001/jamainternmed.2017.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Morais Pereira Simões MDS, de Barros Gonze B, Leite Proença N, Tonon Lauria V, Demarchi Silva Terra V, da Costa Padovani R, et al. Use of a smartphone app combined with gamification to increase the level of physical activity of adults and older adults: protocol of a sequential multiple assignment randomized trial. Trials . 2019;20:780. doi: 10.1186/s13063-019-3879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wyatt G, Lehto R, Guha-Niyogi P, Brewer S, Victorson D, Pace T, et al. Reflexology and meditative practices for symptom management among people with cancer: results from a sequential multiple assignment randomized trial. Res Nurs Health . 2021;44:796–810. doi: 10.1002/nur.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Collins LM, et al. A randomized controlled trial of an optimized smoking treatment delivered in primary care. Ann Behav Med . 2018;52:854–864. doi: 10.1093/abm/kax059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness–implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care . 2012;50:217–226. doi: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Almirall D, Nahum-Shani I, Sherwood NE, Murphy SA. Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Transl Behav Med . 2014;4:260–274. doi: 10.1007/s13142-014-0265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Collins LM, Baker TB, Mermelstein RJ, Piper ME, Jorenby DE, Smith SS, et al. The multiphase optimization strategy for engineering effective tobacco use interventions. Ann Behav Med . 2011;41:208–226. doi: 10.1007/s12160-010-9253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Landes SJ, McBain SA, Curran GM. An introduction to effectiveness–implementation hybrid designs. Psychiatry Res . 2019;280:112513. doi: 10.1016/j.psychres.2019.112513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Armijo-Olivo S, Dennett L, Arienti C, Dahchi M, Arokoski J, Heinemann AW, et al. Blinding in rehabilitation research: empirical evidence on the association between blinding and treatment effect estimates. Am J Phys Med Rehabil . 2020;99:198–209. doi: 10.1097/PHM.0000000000001377. [DOI] [PubMed] [Google Scholar]
- 143. Campbell KL, Cormie P, Weller S, Alibhai SMH, Bolam KA, Campbell A, et al. Exercise recommendation for people with bone metastases: expert consensus for health care providers and exercise professionals. JCO Oncol Pract . 2022;18:e697–e709. doi: 10.1200/OP.21.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Nici L, Singh SJ, Holland AE, ZuWallack RL. Opportunities and challenges in expanding pulmonary rehabilitation into the home and community. Am J Respir Crit Care Med . 2019;200:822–827. doi: 10.1164/rccm.201903-0548PP. [DOI] [PubMed] [Google Scholar]
- 145.National Library of Medicine, National Center for Biotechnology Information. Telerehabilitation. Bethesda, MD: National Library of Medicine; 2022. [accessed 2022 Jun 16]. Available from: https://www.ncbi.nlm.nih.gov/mesh/2009969 [Google Scholar]
- 146. Cox NS, Dal Corso S, Hansen H, McDonald CF, Hill CJ, Zanaboni P, et al. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev . 2021;1:CD013040. doi: 10.1002/14651858.CD013040.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hwang R, Bruning J, Morris N, Mandrusiak A, Russell T. A systematic review of the effects of telerehabilitation in patients with cardiopulmonary diseases. J Cardiopulm Rehabil Prev . 2015;35:380–389. doi: 10.1097/HCR.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 148. Holland AE, Mahal A, Hill CJ, Lee AL, Burge AT, Cox NS, et al. Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial. Thorax . 2017;72:57–65. doi: 10.1136/thoraxjnl-2016-208514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Moy ML, Collins RJ, Martinez CH, Kadri R, Roman P, Holleman RG, et al. An Internet-mediated pedometer-based program improves health-related quality-of-life domains and daily step counts in COPD: a randomized controlled trial. Chest . 2015;148:128–137. doi: 10.1378/chest.14-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Cheville AL, Moynihan T, Herrin J, Loprinzi C, Kroenke K. Effect of collaborative telerehabilitation on functional impairment and pain among patients with advanced-stage cancer: a randomized clinical trial. JAMA Oncol . 2019;5:644–652. doi: 10.1001/jamaoncol.2019.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Driessen EJ, Peeters ME, Bongers BC, Maas HA, Bootsma GP, van Meeteren NL, et al. Effects of prehabilitation and rehabilitation including a home-based component on physical fitness, adherence, treatment tolerance, and recovery in patients with non-small cell lung cancer: a systematic review. Crit Rev Oncol Hematol . 2017;114:63–76. doi: 10.1016/j.critrevonc.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 152. Granger CL, Denehy L, Remedios L, Retica S, Phongpagdi P, Hart N, et al. Barriers to translation of physical activity into the lung cancer model of care: a qualitative study of clinicians’ perspectives. Ann Am Thorac Soc . 2016;13:2215–2222. doi: 10.1513/AnnalsATS.201607-540OC. [DOI] [PubMed] [Google Scholar]
- 153. Hamann HA, Ver Hoeve ES, Carter-Harris L, Studts JL, Ostroff JS. Multilevel opportunities to address lung cancer stigma across the cancer control continuum. J Thorac Oncol . 2018;13:1062–1075. doi: 10.1016/j.jtho.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. McBride CM, Ostroff JS. Teachable moments for promoting smoking cessation: the context of cancer care and survivorship. Cancer Contr . 2003;10:325–333. doi: 10.1177/107327480301000407. [DOI] [PubMed] [Google Scholar]
- 155. Dresler C, Warren GW, Arenberg D, Yang P, Steliga MA, Cummings KM, et al. “Teachable moment” interventions in lung cancer: why action matters. J Thorac Oncol . 2018;13:603–605. doi: 10.1016/j.jtho.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 156. Cheville AL, Rhudy L, Basford JR, Griffin JM, Flores AM. How receptive are patients with late stage cancer to rehabilitation services and what are the sources of their resistance? Arch Phys Med Rehabil . 2017;98:203–210. doi: 10.1016/j.apmr.2016.08.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lenze EJ, Lenard E, Bland M, Barco P, Miller JP, Yingling M, et al. Effect of enhanced medical rehabilitation on functional recovery in older adults receiving skilled nursing care after acute rehabilitation: a randomized clinical trial. JAMA Netw Open . 2019;2:e198199. doi: 10.1001/jamanetworkopen.2019.8199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Mieras A, Pasman HRW, Klop HT, Onwuteaka-Philipsen BD, Tarasevych S, Tiemessen MA, et al. What goals do patients and oncologists have when starting medical treatment for metastatic lung cancer? Clin Lung Cancer . 2021;22:242–251.e5. doi: 10.1016/j.cllc.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 159. Garvey C, Novitch RS, Porte P, Casaburi R. Healing pulmonary rehabilitation in the United States: a call to action for ATS members. Am J Respir Crit Care Med . 2019;199:944–946. doi: 10.1164/rccm.201809-1711ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Spruit MA, Pitta F, McAuley E, ZuWallack RL, Nici L. Pulmonary rehabilitation and physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2015;192:924–933. doi: 10.1164/rccm.201505-0929CI. [DOI] [PubMed] [Google Scholar]
- 161. Burge AT, Holland AE, McDonald CF, Abramson MJ, Hill CJ, Lee AL, et al. Home-based pulmonary rehabilitation for COPD using minimal resources: an economic analysis. Respirology . 2020;25:183–190. doi: 10.1111/resp.13667. [DOI] [PubMed] [Google Scholar]
- 162. Coll-Planas L, Blancafort Alias S, Tully M, Caserotti P, Giné-Garriga M, Blackburn N, et al. SITLESS group Exercise referral schemes enhanced by self-management strategies to reduce sedentary behaviour and increase physical activity among community-dwelling older adults from four European countries: protocol for the process evaluation of the SITLESS randomised controlled trial. BMJ Open . 2019;9:e027073. doi: 10.1136/bmjopen-2018-027073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bickerdike L, Booth A, Wilson PM, Farley K, Wright K. Social prescribing: less rhetoric and more reality: a systematic review of the evidence. BMJ Open . 2017;7:e013384. doi: 10.1136/bmjopen-2016-013384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Quist M, Adamsen L, Rørth M, Laursen JH, Christensen KB, Langer SW. The impact of a multidimensional exercise intervention on physical and functional capacity, anxiety, and depression in patients with advanced-stage lung cancer undergoing chemotherapy. Integr Cancer Ther . 2015;14:341–349. doi: 10.1177/1534735415572887. [DOI] [PubMed] [Google Scholar]
- 165. Temel JS, Greer JA, Goldberg S, Vogel PD, Sullivan M, Pirl WF, et al. A structured exercise program for patients with advanced non-small cell lung cancer. J Thorac Oncol . 2009;4:595–601. doi: 10.1097/JTO.0b013e31819d18e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Elshahat S, Treanor C, Donnelly M. Factors influencing physical activity participation among people living with or beyond cancer: a systematic scoping review. Int J Behav Nutr Phys Act . 2021;18:50. doi: 10.1186/s12966-021-01116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Wong JN, McAuley E, Trinh L. Physical activity programming and counseling preferences among cancer survivors: a systematic review. Int J Behav Nutr Phys Act . 2018;15:48. doi: 10.1186/s12966-018-0680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Turner RR, Steed L, Quirk H, Greasley RU, Saxton JM, Taylor SJ, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev . 2018;9:CD010192. doi: 10.1002/14651858.CD010192.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Davidson JR, MacLean AW, Brundage MD, Schulze K. Sleep disturbance in cancer patients. Soc Sci Med . 2002;54:1309–1321. doi: 10.1016/s0277-9536(01)00043-0. [DOI] [PubMed] [Google Scholar]
- 170. Palesh OG, Roscoe JA, Mustian KM, Roth T, Savard J, Ancoli-Israel S, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol . 2010;28:292–298. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ha DM, Prochazka AV, Bekelman DB, Stevens-Lapsley JE, Studts JL, Keith RL. Modifiable factors associated with health-related quality of life among lung cancer survivors following curative intent therapy. Lung Cancer . 2022;163:42–50. doi: 10.1016/j.lungcan.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 172. Silberfarb PM, Hauri PJ, Oxman TE, Schnurr P. Assessment of sleep in patients with lung cancer and breast cancer. J Clin Oncol . 1993;11:997–1004. doi: 10.1200/JCO.1993.11.5.997. [DOI] [PubMed] [Google Scholar]
- 173. Chen ML, Yu CT, Yang CH. Sleep disturbances and quality of life in lung cancer patients undergoing chemotherapy. Lung Cancer . 2008;62:391–400. doi: 10.1016/j.lungcan.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 174. Le Guen Y, Gagnadoux F, Hureaux J, Jeanfaivre T, Meslier N, Racineux JL, et al. Sleep disturbances and impaired daytime functioning in outpatients with newly diagnosed lung cancer. Lung Cancer . 2007;58:139–143. doi: 10.1016/j.lungcan.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 175. Sarna L. Correlates of symptom distress in women with lung cancer. Cancer Pract . 1993;1:21–28. [PubMed] [Google Scholar]
- 176. Ginsburg ML, Quirt C, Ginsburg AD, MacKillop WJ. Psychiatric illness and psychosocial concerns of patients with newly diagnosed lung cancer. CMAJ . 1995;152:701–708. [PMC free article] [PubMed] [Google Scholar]
- 177. Vena C, Parker K, Allen R, Bliwise D, Jain S, Kimble L. Sleep-wake disturbances and quality of life in patients with advanced lung cancer. Oncol Nurs Forum . 2006;33:761–769. doi: 10.1188/06.ONF.761-769. [DOI] [PubMed] [Google Scholar]
- 178. Halle IH, Westgaard TK, Wahba A, Oksholm T, Rustøen T, Gjeilo KH. Trajectory of sleep disturbances in patients undergoing lung cancer surgery: a prospective study. Interact Cardiovasc Thorac Surg . 2017;25:285–291. doi: 10.1093/icvts/ivx076. [DOI] [PubMed] [Google Scholar]
- 179. Dean GE, Abu Sabbah E, Yingrengreung S, Ziegler P, Chen H, Steinbrenner LM, et al. Sleeping with the enemy: sleep and quality of life in patients with lung cancer. Cancer Nurs . 2015;38:60–70. doi: 10.1097/NCC.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 180. Dean GE, Redeker NS, Wang YJ, Rogers AE, Dickerson SS, Steinbrenner LM, et al. Sleep, mood, and quality of life in patients receiving treatment for lung cancer. Oncol Nurs Forum . 2013;40:441–451. doi: 10.1188/13.ONF.441-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Dreher M, Krüger S, Schulze-Olden S, Keszei A, Storre JH, Woehrle H, et al. Sleep-disordered breathing in patients with newly diagnosed lung cancer. BMC Pulm Med . 2018;18:72. doi: 10.1186/s12890-018-0645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Huang HY, Lin SW, Chuang LP, Wang CL, Sun MH, Li HY, et al. Severe OSA associated with higher risk of mortality in stage III and IV lung cancer. J Clin Sleep Med . 2020;16:1091–1098. doi: 10.5664/jcsm.8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wu IHC, Balachandran DD, Faiz SA, Bashoura L, Escalante CP, Manzullo EF. Characteristics of cancer-related fatigue and concomitant sleep disturbance in cancer patients. J Pain Symptom Manage . 2022;63:e1–e8. doi: 10.1016/j.jpainsymman.2021.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Barsevick A. Defining the symptom cluster: how far have we come? Semin Oncol Nurs . 2016;32:334–350. doi: 10.1016/j.soncn.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 185. Choi S, Ryu E. Effects of symptom clusters and depression on the quality of life in patients with advanced lung cancer. Eur J Cancer Care (Engl) . 2018;27 doi: 10.1111/ecc.12508. [DOI] [PubMed] [Google Scholar]
- 186. Barsevick A, Beck SL, Dudley WN, Wong B, Berger AM, Whitmer K, et al. Efficacy of an intervention for fatigue and sleep disturbance during cancer chemotherapy. J Pain Symptom Manage . 2010;40:200–216. doi: 10.1016/j.jpainsymman.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Papadopoulos D, Kiagia M, Charpidou A, Gkiozos I, Syrigos K. Psychological correlates of sleep quality in lung cancer patients under chemotherapy: a single‐center cross‐sectional study. Psychooncology . 2019;28:1879–1886. doi: 10.1002/pon.5167. [DOI] [PubMed] [Google Scholar]
- 188. Davis MP, Behm B, Balachandran D. Looking both ways before crossing the street: assessing the benefits and risk of opioids in treating patients at risk of sleep-disordered breathing for pain and dyspnea. J Opioid Manag . 2017;13:183–196. doi: 10.5055/jom.2017.0385. [DOI] [PubMed] [Google Scholar]
- 189. Irwin MR, Olmstead RE, Ganz PA, Haque R. Sleep disturbance, inflammation and depression risk in cancer survivors. Brain Behav Immun . 2013;30:S58–S67. doi: 10.1016/j.bbi.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Miaskowski C, Barsevick A, Berger A, Casagrande R, Grady PA, Jacobsen P, et al. Advancing symptom science through symptom cluster research: expert panel proceedings and recommendations. J Natl Cancer Inst . 2017;109:djw253. doi: 10.1093/jnci/djw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Hopwood P, Stephens RJ. Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. J Clin Oncol . 2000;18:893–903. doi: 10.1200/JCO.2000.18.4.893. [DOI] [PubMed] [Google Scholar]
- 192. Berger AM, Yennu S, Million R. Update on interventions focused on symptom clusters: what has been tried and what have we learned? Curr Opin Support Palliat Care . 2013;7:60–66. doi: 10.1097/SPC.0b013e32835c7d88. [DOI] [PubMed] [Google Scholar]
- 193. Yang P, Cheville AL, Wampfler JA, Garces YI, Jatoi A, Clark MM, et al. Quality of life and symptom burden among long-term lung cancer survivors. J Thorac Oncol . 2012;7:64–70. doi: 10.1097/JTO.0b013e3182397b3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Chang WP, Lin CC. Changes in the sleep-wake rhythm, sleep quality, mood, and quality of life of patients receiving treatment for lung cancer: a longitudinal study. Chronobiol Int . 2017;34:451–461. doi: 10.1080/07420528.2017.1293678. [DOI] [PubMed] [Google Scholar]
- 195. Parker KP, Bliwise DL, Ribeiro M, Jain SR, Vena CI, Kohles-Baker MK, et al. Sleep/wake patterns of individuals with advanced cancer measured by ambulatory polysomnography. J Clin Oncol . 2008;26:2464–2472. doi: 10.1200/JCO.2007.12.2135. [DOI] [PubMed] [Google Scholar]
- 196. Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun . 2003;17:321–328. doi: 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 197. Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun . 2013;30:S163–S170. doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 198. Balachandran DD, Miller MA, Faiz SA, Yennurajalingam S, Innominato PF. Evaluation and management of sleep and circadian rhythm disturbance in cancer. Curr Treat Options Oncol . 2021;22:81. doi: 10.1007/s11864-021-00872-x. [DOI] [PubMed] [Google Scholar]
- 199. Fouladbakhsh JM, Davis JE, Yarandi HN. A pilot study of the feasibility and outcomes of yoga for lung cancer survivors. Oncol Nurs Forum . 2014;41:162–174. doi: 10.1188/14.ONF.162-174. [DOI] [PubMed] [Google Scholar]
- 200. Milbury K, Chaoul A, Engle R, Liao Z, Yang C, Carmack C, et al. Couple-based Tibetan yoga program for lung cancer patients and their caregivers. Psychooncology . 2015;24:117–120. doi: 10.1002/pon.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Sullivan DR, Medysky ME, Tyzik AL, Dieckmann NF, Denfeld QE, Winters-Stone K. Feasibility and potential benefits of partner-supported yoga on psychosocial and physical function among lung cancer patients. Psychooncology . 2021;30:789–793. doi: 10.1002/pon.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. McNicholas WT, Verbraecken J, Marin JM. Sleep disorders in COPD: the forgotten dimension. Eur Respir Rev . 2013;22:365–375. doi: 10.1183/09059180.00003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. McNicholas WT, Hansson D, Schiza S, Grote L. Sleep in chronic respiratory disease: COPD and hypoventilation disorders. Eur Respir Rev . 2019;28:190064. doi: 10.1183/16000617.0064-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Shawon MS, Perret JL, Senaratna CV, Lodge C, Hamilton GS, Dharmage SC. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: a systematic review. Sleep Med Rev . 2017;32:58–68. doi: 10.1016/j.smrv.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 205. Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med . 2010;182:325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 206. Machado MC, Vollmer WM, Togeiro SM, Bilderback AL, Oliveira MV, Leitão FS, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Respir J . 2010;35:132–137. doi: 10.1183/09031936.00192008. [DOI] [PubMed] [Google Scholar]
- 207. Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farré R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med . 2012;186:190–194. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, Duran-Cantolla J, Peña MdeL, Masdeu MJ, et al. Spanish Sleep Network Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med . 2013;187:99–105. doi: 10.1164/rccm.201209-1671OC. [DOI] [PubMed] [Google Scholar]
- 209. Gozal D, Farré R, Nieto FJ. Putative links between sleep apnea and cancer: from hypotheses to evolving evidence. Chest . 2015;148:1140–1147. doi: 10.1378/chest.15-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Almendros I, Montserrat JM, Ramírez J, Torres M, Duran-Cantolla J, Navajas D, et al. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J . 2012;39:215–217. doi: 10.1183/09031936.00185110. [DOI] [PubMed] [Google Scholar]
- 211. Hakim F, Wang Y, Zhang SX, Zheng J, Yolcu ES, Carreras A, et al. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res . 2014;74:1329–1337. doi: 10.1158/0008-5472.CAN-13-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Martínez-García MA, Campos-Rodriguez F, Durán-Cantolla J, de la Peña M, Masdeu MJ, González M, et al. Spanish Sleep Network Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med . 2014;15:742–748. doi: 10.1016/j.sleep.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 213. Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med . 2014;10:355–362. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Sillah A, Watson NF, Gozal D, Phipps AI. Obstructive sleep apnea severity and subsequent risk for cancer incidence. Prev Med Rep . 2019;15:100886. doi: 10.1016/j.pmedr.2019.100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Kendzerska T, Povitz M, Leung RS, Boulos MI, McIsaac DI, Murray BJ, et al. Obstructive sleep apnea and incident cancer: a large retrospective multicenter clinical cohort study. Cancer Epidemiol Biomarkers Prev . 2021;30:295–304. doi: 10.1158/1055-9965.EPI-20-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Pérez-Warnisher MT, Cabezas E, Troncoso MF, Gómez T, Melchor R, Pinillos EJ, et al. Sleep disordered breathing and nocturnal hypoxemia are very prevalent in a lung cancer screening population and may condition lung cancer screening findings: results of the prospective Sleep Apnea In Lung Cancer Screening (SAILS) study. Sleep Med . 2019;54:181–186. doi: 10.1016/j.sleep.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 217. Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. ATS/ERS Task Force on Pulmonary Rehabilitation An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med . 2013;188:e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 218.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 219. Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage . 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Fisher MI, Cohn JC, Harrington SE, Lee JQ, Malone D. Screening and assessment of cancer-related fatigue: a clinical practice guideline for health care providers. Phys Ther . 2022;102:pzac120. doi: 10.1093/ptj/pzac120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Seyidova-Khoshknabi D, Davis MP, Walsh D. Review article: a systematic review of cancer-related fatigue measurement questionnaires. Am J Hosp Palliat Care . 2011;28:119–129. doi: 10.1177/1049909110381590. [DOI] [PubMed] [Google Scholar]